- Research article
- Open access
- Published: 30 April 2021

A scoping review of the literature featuring research ethics and research integrity cases
- Anna Catharina Vieira Armond ORCID: orcid.org/0000-0002-7121-5354 1 ,
- Bert Gordijn 2 ,
- Jonathan Lewis 2 ,
- Mohammad Hosseini 2 ,
- János Kristóf Bodnár 1 ,
- Soren Holm 3 , 4 &
- Péter Kakuk 5
BMC Medical Ethics volume 22 , Article number: 50 ( 2021 ) Cite this article
13k Accesses
23 Citations
28 Altmetric
Metrics details
The areas of Research Ethics (RE) and Research Integrity (RI) are rapidly evolving. Cases of research misconduct, other transgressions related to RE and RI, and forms of ethically questionable behaviors have been frequently published. The objective of this scoping review was to collect RE and RI cases, analyze their main characteristics, and discuss how these cases are represented in the scientific literature.
The search included cases involving a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework. A search was conducted in PubMed, Web of Science, SCOPUS, JSTOR, Ovid, and Science Direct in March 2018, without language or date restriction. Data relating to the articles and the cases were extracted from case descriptions.
A total of 14,719 records were identified, and 388 items were included in the qualitative synthesis. The papers contained 500 case descriptions. After applying the eligibility criteria, 238 cases were included in the analysis. In the case analysis, fabrication and falsification were the most frequently tagged violations (44.9%). The non-adherence to pertinent laws and regulations, such as lack of informed consent and REC approval, was the second most frequently tagged violation (15.7%), followed by patient safety issues (11.1%) and plagiarism (6.9%). 80.8% of cases were from the Medical and Health Sciences, 11.5% from the Natural Sciences, 4.3% from Social Sciences, 2.1% from Engineering and Technology, and 1.3% from Humanities. Paper retraction was the most prevalent sanction (45.4%), followed by exclusion from funding applications (35.5%).
Conclusions
Case descriptions found in academic journals are dominated by discussions regarding prominent cases and are mainly published in the news section of journals. Our results show that there is an overrepresentation of biomedical research cases over other scientific fields compared to its proportion in scientific publications. The cases mostly involve fabrication, falsification, and patient safety issues. This finding could have a significant impact on the academic representation of misbehaviors. The predominance of fabrication and falsification cases might diverge the attention of the academic community from relevant but less visible violations, and from recently emerging forms of misbehaviors.
Peer Review reports
There has been an increase in academic interest in research ethics (RE) and research integrity (RI) over the past decade. This is due, among other reasons, to the changing research environment with new and complex technologies, increased pressure to publish, greater competition in grant applications, increased university-industry collaborative programs, and growth in international collaborations [ 1 ]. In addition, part of the academic interest in RE and RI is due to highly publicized cases of misconduct [ 2 ].
There is a growing body of published RE and RI cases, which may contribute to public attitudes regarding both science and scientists [ 3 ]. Different approaches have been used in order to analyze RE and RI cases. Studies focusing on ORI files (Office of Research Integrity) [ 2 ], retracted papers [ 4 ], quantitative surveys [ 5 ], data audits [ 6 ], and media coverage [ 3 ] have been conducted to understand the context, causes, and consequences of these cases.
Analyses of RE and RI cases often influence policies on responsible conduct of research [ 1 ]. Moreover, details about cases facilitate a broader understanding of issues related to RE and RI and can drive interventions to address them. Currently, there are no comprehensive studies that have collected and evaluated the RE and RI cases available in the academic literature. This review has been developed by members of the EnTIRE consortium to generate information on the cases that will be made available on the Embassy of Good Science platform ( www.embassy.science ). Two separate analyses have been conducted. The first analysis uses identified research articles to explore how the literature presents cases of RE and RI, in relation to the year of publication, country, article genre, and violation involved. The second analysis uses the cases extracted from the literature in order to characterize the cases and analyze them concerning the violations involved, sanctions, and field of science.
This scoping review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and PRISMA Extension for Scoping Reviews (PRISMA-ScR). The full protocol was pre-registered and it is available at https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5bde92120&appId=PPGMS .
Eligibility
Articles with non-fictional case(s) involving a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework, were included. Cases unrelated to scientific activities, research institutions, academic or industrial research and publication were excluded. Articles that did not contain a substantial description of the case were also excluded.
A normative framework consists of explicit rules, formulated in laws, regulations, codes, and guidelines, as well as implicit rules, which structure local research practices and influence the application of explicitly formulated rules. Therefore, if a case involves a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework, then it does so on the basis of explicit and/or implicit rules governing RE and RI practice.
Search strategy
A search was conducted in PubMed, Web of Science, SCOPUS, JSTOR, Ovid, and Science Direct in March 2018, without any language or date restrictions. Two parallel searches were performed with two sets of medical subject heading (MeSH) terms, one for RE and another for RI. The parallel searches generated two sets of data thereby enabling us to analyze and further investigate the overlaps in, differences in, and evolution of, the representation of RE and RI cases in the academic literature. The terms used in the first search were: (("research ethics") AND (violation OR unethical OR misconduct)). The terms used in the parallel search were: (("research integrity") AND (violation OR unethical OR misconduct)). The search strategy’s validity was tested in a pilot search, in which different keyword combinations and search strings were used, and the abstracts of the first hundred hits in each database were read (Additional file 1 ).
After searching the databases with these two search strings, the titles and abstracts of extracted items were read by three contributors independently (ACVA, PK, and KB). Articles that could potentially meet the inclusion criteria were identified. After independent reading, the three contributors compared their results to determine which studies were to be included in the next stage. In case of a disagreement, items were reassessed in order to reach a consensus. Subsequently, qualified items were read in full.
Data extraction
Data extraction processes were divided by three assessors (ACVA, PK and KB). Each list of extracted data generated by one assessor was cross-checked by the other two. In case of any inconsistencies, the case was reassessed to reach a consensus. The following categories were employed to analyze the data of each extracted item (where available): (I) author(s); (II) title; (III) year of publication; (IV) country (according to the first author's affiliation); (V) article genre; (VI) year of the case; (VII) country in which the case took place; (VIII) institution(s) and person(s) involved; (IX) field of science (FOS-OECD classification)[ 7 ]; (X) types of violation (see below); (XI) case description; and (XII) consequences for persons or institutions involved in the case.
Two sets of data were created after the data extraction process. One set was used for the analysis of articles and their representation in the literature, and the other set was created for the analysis of cases. In the set for the analysis of articles, all eligible items, including duplicate cases (cases found in more than one paper, e.g. Hwang case, Baltimore case) were included. The aim was to understand the historical aspects of violations reported in the literature as well as the paper genre in which cases are described and discussed. For this set, the variables of the year of publication (III); country (IV); article genre (V); and types of violation (X) were analyzed.
For the analysis of cases, all duplicated cases and cases that did not contain enough information about particularities to differentiate them from others (e.g. names of the people or institutions involved, country, date) were excluded. In this set, prominent cases (i.e. those found in more than one paper) were listed only once, generating a set containing solely unique cases. These additional exclusion criteria were applied to avoid multiple representations of cases. For the analysis of cases, the variables: (VI) year of the case; (VII) country in which the case took place; (VIII) institution(s) and person(s) involved; (IX) field of science (FOS-OECD classification); (X) types of violation; (XI) case details; and (XII) consequences for persons or institutions involved in the case were considered.
Article genre classification
We used ten categories to capture the differences in genre. We included a case description in a “news” genre if a case was published in the news section of a scientific journal or newspaper. Although we have not developed a search strategy for newspaper articles, some of them (e.g. New York Times) are indexed in scientific databases such as Pubmed. The same method was used to allocate case descriptions to “editorial”, “commentary”, “misconduct notice”, “retraction notice”, “review”, “letter” or “book review”. We applied the “case analysis” genre if a case description included a normative analysis of the case. The “educational” genre was used when a case description was incorporated to illustrate RE and RI guidelines or institutional policies.
Categorization of violations
For the extraction process, we used the articles’ own terminology when describing violations/ethical issues involved in the event (e.g. plagiarism, falsification, ghost authorship, conflict of interest, etc.) to tag each article. In case the terminology was incompatible with the case description, other categories were added to the original terminology for the same case. Subsequently, the resulting list of terms was standardized using the list of major and minor misbehaviors developed by Bouter and colleagues [ 8 ]. This list consists of 60 items classified into four categories: Study design, data collection, reporting, and collaboration issues. (Additional file 2 ).
Systematic search
A total of 11,641 records were identified through the RE search and 3078 in the RI search. The results of the parallel searches were combined and the duplicates removed. The remaining 10,556 records were screened, and at this stage, 9750 items were excluded because they did not fulfill the inclusion criteria. 806 items were selected for full-text reading. Subsequently, 388 articles were included in the qualitative synthesis (Fig. 1 ).
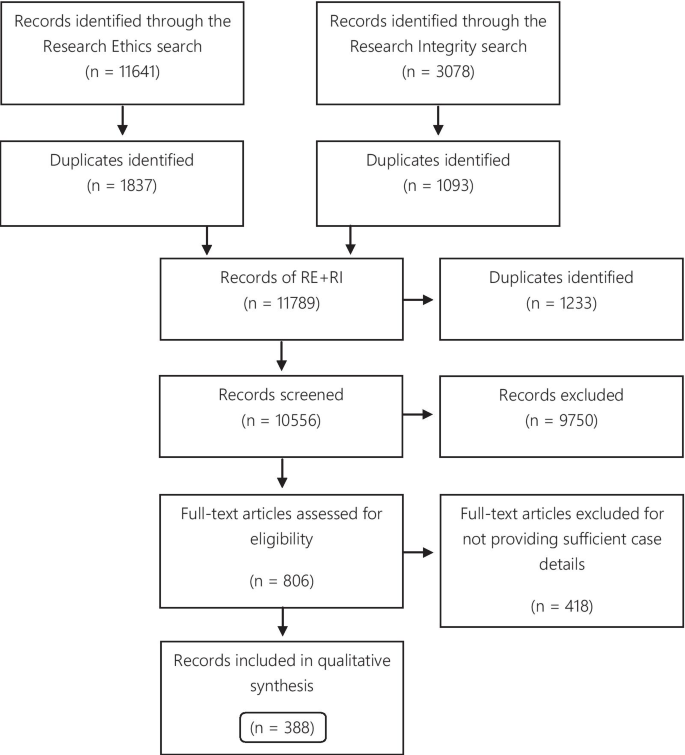
Flow diagram
Of the 388 articles, 157 were only identified via the RE search, 87 exclusively via the RI search, and 144 were identified via both search strategies. The eligible articles contained 500 case descriptions, which were used for the analysis of the publications articles analysis. 256 case descriptions discussed the same 50 cases. The Hwang case was the most frequently described case, discussed in 27 articles. Furthermore, the top 10 most described cases were found in 132 articles (Table 1 ).
For the analysis of cases, 206 (41.2% of the case descriptions) duplicates were excluded, and 56 (11.2%) cases were excluded for not providing enough information to distinguish them from other cases, resulting in 238 eligible cases.
Analysis of the articles
The categories used to classify the violations include those that pertain to the different kinds of scientific misconduct (falsification, fabrication, plagiarism), detrimental research practices (authorship issues, duplication, peer-review, errors in experimental design, and mentoring), and “other misconduct” (according to the definitions from the National Academies of Sciences and Medicine, [ 1 ]). Each case could involve more than one type of violation. The majority of cases presented more than one violation or ethical issue, with a mean of 1.56 violations per case. Figure 2 presents the frequency of each violation tagged to the articles. Falsification and fabrication were the most frequently tagged violations. The violations accounted respectively for 29.1% and 30.0% of the number of taggings (n = 780), and they were involved in 46.8% and 45.4% of the articles (n = 500 case descriptions). Problems with informed consent represented 9.1% of the number of taggings and 14% of the articles, followed by patient safety (6.7% and 10.4%) and plagiarism (5.4% and 8.4%). Detrimental research practices, such as authorship issues, duplication, peer-review, errors in experimental design, mentoring, and self-citation were mentioned cumulatively in 7.0% of the articles.
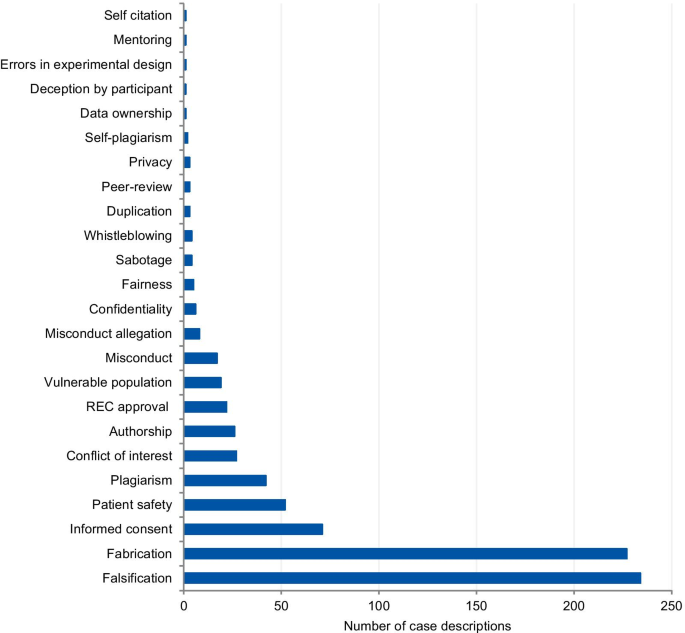
Tagged violations from the article analysis
Analysis of the cases
Figure 3 presents the frequency and percentage of each violation found in the cases. Each case could include more than one item from the list. The 238 cases were tagged 305 times, with a mean of 1.28 items per case. Fabrication and falsification were the most frequently tagged violations (44.9%), involved in 57.7% of the cases (n = 238). The non-adherence to pertinent laws and regulations, such as lack of informed consent and REC approval, was the second most frequently tagged violation (15.7%) and involved in 20.2% of the cases. Patient safety issues were the third most frequently tagged violations (11.1%), involved in 14.3% of the cases, followed by plagiarism (6.9% and 8.8%). The list of major and minor misbehaviors [ 8 ] classifies the items into study design, data collection, reporting, and collaboration issues. Our results show that 56.0% of the tagged violations involved issues in reporting, 16.4% in data collection, 15.1% involved collaboration issues, and 12.5% in the study design. The items in the original list that were not listed in the results were not involved in any case collected.
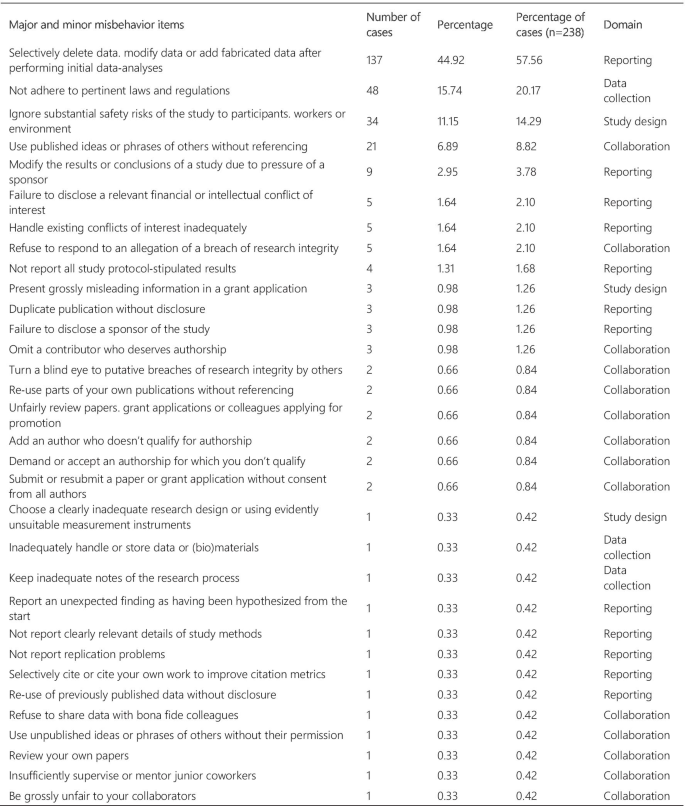
Major and minor misbehavior items from the analysis of cases
Article genre
The articles were mostly classified into “news” (33.0%), followed by “case analysis” (20.9%), “editorial” (12.1%), “commentary” (10.8%), “misconduct notice” (10.3%), “retraction notice” (6.4%), “letter” (3.6%), “educational paper” (1.3%), “review” (1%), and “book review” (0.3%) (Fig. 4 ). The articles classified into “news” and “case analysis” included predominantly prominent cases. Items classified into “news” often explored all the investigation findings step by step for the associated cases as the case progressed through investigations, and this might explain its high prevalence. The case analyses included mainly normative assessments of prominent cases. The misconduct and retraction notices included the largest number of unique cases, although a relatively large portion of the retraction and misconduct records could not be included because of insufficient case details. The articles classified into “editorial”, “commentary” and “letter” also included unique cases.
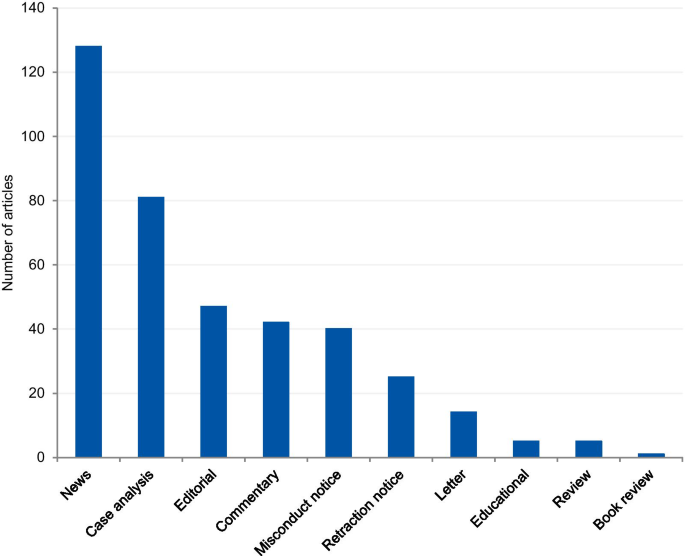
Article genre of included articles
Article analysis
The dates of the eligible articles range from 1983 to 2018 with notable peaks between 1990 and 1996, most probably associated with the Gallo [ 9 ] and Imanishi-Kari cases [ 10 ], and around 2005 with the Hwang [ 11 ], Wakefield [ 12 ], and CNEP trial cases [ 13 ] (Fig. 5 ). The trend line shows an increase in the number of articles over the years.
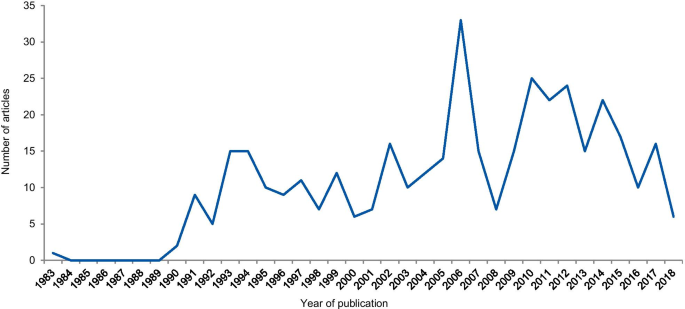
Frequency of articles according to the year of publication
Case analysis
The dates of included cases range from 1798 to 2016. Two cases occurred before 1910, one in 1798 and the other in 1845. Figure 6 shows the number of cases per year from 1910. An increase in the curve started in the early 1980s, reaching the highest frequency in 2004 with 13 cases.
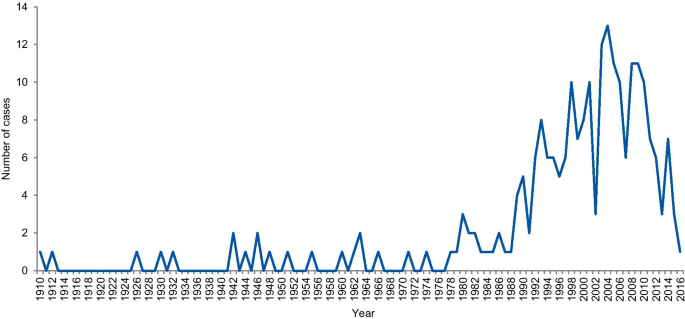
Frequency of cases per year
Geographical distribution
The first analysis concerned the authors’ affiliation and the corresponding author’s address. Where the article contained more than one country in the affiliation list, only the first author’s location was considered. Eighty-one articles were excluded because the authors’ affiliations were not available, and 307 articles were included in the analysis. The articles originated from 26 different countries (Additional file 3 ). Most of the articles emanated from the USA and the UK (61.9% and 14.3% of articles, respectively), followed by Canada (4.9%), Australia (3.3%), China (1.6%), Japan (1.6%), Korea (1.3%), and New Zealand (1.3%). Some of the most discussed cases occurred in the USA; the Imanishi-Kari, Gallo, and Schön cases [ 9 , 10 ]. Intensely discussed cases are also associated with Canada (Fisher/Poisson and Olivieri cases), the UK (Wakefield and CNEP trial cases), South Korea (Hwang case), and Japan (RIKEN case) [ 12 , 14 ]. In terms of percentages, North America and Europe stand out in the number of articles (Fig. 7 ).
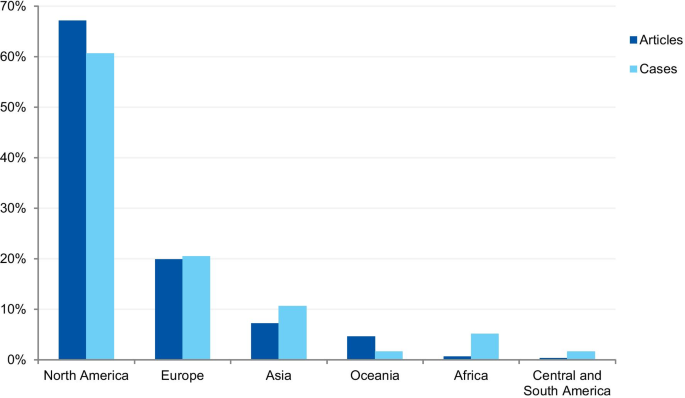
Percentage of articles and cases by continent
The case analysis involved the location where the case took place, taking into account the institutions involved in the case. For cases involving more than one country, all the countries were considered. Three cases were excluded from the analysis due to insufficient information. In the case analysis, 40 countries were involved in 235 different cases (Additional file 4 ). Our findings show that most of the reported cases occurred in the USA and the United Kingdom (59.6% and 9.8% of cases, respectively). In addition, a number of cases occurred in Canada (6.0%), Japan (5.5%), China (2.1%), and Germany (2.1%). In terms of percentages, North America and Europe stand out in the number of cases (Fig. 7 ). To enable comparison, we have additionally collected the number of published documents according to country distribution, available on SCImago Journal & Country Rank [ 16 ]. The numbers correspond to the documents published from 1996 to 2019. The USA occupies the first place in the number of documents, with 21.9%, followed by China (11.1%), UK (6.3%), Germany (5.5%), and Japan (4.9%).
Field of science
The cases were classified according to the field of science. Four cases (1.7%) could not be classified due to insufficient information. Where information was available, 80.8% of cases were from the Medical and Health Sciences, 11.5% from the Natural Sciences, 4.3% from Social Sciences, 2.1% from Engineering and Technology, and 1.3% from Humanities (Fig. 8 ). Additionally, we have retrieved the number of published documents according to scientific field distribution, available on SCImago [ 16 ]. Of the total number of scientific publications, 41.5% are related to natural sciences, 22% to engineering, 25.1% to health and medical sciences, 7.8% to social sciences, 1.9% to agricultural sciences, and 1.7% to the humanities.
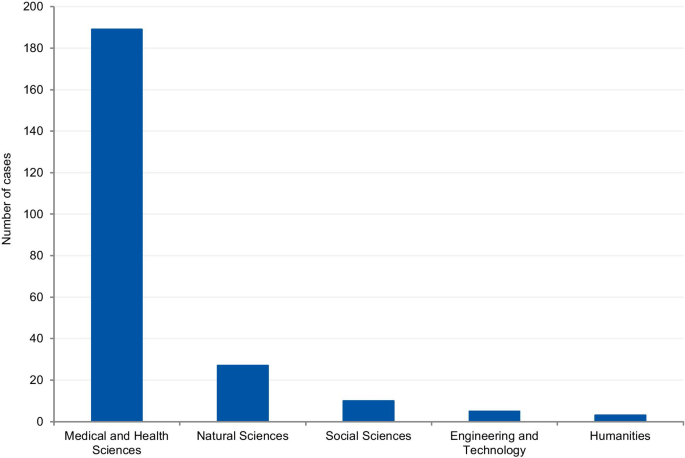
Field of science from the analysis of cases
This variable aimed to collect information on possible consequences and sanctions imposed by funding agencies, scientific journals and/or institutions. 97 cases could not be classified due to insufficient information. 141 cases were included. Each case could potentially include more than one outcome. Most of cases (45.4%) involved paper retraction, followed by exclusion from funding applications (35.5%). (Table 2 ).
RE and RI cases have been increasingly discussed publicly, affecting public attitudes towards scientists and raising awareness about ethical issues, violations, and their wider consequences [ 5 ]. Different approaches have been applied in order to quantify and address research misbehaviors [ 5 , 17 , 18 , 19 ]. However, most cases are investigated confidentially and the findings remain undisclosed even after the investigation [ 19 , 20 ]. Therefore, the study aimed to collect the RE and RI cases available in the scientific literature, understand how the cases are discussed, and identify the potential of case descriptions to raise awareness on RE and RI.
We collected and analyzed 500 detailed case descriptions from 388 articles and our results show that they mostly relate to extensively discussed and notorious cases. Approximately half of all included cases was mentioned in at least two different articles, and the top ten most commonly mentioned cases were discussed in 132 articles.
The prominence of certain cases in the literature, based on the number of duplicated cases we found (e.g. Hwang case), can be explained by the type of article in which cases are discussed and the type of violation involved in the case. In the article genre analysis, 33% of the cases were described in the news section of scientific publications. Our findings show that almost all article genres discuss those cases that are new and in vogue. Once the case appears in the public domain, it is intensely discussed in the media and by scientists, and some prominent cases have been discussed for more than 20 years (Table 1 ). Misconduct and retraction notices were exceptions in the article genre analysis, as they presented mostly unique cases. The misconduct notices were mainly found on the NIH repository, which is indexed in the searched databases. Some federal funding agencies like NIH usually publicize investigation findings associated with the research they fund. The results derived from the NIH repository also explains the large proportion of articles from the US (61.9%). However, in some cases, only a few details are provided about the case. For cases that have not received federal funding and have not been reported to federal authorities, the investigation is conducted by local institutions. In such instances, the reporting of findings depends on each institution’s policy and willingness to disclose information [ 21 ]. The other exception involves retraction notices. Despite the existence of ethical guidelines [ 22 ], there is no uniform and a common approach to how a journal should report a retraction. The Retraction Watch website suggests two lists of information that should be included in a retraction notice to satisfy the minimum and optimum requirements [ 22 , 23 ]. As well as disclosing the reason for the retraction and information regarding the retraction process, optimal notices should include: (I) the date when the journal was first alerted to potential problems; (II) details regarding institutional investigations and associated outcomes; (III) the effects on other papers published by the same authors; (IV) statements about more recent replications only if and when these have been validated by a third party; (V) details regarding the journal’s sanctions; and (VI) details regarding any lawsuits that have been filed regarding the case. The lack of transparency and information in retraction notices was also noted in studies that collected and evaluated retractions [ 24 ]. According to Resnik and Dinse [ 25 ], retractions notices related to cases of misconduct tend to avoid naming the specific violation involved in the case. This study found that only 32.8% of the notices identify the actual problem, such as fabrication, falsification, and plagiarism, and 58.8% reported the case as replication failure, loss of data, or error. Potential explanations for euphemisms and vague claims in retraction notices authored by editors could pertain to the possibility of legal actions from the authors, honest or self-reported errors, and lack of resources to conduct thorough investigations. In addition, the lack of transparency can also be explained by the conflicts of interests of the article’s author(s), since the notices are often written by the authors of the retracted article.
The analysis of violations/ethical issues shows the dominance of fabrication and falsification cases and explains the high prevalence of prominent cases. Non-adherence to laws and regulations (REC approval, informed consent, and data protection) was the second most prevalent issue, followed by patient safety, plagiarism, and conflicts of interest. The prevalence of the five most tagged violations in the case analysis was higher than the prevalence found in the analysis of articles that involved the same violations. The only exceptions are fabrication and falsification cases, which represented 45% of the tagged violations in the analysis of cases, and 59.1% in the article analysis. This disproportion shows a predilection for the publication of discussions related to fabrication and falsification when compared to other serious violations. Complex cases involving these types of violations make good headlines and this follows a custom pattern of writing about cases that catch the public and media’s attention [ 26 ]. The way cases of RE and RI violations are explored in the literature gives a sense that only a few scientists are “the bad apples” and they are usually discovered, investigated, and sanctioned accordingly. This implies that the integrity of science, in general, remains relatively untouched by these violations. However, studies on misconduct determinants show that scientific misconduct is a systemic problem, which involves not only individual factors, but structural and institutional factors as well, and that a combined effort is necessary to change this scenario [ 27 , 28 ].
Analysis of cases
A notable increase in RE and RI cases occurred in the 1990s, with a gradual increase until approximately 2006. This result is in agreement with studies that evaluated paper retractions [ 24 , 29 ]. Although our study did not focus only on retractions, the trend is similar. This increase in cases should not be attributed only to the increase in the number of publications, since studies that evaluated retractions show that the percentage of retraction due to fraud has increased almost ten times since 1975, compared to the total number of articles. Our results also show a gradual reduction in the number of cases from 2011 and a greater drop in 2015. However, this reduction should be considered cautiously because many investigations take years to complete and have their findings disclosed. ORI has shown that from 2001 to 2010 the investigation of their cases took an average of 20.48 months with a maximum investigation time of more than 9 years [ 24 ].
The countries from which most cases were reported were the USA (59.6%), the UK (9.8%), Canada (6.0%), Japan (5.5%), and China (2.1%). When analyzed by continent, the highest percentage of cases took place in North America, followed by Europe, Asia, Oceania, Latin America, and Africa. The predominance of cases from the USA is predictable, since the country publishes more scientific articles than any other country, with 21.8% of the total documents, according to SCImago [ 16 ]. However, the same interpretation does not apply to China, which occupies the second position in the ranking, with 11.2%. These differences in the geographical distribution were also found in a study that collected published research on research integrity [ 30 ]. The results found by Aubert Bonn and Pinxten (2019) show that studies in the United States accounted for more than half of the sample collected, and although China is one of the leaders in scientific publications, it represented only 0.7% of the sample. Our findings can also be explained by the search strategy that included only keywords in English. Since the majority of RE and RI cases are investigated and have their findings locally disclosed, the employment of English keywords and terms in the search strategy is a limitation. Moreover, our findings do not allow us to draw inferences regarding the incidence or prevalence of misconduct around the world. Instead, it shows where there is a culture of publicly disclosing information and openly discussing RE and RI cases in English documents.
Scientific field analysis
The results show that 80.8% of reported cases occurred in the medical and health sciences whilst only 1.3% occurred in the humanities. This disciplinary difference has also been observed in studies on research integrity climates. A study conducted by Haven and colleagues, [ 28 ] associated seven subscales of research climate with the disciplinary field. The subscales included: (1) Responsible Conduct of Research (RCR) resources, (2) regulatory quality, (3) integrity norms, (4) integrity socialization, (5) supervisor/supervisee relations, (6) (lack of) integrity inhibitors, and (7) expectations. The results, based on the seven subscale scores, show that researchers from the humanities and social sciences have the lowest perception of the RI climate. By contrast, the natural sciences expressed the highest perception of the RI climate, followed by the biomedical sciences. There are also significant differences in the depth and extent of the regulatory environments of different disciplines (e.g. the existence of laws, codes of conduct, policies, relevant ethics committees, or authorities). These findings corroborate our results, as those areas of science most familiar with RI tend to explore the subject further, and, consequently, are more likely to publish case details. Although the volume of published research in each research area also influences the number of cases, the predominance of medical and health sciences cases is not aligned with the trends regarding the volume of published research. According to SCImago Journal & Country Rank [ 16 ], natural sciences occupy the first place in the number of publications (41,5%), followed by the medical and health sciences (25,1%), engineering (22%), social sciences (7,8%), and the humanities (1,7%). Moreover, biomedical journals are overrepresented in the top scientific journals by IF ranking, and these journals usually have clear policies for research misconduct. High-impact journals are more likely to have higher visibility and scrutiny, and consequently, more likely to have been the subject of misconduct investigations. Additionally, the most well-known general medical journals, including NEJM, The Lancet, and the BMJ, employ journalists to write their news sections. Since these journals have the resources to produce extensive news sections, it is, therefore, more likely that medical cases will be discussed.
Violations analysis
In the analysis of violations, the cases were categorized into major and minor misbehaviors. Most cases involved data fabrication and falsification, followed by cases involving non-adherence to laws and regulations, patient safety, plagiarism, and conflicts of interest. When classified by categories, 12.5% of the tagged violations involved issues in the study design, 16.4% in data collection, 56.0% in reporting, and 15.1% involved collaboration issues. Approximately 80% of the tagged violations involved serious research misbehaviors, based on the ranking of research misbehaviors proposed by Bouter and colleagues. However, as demonstrated in a meta-analysis by Fanelli (2009), most self-declared cases involve questionable research practices. In the meta-analysis, 33.7% of scientists admitted questionable research practices, and 72% admitted when asked about the behavior of colleagues. This finding contrasts with an admission rate of 1.97% and 14.12% for cases involving fabrication, falsification, and plagiarism. However, Fanelli’s meta-analysis does not include data about research misbehaviors in its wider sense but focuses on behaviors that bias research results (i.e. fabrication and falsification, intentional non-publication of results, biased methodology, misleading reporting). In our study, the majority of cases involved FFP (66.4%). Overrepresentation of some types of violations, and underrepresentation of others, might lead to misguided efforts, as cases that receive intense publicity eventually influence policies relating to scientific misconduct and RI [ 20 ].
Sanctions analysis
The five most prevalent outcomes were paper retraction, followed by exclusion from funding applications, exclusion from service or position, dismissal and suspension, and paper correction. This result is similar to that found by Redman and Merz [ 31 ], who collected data from misconduct cases provided by the ORI. Moreover, their results show that fabrication and falsification cases are 8.8 times more likely than others to receive funding exclusions. Such cases also received, on average, 0.6 more sanctions per case. Punishments for misconduct remain under discussion, ranging from the criminalization of more serious forms of misconduct [ 32 ] to social punishments, such as those recently introduced by China [ 33 ]. The most common sanction identified by our analysis—paper retraction—is consistent with the most prevalent types of violation, that is, falsification and fabrication.
Publicizing scientific misconduct
The lack of publicly available summaries of misconduct investigations makes it difficult to share experiences and evaluate the effectiveness of policies and training programs. Publicizing scientific misconduct can have serious consequences and creates a stigma around those involved in the case. For instance, publicized allegations can damage the reputation of the accused even when they are later exonerated [ 21 ]. Thus, for published cases, it is the responsibility of the authors and editors to determine whether the name(s) of those involved should be disclosed. On the one hand, it is envisaged that disclosing the name(s) of those involved will encourage others in the community to foster good standards. On the other hand, it is suggested that someone who has made a mistake should have the right to a chance to defend his/her reputation. Regardless of whether a person's name is left out or disclosed, case reports have an important educational function and can help guide RE- and RI-related policies [ 34 ]. A recent paper published by Gunsalus [ 35 ] proposes a three-part approach to strengthen transparency in misconduct investigations. The first part consists of a checklist [ 36 ]. The second suggests that an external peer reviewer should be involved in investigative reporting. The third part calls for the publication of the peer reviewer’s findings.
Limitations
One of the possible limitations of our study may be our search strategy. Although we have conducted pilot searches and sensitivity tests to reach the most feasible and precise search strategy, we cannot exclude the possibility of having missed important cases. Furthermore, the use of English keywords was another limitation of our search. Since most investigations are performed locally and published in local repositories, our search only allowed us to access cases from English-speaking countries or discussed in academic publications written in English. Additionally, it is important to note that the published cases are not representative of all instances of misconduct, since most of them are never discovered, and when discovered, not all are fully investigated or have their findings published. It is also important to note that the lack of information from the extracted case descriptions is a limitation that affects the interpretation of our results. In our review, only 25 retraction notices contained sufficient information that allowed us to include them in our analysis in conformance with the inclusion criteria. Although our search strategy was not focused specifically on retraction and misconduct notices, we believe that if sufficiently detailed information was available in such notices, the search strategy would have identified them.
Case descriptions found in academic journals are dominated by discussions regarding prominent cases and are mainly published in the news section of journals. Our results show that there is an overrepresentation of biomedical research cases over other scientific fields when compared with the volume of publications produced by each field. Moreover, published cases mostly involve fabrication, falsification, and patient safety issues. This finding could have a significant impact on the academic representation of ethical issues for RE and RI. The predominance of fabrication and falsification cases might diverge the attention of the academic community from relevant but less visible violations and ethical issues, and recently emerging forms of misbehaviors.
Availability of data and materials
This review has been developed by members of the EnTIRE project in order to generate information on the cases that will be made available on the Embassy of Good Science platform ( www.embassy.science ). The dataset supporting the conclusions of this article is available in the Open Science Framework (OSF) repository in https://osf.io/3xatj/?view_only=313a0477ab554b7489ee52d3046398b9 .
National Academies of Sciences E, Medicine. Fostering integrity in research. National Academies Press; 2017.
Davis MS, Riske-Morris M, Diaz SR. Causal factors implicated in research misconduct: evidence from ORI case files. Sci Eng Ethics. 2007;13(4):395–414. https://doi.org/10.1007/s11948-007-9045-2 .
Article Google Scholar
Ampollini I, Bucchi M. When public discourse mirrors academic debate: research integrity in the media. Sci Eng Ethics. 2020;26(1):451–74. https://doi.org/10.1007/s11948-019-00103-5 .
Hesselmann F, Graf V, Schmidt M, Reinhart M. The visibility of scientific misconduct: a review of the literature on retracted journal articles. Curr Sociol La Sociologie contemporaine. 2017;65(6):814–45. https://doi.org/10.1177/0011392116663807 .
Martinson BC, Anderson MS, de Vries R. Scientists behaving badly. Nature. 2005;435(7043):737–8. https://doi.org/10.1038/435737a .
Loikith L, Bauchwitz R. The essential need for research misconduct allegation audits. Sci Eng Ethics. 2016;22(4):1027–49. https://doi.org/10.1007/s11948-016-9798-6 .
OECD. Revised field of science and technology (FoS) classification in the Frascati manual. Working Party of National Experts on Science and Technology Indicators 2007. p. 1–12.
Bouter LM, Tijdink J, Axelsen N, Martinson BC, ter Riet G. Ranking major and minor research misbehaviors: results from a survey among participants of four World Conferences on Research Integrity. Res Integrity Peer Rev. 2016;1(1):17. https://doi.org/10.1186/s41073-016-0024-5 .
Greenberg DS. Resounding echoes of Gallo case. Lancet. 1995;345(8950):639.
Dresser R. Giving scientists their due. The Imanishi-Kari decision. Hastings Center Rep. 1997;27(3):26–8.
Hong ST. We should not forget lessons learned from the Woo Suk Hwang’s case of research misconduct and bioethics law violation. J Korean Med Sci. 2016;31(11):1671–2. https://doi.org/10.3346/jkms.2016.31.11.1671 .
Opel DJ, Diekema DS, Marcuse EK. Assuring research integrity in the wake of Wakefield. BMJ (Clinical research ed). 2011;342(7790):179. https://doi.org/10.1136/bmj.d2 .
Wells F. The Stoke CNEP Saga: did it need to take so long? J R Soc Med. 2010;103(9):352–6. https://doi.org/10.1258/jrsm.2010.10k010 .
Normile D. RIKEN panel finds misconduct in controversial paper. Science. 2014;344(6179):23. https://doi.org/10.1126/science.344.6179.23 .
Wager E. The Committee on Publication Ethics (COPE): Objectives and achievements 1997–2012. La Presse Médicale. 2012;41(9):861–6. https://doi.org/10.1016/j.lpm.2012.02.049 .
SCImago nd. SJR — SCImago Journal & Country Rank [Portal]. http://www.scimagojr.com . Accessed 03 Feb 2021.
Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta-analysis of survey data. PLoS ONE. 2009;4(5):e5738. https://doi.org/10.1371/journal.pone.0005738 .
Steneck NH. Fostering integrity in research: definitions, current knowledge, and future directions. Sci Eng Ethics. 2006;12(1):53–74. https://doi.org/10.1007/PL00022268 .
DuBois JM, Anderson EE, Chibnall J, Carroll K, Gibb T, Ogbuka C, et al. Understanding research misconduct: a comparative analysis of 120 cases of professional wrongdoing. Account Res. 2013;20(5–6):320–38. https://doi.org/10.1080/08989621.2013.822248 .
National Academy of Sciences NAoE, Institute of Medicine Panel on Scientific R, the Conduct of R. Responsible Science: Ensuring the Integrity of the Research Process: Volume I. Washington (DC): National Academies Press (US) Copyright (c) 1992 by the National Academy of Sciences; 1992.
Bauchner H, Fontanarosa PB, Flanagin A, Thornton J. Scientific misconduct and medical journals. JAMA. 2018;320(19):1985–7. https://doi.org/10.1001/jama.2018.14350 .
COPE Council. COPE Guidelines: Retraction Guidelines. 2019. https://doi.org/10.24318/cope.2019.1.4 .
Retraction Watch. What should an ideal retraction notice look like? 2015, May 21. https://retractionwatch.com/2015/05/21/what-should-an-ideal-retraction-notice-look-like/ .
Fang FC, Steen RG, Casadevall A. Misconduct accounts for the majority of retracted scientific publications. Proc Natl Acad Sci USA. 2012;109(42):17028–33. https://doi.org/10.1073/pnas.1212247109 .
Resnik DB, Dinse GE. Scientific retractions and corrections related to misconduct findings. J Med Ethics. 2013;39(1):46–50. https://doi.org/10.1136/medethics-2012-100766 .
de Vries R, Anderson MS, Martinson BC. Normal misbehavior: scientists talk about the ethics of research. J Empir Res Hum Res Ethics JERHRE. 2006;1(1):43–50. https://doi.org/10.1525/jer.2006.1.1.43 .
Sovacool BK. Exploring scientific misconduct: isolated individuals, impure institutions, or an inevitable idiom of modern science? J Bioethical Inquiry. 2008;5(4):271. https://doi.org/10.1007/s11673-008-9113-6 .
Haven TL, Tijdink JK, Martinson BC, Bouter LM. Perceptions of research integrity climate differ between academic ranks and disciplinary fields: results from a survey among academic researchers in Amsterdam. PLoS ONE. 2019;14(1):e0210599. https://doi.org/10.1371/journal.pone.0210599 .
Trikalinos NA, Evangelou E, Ioannidis JPA. Falsified papers in high-impact journals were slow to retract and indistinguishable from nonfraudulent papers. J Clin Epidemiol. 2008;61(5):464–70. https://doi.org/10.1016/j.jclinepi.2007.11.019 .
Aubert Bonn N, Pinxten W. A decade of empirical research on research integrity: What have we (not) looked at? J Empir Res Hum Res Ethics. 2019;14(4):338–52. https://doi.org/10.1177/1556264619858534 .
Redman BK, Merz JF. Scientific misconduct: do the punishments fit the crime? Science. 2008;321(5890):775. https://doi.org/10.1126/science.1158052 .
Bülow W, Helgesson G. Criminalization of scientific misconduct. Med Health Care Philos. 2019;22(2):245–52. https://doi.org/10.1007/s11019-018-9865-7 .
Cyranoski D. China introduces “social” punishments for scientific misconduct. Nature. 2018;564(7736):312. https://doi.org/10.1038/d41586-018-07740-z .
Bird SJ. Publicizing scientific misconduct and its consequences. Sci Eng Ethics. 2004;10(3):435–6. https://doi.org/10.1007/s11948-004-0001-0 .
Gunsalus CK. Make reports of research misconduct public. Nature. 2019;570(7759):7. https://doi.org/10.1038/d41586-019-01728-z .
Gunsalus CK, Marcus AR, Oransky I. Institutional research misconduct reports need more credibility. JAMA. 2018;319(13):1315–6. https://doi.org/10.1001/jama.2018.0358 .
Download references
Acknowledgements
The authors wish to thank the EnTIRE research group. The EnTIRE project (Mapping Normative Frameworks for Ethics and Integrity of Research) aims to create an online platform that makes RE+RI information easily accessible to the research community. The EnTIRE Consortium is composed by VU Medical Center, Amsterdam, gesinn. It Gmbh & Co Kg, KU Leuven, University of Split School of Medicine, Dublin City University, Central European University, University of Oslo, University of Manchester, European Network of Research Ethics Committees.
EnTIRE project (Mapping Normative Frameworks for Ethics and Integrity of Research) has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N 741782. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Department of Behavioural Sciences, Faculty of Medicine, University of Debrecen, Móricz Zsigmond krt. 22. III. Apartman Diákszálló, Debrecen, 4032, Hungary
Anna Catharina Vieira Armond & János Kristóf Bodnár
Institute of Ethics, School of Theology, Philosophy and Music, Dublin City University, Dublin, Ireland
Bert Gordijn, Jonathan Lewis & Mohammad Hosseini
Centre for Social Ethics and Policy, School of Law, University of Manchester, Manchester, UK
Center for Medical Ethics, HELSAM, Faculty of Medicine, University of Oslo, Oslo, Norway
Center for Ethics and Law in Biomedicine, Central European University, Budapest, Hungary
Péter Kakuk
You can also search for this author in PubMed Google Scholar
Contributions
All authors (ACVA, BG, JL, MH, JKB, SH and PK) developed the idea for the article. ACVA, PK, JKB performed the literature search and data analysis, ACVA and PK produced the draft, and all authors critically revised it. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Anna Catharina Vieira Armond .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Pilot search and search strategy.
Additional file 2
. List of Major and minor misbehavior items (Developed by Bouter LM, Tijdink J, Axelsen N, Martinson BC, ter Riet G. Ranking major and minor research misbehaviors: results from a survey among participants of four World Conferences on Research Integrity. Research integrity and peer review. 2016;1(1):17. https://doi.org/10.1186/s41073-016-0024-5 ).
Additional file 3
. Table containing the number and percentage of countries included in the analysis of articles.
Additional file 4
. Table containing the number and percentage of countries included in the analysis of the cases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Armond, A.C.V., Gordijn, B., Lewis, J. et al. A scoping review of the literature featuring research ethics and research integrity cases. BMC Med Ethics 22 , 50 (2021). https://doi.org/10.1186/s12910-021-00620-8
Download citation
Received : 06 October 2020
Accepted : 21 April 2021
Published : 30 April 2021
DOI : https://doi.org/10.1186/s12910-021-00620-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics
- Research integrity
- Scientific misconduct
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
Understanding Research Ethics
- First Online: 22 April 2022
Cite this chapter

- Sarah Cuschieri 2
436 Accesses
As a researcher, whatever your career stage, you need to understand and practice good research ethics. Moral and ethical principles are requisite in research to ensure no deception or harm to participants, scientific community, and society occurs. Failure to follow such principles leads to research misconduct, in which case the researcher faces repercussions ranging from withdrawal of an article from publication to potential job loss. This chapter describes the various types of research misconduct that you should be aware of, i.e., data fabrication and falsification, plagiarism, research bias, data integrity, researcher and funder conflicts of interest. A sound comprehension of research ethics will take you a long way in your career.
- Research ethics
- Scientific bias
- Conflict of interest
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Author information
Authors and affiliations.
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
Sarah Cuschieri
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cuschieri, S. (2022). Understanding Research Ethics. In: A Roadmap to Successful Scientific Publishing. Springer, Cham. https://doi.org/10.1007/978-3-030-99295-8_2
Download citation
DOI : https://doi.org/10.1007/978-3-030-99295-8_2
Published : 22 April 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-99294-1
Online ISBN : 978-3-030-99295-8
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Technical Support
- Find My Rep
You are here
Research Ethics
Preview this book.
- Description
- Aims and Scope
- Editorial Board
- Abstracting / Indexing
- Submission Guidelines
Research Ethics provides a platform for sharing experiences and analysis of ethical issues that are related to the design, conduct, impact and oversight of research. Through open and transparent narrative and analysis of ethical issues in research, it serves to raise awareness, challenge assumptions and help find solutions for complex ethical issues.
Ethical issues are not limited to a specific discipline or type of research. The Editors welcome submissions from any research field (for instance, biomedical, social science, environmental, information technology, or the arts) and a broad range of methodological approaches (for instance, clinical trials, animal experimentation, qualitative studies, laboratory or desk-based research).
Some examples of the topics addressed in Research Ethics include:
- Ethical issues related to the inclusion of vulnerable populations in research
- Ethics dumping
- Benefit sharing
- Ethical issues related to social media research
- Research integrity and research misconduct
- The education and training of researchers and ethics reviewers
- The development and implementation of governance mechanisms
In addition to these applied ethics issues, the journal also welcomes original theoretical papers that contribute to the debate around the normative underpinnings or ethical frameworks for research ethics.
Research Ethics publishes original papers and review articles as well as informative case studies and offers a home for submissions from authors from around the world. The quality of submitted articles is evaluated independently by double-blind peer review. This journal is a member of the Committee on Publication Ethics (COPE).
Research Ethics is aimed at readers and authors who are interested in ethical issues associated with the design and conduct of research, the regulation of research, the procedures and process of ethical review and issues related to scientific integrity. The journal aims to promote, inspire, host and engage in open and public debate about research ethics on an international scale and to contribute to the education of researchers and reviewers of research.
Research Ethics offers a home for submissions from authors from around the world and publishes original papers and review articles as well as informative case studies. The quality of submitted articles is evaluated independently by double-blind peer review.
- Clarivate Analytics: Emerging Sources Citation Index (ESCI)
- Directory of Open Access Journals (DOAJ)
- ERIC (Education Resources Information Center)
Manuscript Submission Guidelines: Research Ethics
This Journal is a member of the Committee on Publication Ethics .
Please read the guidelines below then visit the Journal’s submission site http://mc.manuscriptcentral.com/rea to upload your manuscript. Please note that manuscripts not conforming to these guidelines may be returned.
Only manuscripts of sufficient quality that meet the aims and scope of Research Ethics will be reviewed.
All articles published in Research Ethics are published fully open access under a Creative Commons licence and available worldwide, with readers having barrier-free access to the full-text articles immediately upon publication. From 1st January 2024, an article processing charge (APC) is levied on all article types that are published in the journal.
For authors that are currently eligible for an Open Access Agreement at your institution with Sage , your article will be published at either no direct cost to you or at a deeply discounted rate, depending on the terms of the agreement.
Authors based at institutions in developing countries may also be eligible for an APC waiver, please see our website page on Gold Open Access Article Processing Charge Waivers for further information. Please refer to our page regarding our partnerships around the world if you would like to know more about the Research4Life initiative.
For authors not eligible for a Sage Open Access Agreement, the APC is USD $500.
- Open Access
- Article processing charge (APC)
- What do we publish? 3.1 Aims & Scope 3.2 Article types 3.3 Writing your paper
- Editorial policies 4.1 Peer review policy 4.2 Authorship 4.3 Acknowledgements 4.4 Declaration of conflicting interests 4.5 Research ethics and patient consent 4.6 Clinical trials
- Publishing policies 5.1 Publication ethics 5.2 Contributor's publishing agreement
- Preparing your manuscript 6.1 Main File 6.2 Title Page 6.3 Formatting 6.4 Language 6.5 Artwork, figures and other graphics 6.6 Supplementary material 6.7 Reference style 6.8 English language editing services
- Submitting your manuscript 7.1 ORCID 7.2 Title, keywords and abstracts 7.3 Information required for completing your submission 7.4 Permissions
- On acceptance and publication 8.1 Sage Production 8.2 Online publication 8.3 Promoting your article
- Further information
1. Open Access
Research Ethics is an open access, peer-reviewed journal. Each article accepted by peer review is made freely available online immediately upon publication, is published under a Creative Commons license and will be hosted online in perpetuity.
For general information on open access at Sage please visit the Open Access page or view our Open Access FAQs.
Back to top
2. Article processing charge (APC)
For authors not eligible for a Sage Open Access Agreement, the article processing charge (APC) is USD $500.
3. What do we publish?
3.1 Aims & Scope
Before submitting your manuscript to Research Ethics , please ensure you have read the Aims & Scope .
3.2 Article Types
Research Ethics publishes original articles and review articles as well as informative case studies on the ethical issues associated with the design and conduct of research, the regulation of research, the procedures and process of ethical review, and issues related to scientific integrity. The journal encourages the submission of the following types of manuscripts:
- Original articles: these manuscripts present original empirical content and/or an original theoretical perspective. Submissions that present original empirical content should not exceed 12,000 words (including references). Submissions that present an original theoretical perspective should not exceed 6,000 words (including references). Longer manuscripts may occasionally be considered at the discretion of the Editors.
- Topic Pieces: these articles are intended to form a snapshot of, or perspective on, contemporary thinking on a topic or issue in research ethics or research integrity. Submissions of topic pieces should be no longer than 2,000 words (including references).
- Case studies: these articles provide examples of real-world ethical challenges in research or research ethics review, as well as real-world case studies in research integrity or research misconduct. Case studies should include ethics analysis of the identified challenges and, where possible, recommendations for dealing with them. Submissions of case studies should be no longer than 3,000 words (including references).
- Review articles: these articles address key issues in research ethics or integrity with a focus on under-researched topics. Rather than introducing new material, review articles offer critical analysis of specific issues in particular areas with significant referencing to existing published literature. Review articles should be between 3,000 and 6,000 words (including references).
3.3 Writing your paper
The Sage Author Gateway has some general advice and on how to get published , plus links to further resources. Sage Author Services also offers authors a variety of ways to improve and enhance their article including English language editing, plagiarism detection, and video abstract and infographic preparation.
3.3.1 Make your article discoverable
When writing up your paper, think about how you can make it discoverable. The title, keywords and abstract are key to ensuring readers find your article through search engines such as Google. For information and guidance on how best to title your article, write your abstract and select your keywords, have a look at this page on the Gateway: How to Help Readers Find Your Article Online .
4. Editorial policies
4 .1 Peer review policy
Manuscripts submitted for publication in Research Ethics are subject to anonymised peer review.
Submissions are initially reviewed by the editors, who determine whether the submission meets the basic guidelines (e.g. addresses a topic relevant to research ethics or research integrity, is written in English, and is sufficiently understandable). If so, the editors will consult among themselves to determine if the submission, based on its quality, should go on to peer review. Submissions passing this first level of review will be assigned to at least two peer reviewers with the appropriate expertise. The reviewers assess submissions based on scholarly quality, relevance, timeliness, novelty, importance, engagement with the relevant literature, and similar factors. Upon receipt of the peer review responses, the editors will decide whether the manuscript should be rejected, or accepted with or without required revisions.
Research Ethics operates a strictly fully anonymous peer review process in which the reviewer’s name is withheld from the author and, the author’s name from the reviewer. The reviewer may at their own discretion opt to reveal their name to the author in their review, but our standard policy practice is for both identities to remain concealed. The desired turnaround time for manuscripts is a maximum of eight weeks from submission to initial decision. If the manuscript is accepted for publication, the editors reserve the right to make minor adjustments (e.g. grammar, tone) and, if necessary, to shorten the manuscript without changing the meaning.
4.2 Authorship
All parties who have made a substantive contribution to the manuscript should be listed as authors. Principal authorship, authorship order, and other publication credits should be based on the relative scientific or professional contributions of the individuals involved, regardless of their status. A student is usually listed as principal author on any multiple-authored publication that substantially derives from the student’s dissertation or thesis. See also the COPE discussion document on authorship, available here .
Please note that AI chatbots and Large Language Models (LLMs), such as ChatGPT, do not currently satisfy our authorship criteria. Use of any AI chatbot or LLM in helping prepare a manuscript for submission should be clearly indicated in the manuscript, including which model was used and for what purpose. Please use the methods or acknowledgements section, as appropriate. For more information see the Sage policy on Use of ChatGPT and Generative AI .
4.3 Acknowledgements
Contributors or advisors who do not meet the criteria for authorship can be listed in an Acknowledgements section. Examples of those who might be acknowledged include a person who provided purely technical help, general support or feedback on an early draft. Please ensure that persons who are acknowledged have given permission for mention in the article and upload their confirmation (as supplementary materials) at submission.
4.3.1 Third party submissions
Where an individual who is not listed as an author submits a manuscript on behalf of the author(s), a statement must be included in the Acknowledgements section of the manuscript and in the accompanying cover letter. The statements must:
- Disclose this type of editorial assistance – including the individual’s name, company and level of input
- Identify any entities that paid for this assistance
- Confirm that the listed authors have authorized the submission of their manuscript via third party and approved any statements or declarations, e.g. conflicting interests, funding, etc.
Where appropriate, Sage reserves the right to deny consideration to manuscripts submitted by a third party rather than by the authors themselves .
4.3.2 Writing assistance
Individuals who provided writing assistance, e.g. from a specialist communications company, do not qualify as authors and so should only be included in the Acknowledgements section. Authors must disclose any writing assistance – including the individual’s name, company and level of input – and identify the entity that paid for this assistance.
It is not necessary to disclose use of language polishing services.
4.4 Declaration of conflicting interests
It is the policy of Research Ethics to require a declaration of conflicting interests from all authors enabling a statement to be carried within the paginated pages of all published articles.
The declaration of conflicting interests must be provided at the submission stage in two ways:
- Authors should provide a full statement disclosing the existence of any financial or non-financial interest within the title page and/or cover letter, which is not sent to reviewers, to detail these and to declare any potential conflicts of interest to the editor.
- Authors should provide a minimal statement (either "The authors declare the existence of a financial/non-financial competing interest" OR "The authors declare no competing interests") within the anonymised manuscript. The minimal statement will be shared with peer-reviewers and must not include any information which may enable the disclosure of author identities.
In addition to any declarations in the submission system, all authors are required to include a ‘Declaration of Conflicting Interests’ statement at the end of their published article using one of the following standard sentences:
- The authors declare the following competing interests.
- The authors declare no competing interests.
For guidance on conflict of interest statements, please see Sage’s Publishing Policies and the ICMJE recommendations here .
4.5 Research ethics and patient approvals
It is the policy of Research Ethics to require a declaration about research ethics approval enabling a statement to be carried within the paginated pages of all published articles. To ensure anonymous peer-review, please include these details on the title page of your submission. This should include the name of the ethics committee that approved the study and, where possible, the date of approval and reference number of the application.
Should research ethics approval not be relevant/required for your study/article, please include a statement to this effect at the submission stage, along with any accompanying evidence if possible (e.g. relevant link to policy position of institution or legal framework clarifying ethics approval is not required or expected for the kind of study/article being submitted). The following standard sentence can be used:
- The authors declare that research ethics approval was not required for this study.
4.5.1 Medical research
Medical research involving human participants or data must be conducted according to the World Medical Association Declaration of Helsinki .
Submitted manuscripts should conform to the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals , and all articles reporting on studies involving human participants must state that the relevant ethics committee provided ethics approval (or waived its requirement). Please ensure that you have provided the full name and institution of the ethics committee, in addition to the approval number and date of approval. Please include these details on the title page of your submission. For research articles, authors are also required to state in the methods section whether participants provided informed consent and whether the consent was written or verbal.
Information on informed consent to report on individual cases or case series should be included in the manuscript text. A statement is required regarding whether written informed consent for patient information and images to be published was provided by the participant(s) or a legally authorized representative.
Please also refer to the ICMJE Recommendations for the Protection of Research Participants
4.5.2 Non-medical research involving humans and human data
All manuscripts reporting studies with humans or human data, including studies that involve primary collection of personal data such as surveys or interviews, must state the relevant ethics committee provided (or waived) approval.
If ethics approval was obtained, please provide the name(s) of the ethics committee(s)/IRB(s) plus the approval number(s)/ID(s). If the study received exemption from ethics approval, please provide the name(s) of the ethics committee(s)/IRB(s) or other authorized body and the reason for exemption. If ethics approval was not sought for the present study, please specify why it was not required and cite the relevant guidelines or legislation where applicable, for the benefit of an international readership. Please include these details on the title page of your submission.
For empirical research articles, authors are also required to state in the methods section whether participants provided informed consent and whether the consent was written or verbal.
If you are unsure whether ethics approval is required for your study, please refer to this Editorial .
4.5.3 Animal studies
All manuscripts reporting on studies with animals must provide details of the relevant research ethics approval (or waiver). Please include these details on the title page of your submission.
4.6 Clinical trials
Many Sage journals conform to the ICMJE requirement that clinical trials are registered in a WHO-approved public trials registry at or before the time of first patient enrolment as a condition of consideration for publication. The trial registry name and URL, and registration number must be included at the end of the abstract.
Further to the above, other Sage journals may consider retrospectively registered trials if the justification for late registration is acceptable, consistent with the AllTrials campaign . The trial registry name and URL, and registration number must be included at the end of the abstract.
5. Publishing Policies
5.1 Publication ethics
Sage is committed to upholding the integrity of the academic record. We encourage authors to refer to the Committee on Publication Ethics’ International Standards for Authors and view the Publication Ethics page on the Sage Author Gateway .
5.1.1 Plagiarism
Research Ethics and Sage take issues of copyright infringement, plagiarism or other breaches of best practice in publication very seriously. We seek to protect the rights of our authors and we always investigate claims of plagiarism or misuse of published articles. Equally, we seek to protect the reputation of the journal against malpractice. Submitted articles may be checked with duplication-checking software. Where an article, for example, is found to have plagiarised other work or included third-party copyright material without permission or with insufficient acknowledgement, or where the authorship of the article is contested, we reserve the right to take action including, but not limited to: publishing an erratum or corrigendum (correction); retracting the article; taking up the matter with the head of department or dean of the author's institution and/or relevant academic bodies or societies; or taking appropriate legal action.
5.1.2 Prior publication
If material has been previously published it is not generally acceptable for publication in a Sage journal. However, there are certain circumstances where previously published material can be considered for publication. Please refer to the guidance on the Sage Author Gateway or if in doubt, contact the Editor at the address given below.
5.2 Contributor's publishing agreement
Before publication Sage requires the author as the rights holder to sign a Journal Contributor’s Publishing Agreement. Research Ethics publishes manuscripts under Creative Commons licenses . The standard license for the journal is Creative Commons by Attribution Non-Commercial (CC BY-NC), which allows others to re-use the work without permission as long as the work is properly referenced and the use is non-commercial. For more information, you are advised to visit Sage's OA licenses page .
Alternative license arrangements are available, for example, to meet particular funder mandates, made at the author’s request.
6. Preparing your manuscript for submission
You will be asked to upload your anonymised manuscript separately from a title page. Please take note of the requirements for preparation for each of these documents.
6.1 Main file
Your main file is your anonymised manuscript. Please ensure that you do not include any identifiable information in your main manuscript. It will consist of the body of your work plus references. You do not need to include the title or abstract as these will be added during the submission process.
6.2 Title page
Separate from your anonymised manuscript (main file), please also include a separate title page with the following information:
- Name of all the authors with institutional affiliations and contact details
- Ethics approval information
- Conflict of interest information
- Funding information
- Acknowledgements
6.3 Formatting
The preferred format for your manuscript is Word. Files should be submitted in a .doc or .docx file format. Word templates are available on the Manuscript Submission Guidelines page of our Author Gateway. It is preferable that all submissions be typed in sans serif font (e.g. Calibri, Helvetica). Double-spacing is also preferred. Keep formatting simple, and avoid unnecessary advanced word processing features, justification, linked objects, or creating your own symbols.
6.4 Language
All manuscripts must be written in English.
6.4.1 Terminology
Please note that at Research Ethics we actively discourage use of the term ‘research subjects’ when referring to studies that involve human participants. The word ‘subjects’ should only be used when referring to the processing of data (as in ‘data subjects’). As an alternative, please use: humans, persons, participants, research participants or human participants.
6.5 Artwork, figures and other graphics
For guidance on the preparation of illustrations, pictures and graphs in electronic format, please visit Sage’s Manuscript Submission Guidelines .
Figures supplied in colour will appear in colour online.
6.6 Supplementary material
This journal is able to host additional materials online (e.g. datasets, podcasts, videos, images etc) alongside the full-text of the article. For more information please refer to our guidelines on submitting supplementary files .
6.7 Reference style
Research Ethics adheres to the Sage Harvard reference style. View the Sage Harvard guidelines to ensure your manuscript conforms to this reference style.
If you use EndNote to manage references, you can download the Sage Harvard EndNote output file .
6.8 English language editing services
Authors seeking assistance with English language editing, translation, or figure and manuscript formatting to fit the journal’s specifications should consider using Sage Language Services. Visit Sage Language Services on our Journal Author Gateway for further information.
7. Submitting your manuscript
Research Ethics is hosted on Sage Track, a web based online submission and peer review system powered by ScholarOne™ Manuscripts. Visit http://mc.manuscriptcentral.com/rea to login and submit your article online.
IMPORTANT: Please check whether you already have an account in the system before trying to create a new one. If you have reviewed or authored for the journal in the past year it is likely that you will have had an account created. For further guidance on submitting your manuscript online please visit ScholarOne Online Help .
As part of our commitment to ensuring an ethical, transparent and fair peer review process Sage is a supporting member of ORCID, the Open Researcher and Contributor ID . ORCID provides a unique and persistent digital identifier that distinguishes researchers from every other researcher, even those who share the same name, and, through integration in key research workflows such as manuscript and grant submission, supports automated linkages between researchers and their professional activities, ensuring that their work is recognized.
The collection of ORCID iDs from corresponding authors is now part of the submission process of this journal. If you already have an ORCID iD you will be asked to associate that to your submission during the online submission process. We also strongly encourage all co-authors to link their ORCID iD to their accounts in our online peer review platforms. It takes seconds to do: click the link when prompted, sign into your ORCID account and our systems are automatically updated. Your ORCID iD will become part of your accepted publication’s metadata, making your work attributable to you and only you. Your ORCID iD is published with your article so that fellow researchers reading your work can link to your ORCID profile, and from there link to your other publications.
If you do not already have an ORCID iD please follow this link to create one or visit our ORCID homepage to learn more.
7.2 Title, keywords and abstracts
Please supply a title, an abstract and keywords to accompany your manuscript. The title, keywords and abstract are key to ensuring readers find your article online through online search engines such as Google. Please refer to the information and guidance on how best to title your article, write your abstract and select your keywords by visiting the Sage Journal Author Gateway for guidelines on How to Help Readers Find Your Article Online.
All manuscripts should include up to 6 keywords (in alphabetical order) and an abstract of up to 250 words, which is a condensation of the manuscript that contains a statement of purpose, a description of the content, argument, or analysis, and a concise summary of conclusions.
7.3 Co-authors
You will be asked to provide contact details and affiliations for all co-authors via the submission system and identify who is to be the corresponding author. These details must match what appears on your manuscript.
7.3.1. Ethics approval documentation during manuscript submission
As noted above, for all studies requiring ethics approval, evidence of research ethics approval must be uploaded with your manuscript files during the submission stage. This must show (as a minimum) the name of the ethics committee, the name of the study, the name of the applicant, the ethics approval number/ID, and the date of approval. If an ethics waiver was obtained, please upload evidence of the waiver to include the reason for the waiver.
In your anonymised main manuscript file, please also include a statement (under a Methods section or a separate section on Ethics Approval) whether your study received ethics approval or a waiver, but ensure that any information that could identify the specific institution or committee that provided the approval or waiver is also anonymised, e.g. “This study received ethics approval from [anonymised] on 10 October 2023."
7.4 Permissions
Please also ensure that you have obtained any necessary permission from copyright holders for reproducing any illustrations, tables, figures or lengthy quotations previously published elsewhere. For further information including guidance on fair dealing for criticism and review, please see the Copyright and Permissions page on the Sage Author Gateway .
8. On acceptance and publication
8.1 Sage Production
If your article is accepted, your Sage Production Editor will keep you informed as to your article’s progress throughout the production process. Proofs will be sent by PDF to the corresponding author and should be returned promptly. Authors are reminded to check their proofs carefully to confirm that all author information, including names, affiliations, sequence and contact details are correct, and that Funding and Conflict of Interest statements, if any, are accurate. Please note that if there are any changes to the author list at this stage all authors will be required to complete and sign a form authorising the change.
8.2 Online publication
One of the many benefits of publishing your research in an open access journal is the speed to publication. Your article will be published online in a fully citable form with a DOI number as soon as it has completed the production process. At this time it will be completely free to view and download for all.
8.3 Promoting your article
Publication is not the end of the process! You can help disseminate your paper and ensure it is as widely read and cited as possible. The Sage Author Gateway has numerous resources to help you promote your work. Visit the Promote Your Article page on the Gateway for tips and advice.
9. Further information
Any correspondence, queries or additional requests for information on the manuscript submission process should be sent to the Research Ethics editorial office as follows:
The Editors, Research Ethics, [email protected]
- Read Online
- Current Issue
- Email Alert
- Permissions
- Foreign rights
- Reprints and sponsorship
- Advertising
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Ethical Considerations in Research | Types & Examples
Ethical Considerations in Research | Types & Examples
Published on October 18, 2021 by Pritha Bhandari . Revised on June 22, 2023.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviors, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to
- protect the rights of research participants
- enhance research validity
- maintain scientific or academic integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, other interesting articles, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research objectives with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process , so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
You make sure to provide all potential participants with all the relevant information about
- what the study is about
- the risks and benefits of taking part
- how long the study will take
- your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymize data collection . For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymization is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources or counseling or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine academic integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Measures of central tendency
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Thematic analysis
- Cohort study
- Peer review
- Ethnography
Research bias
- Implicit bias
- Cognitive bias
- Conformity bias
- Hawthorne effect
- Availability heuristic
- Attrition bias
- Social desirability bias
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bhandari, P. (2023, June 22). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved April 1, 2024, from https://www.scribbr.com/methodology/research-ethics/
Is this article helpful?

Pritha Bhandari
Other students also liked, data collection | definition, methods & examples, what is self-plagiarism | definition & how to avoid it, how to avoid plagiarism | tips on citing sources, what is your plagiarism score.
Articles on Research ethics
Displaying 1 - 20 of 83 articles.

The San people of southern Africa: where ethics codes for researching indigenous people could fail them
Stasja Koot , Wageningen University

When research study materials don’t speak their participants’ language, data can get lost in translation
Sonia Colina , University of Arizona

We won’t always have to use animals for medical research. Here’s what we can do instead
Greg Williams , CSIRO and Laura Anne Thomas , CSIRO

San and Khoe skeletons: how a South African university sought to restore dignity and redress the past
Victoria Gibbon , University of Cape Town
Promising assisted reproductive technologies come with ethical, legal and social challenges – a developmental biologist and a bioethicist discuss IVF, abortion and the mice with two dads
Keith Latham , Michigan State University and Mary Faith Marshall , University of Virginia

Researchers can learn a lot with your genetic information, even when you skip survey questions – yesterday’s mode of informed consent doesn’t quite fit today’s biobank studies
Robbee Wedow , Purdue University

You shed DNA everywhere you go – trace samples in the water, sand and air are enough to identify who you are, raising ethical questions about privacy
Jenny Whilde , University of Florida and Jessica Alice Farrell , University of Florida

I used to work at Google and now I’m an AI researcher. Here’s why slowing down AI development is wise
Rodolfo Ocampo , UNSW Sydney

Human genome editing offers tantalizing possibilities – but without clear guidelines, many ethical questions still remain
André O. Hudson , Rochester Institute of Technology and Gary Skuse , Rochester Institute of Technology

Can we ethically justify harming animals for research?
Julian Koplin , Monash University

What is ethical animal research? A scientist and veterinarian explain
Lana Ruvolo Grasser , National Institutes of Health and Rachelle Stammen , Emory University

‘Gain of function’ research can create experimental viruses. In light of COVID, it should be more strictly regulated – or banned
Colin D. Butler , Australian National University

New ‘ethics guidance’ for top science journals aims to root out harmful research – but can it succeed?
Cordelia Fine , The University of Melbourne

What’s next for ancient DNA studies after Nobel Prize honors groundbreaking field of paleogenomics
Mary Prendergast , Rice University

Five steps every researcher should take to ensure participants are not harmed and are fully heard
Jasper Knight , University of the Witwatersrand

African ubuntu can deepen how research is done
Obaa Akua Konadu-Osei , Stellenbosch University
Uncovering the genetic basis of mental illness requires data and tools that aren’t just based on white people – this international team is collecting DNA samples around the globe
Hailiang Huang , Harvard University

Researchers should be assessed on quality not quantity: here’s how
Lyn Horn , University of Cape Town and Lex Bouter , Vrije Universiteit Amsterdam

How a South African community’s request for its genetic data raises questions about ethical and equitable research
Dana Al-Hindi , University of California, Davis and Brenna Henn , University of California, Davis

Archaeological site along the Nile opens a window on the Nubian civilization that flourished in ancient Sudan
Michele R. Buzon , Purdue University
Related Topics
- Clinical trials
- Human research ethics
- Medical research
- Researchers
- Science research
- Scientific research
Top contributors
Adjunct professor, The University of Western Australia
Professor, School of Economics, UNSW Sydney
Associate Professor of Law, Bond University
Associate Professor, School of Arts and Media, UNSW Sydney
Associate Professor in Biological Anthropology, Division of Clinical Anatomy and Biological Anthropology, University of Cape Town
Associate Professor of Bioethics, University of Portsmouth
Professor of Ethics, Keele University
Associate Professor, School of Law, University of Canberra
Senior Lecturer in Medical Ethics and Law, St George's, University of London
Senior Lecturer in Ethics and Professionalism, University of Adelaide
Professor of Marine Science & Founder/Director of Blue Carbon Lab, Deakin University
Honorary Professor, Australian National University
Associate professor, University of Sydney
Distinguished Professor of Marine Science, King Abdullah University of Science and Technology
Former Foundation Professor of Animal Welfare, University of Queensland, Curtin University
- X (Twitter)
- Unfollow topic Follow topic

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Introduction: What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. This introduction covers what research ethics is, its ethical distinctions, approaches to teaching research ethics, and other resources on this topic.
What is Research Ethics
Why Teach Research Ethics
Animal Subjects
Biosecurity
Collaboration
Conflicts of Interest
Data Management
Human Subjects
Peer Review
Publication
Research Misconduct
Social Responsibility
Stem Cell Research
Whistleblowing
Descriptions of educational settings , including in the classroom, and in research contexts.
Case Studies
Other Discussion Tools
Information about the history and authors of the Resources for Research Ethics Collection
What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include:
- Protections of human and animal subjects
However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research subjects. Other ethical challenges are rooted in many dimensions of research, including the:
- Collection, use, and interpretation of research data
- Methods for reporting and reviewing research plans or findings
- Relationships among researchers with one another
- Relationships between researchers and those that will be affected by their research
- Means for responding to misunderstandings, disputes, or misconduct
- Options for promoting ethical conduct in research
The domain of research ethics is intended to include nothing less than the fostering of research that protects the interests of the public, the subjects of research, and the researchers themselves.
Ethical Distinctions
In discussing or teaching research ethics, it is important to keep some basic distinctions in mind.
- It is important not to confuse moral claims about how people ought to behave with descriptive claims about how they in fact do behave. From the fact that gift authorship or signing off on un-reviewed data may be "common practice" in some contexts, it doesn't follow that they are morally or professionally justified. Nor is morality to be confused with the moral beliefs or ethical codes that a given group or society holds (how some group thinks people should live). A belief in segregation is not morally justified simply because it is widely held by a group of people or given society. Philosophers term this distinction between prescriptive and descriptive claims the 'is-ought distinction.'
- A second important distinction is that between morality and the law. The law may or may not conform to the demands of ethics (Kagan, 1998). To take a contemporary example: many believe that the law prohibiting federally funded stem cell research is objectionable on moral (as well as scientific) grounds, i.e., that such research can save lives and prevent much human misery. History is full of examples of bad laws, that is laws now regarded as morally unjustifiable, e.g., the laws of apartheid, laws prohibiting women from voting or inter-racial couples from marrying.
- It is also helpful to distinguish between two different levels of discussion (or two different kinds of ethical questions): first-order or "ground-level" questions and second-order questions.
- First-order moral questions concern what we should do. Such questions may be very general or quite specific. One might ask whether the tradition of 'senior' authorship should be defended and preserved or, more generally, what are the principles that should go into deciding the issue of 'senior' authorship. Such questions and the substantive proposals regarding how to answer them belong to the domain of what moral philosophers call 'normative ethics.'
- Second-order moral questions concern the nature and purpose of morality itself. When someone claims that falsifying data is wrong, what exactly is the standing of this claim? What exactly does the word 'wrong' mean in the conduct of scientific research? And what are we doing when we make claims about right and wrong, scientific integrity and research misconduct? These second-order questions are quite different from the ground-level questions about how to conduct one's private or professional life raised above. They concern the nature of morality rather than its content, i.e., what acts are required, permitted or prohibited. This is the domain of what moral philosophers call 'metaethics' (Kagan, 1998).
Ethical Approaches
Each of these approaches provides moral principles and ways of thinking about the responsibilities, duties and obligations of moral life. Individually and jointly, they can provide practical guidance in ethical decision-making.
- One of the most influential and familiar approaches to ethics is deontological ethics, associated with Immanuel Kant (1742-1804). Deontological ethics hold certain acts as right or wrong in themselves, e.g., promise breaking or lying. So, for example, in the context of research, fraud, plagiarism and misrepresentation are regarded as morally wrong in themselves, not simply because they (tend to) have bad consequences. The deontological approach is generally grounded in a single fundamental principle: Act as you would wish others to act towards you OR always treat persons as an end, never as a means to an end.
- From such central principles are derived rules or guidelines for what is permitted, required and prohibited. Objections to principle-based or deontological ethics include the difficulty of applying highly general principles to specific cases, e.g.: Does treating persons as ends rule out physician-assisted suicide, or require it? Deontological ethics is generally contrasted to consequentialist ethics (Honderich, 1995).
- According to consequentialist approaches, the rightness or wrongness of an action depends solely on its consequences. One should act in such a way as to bring about the best state of affairs, where the best state of affairs may be understood in various ways, e.g., as the greatest happiness for the greatest number of people, maximizing pleasure and minimizing pain or maximizing the satisfaction of preferences. A theory such as Utilitarianism (with its roots in the work of Jeremy Bentham and John Stuart Mill) is generally taken as the paradigm example of consequentialism. Objections to consequentialist ethics tend to focus on its willingness to regard individual rights and values as "negotiable." So, for example, most people would regard murder as wrong independently of the fact that killing one person might allow several others to be saved (the infamous sacrifice of an ailing patient to provide organs for several other needy patients). Similarly, widespread moral opinion holds certain values important (integrity, justice) not only because they generally lead to good outcomes, but in and of themselves.
- Virtue ethics focuses on moral character rather than action and behavior considered in isolation. Central to this approach is the question what ought we (as individuals, as scientists, as physicians) to be rather than simply what we ought to do. The emphasis here is on inner states, that is, moral dispositions and habits such as courage or a developed sense of personal integrity. Virtue ethics can be a useful approach in the context of RCR and professional ethics, emphasizing the importance of moral virtues such as compassion, honesty, and respect. This approach has also a great deal to offer in discussions of bioethical issues where a traditional emphasis on rights and abstract principles frequently results in polarized, stalled discussions (e.g., abortion debates contrasting the rights of the mother against the rights of the fetus).
- The term 'an ethics of care' grows out of the work of Carol Gilligan, whose empirical work in moral psychology claimed to discover a "different voice," a mode of moral thinking distinct from principle-based moral thinking (e.g., the theories of Kant and Mill). An ethics of care stresses compassion and empathetic understanding, virtues Gilligan associated with traditional care-giving roles, especially those of women.
- This approach differs from traditional moral theories in two important ways. First, it assumes that it is the connections between persons, e.g., lab teams, colleagues, parents and children, student and mentor, not merely the rights and obligations of discrete individuals that matter. The moral world, on this view, is best seen not as the interaction of discrete individuals, each with his or her own interests and rights, but as an interrelated web of obligations and commitment. We interact, much of the time, not as private individuals, but as members of families, couples, institutions, research groups, a given profession and so on. Second, these human relationships, including relationships of dependency, play a crucial role on this account in determining what our moral obligations and responsibilities are. So, for example, individuals have special responsibilities to care for their children, students, patients, and research subjects.
- An ethics of care is thus particularly useful in discussing human and animal subjects research, issues of informed consent, and the treatment of vulnerable populations such as children, the infirm or the ill.
- The case study approach begins from real or hypothetical cases. Its objective is to identify the intuitively plausible principles that should be taken into account in resolving the issues at hand. The case study approach then proceeds to critically evaluate those principles. In discussing whistle-blowing, for example, a good starting point is with recent cases of research misconduct, seeking to identify and evaluate principles such as a commitment to the integrity of science, protecting privacy, or avoiding false or unsubstantiated charges. In the context of RCR instruction, case studies provide one of the most interesting and effective approaches to developing sensitivity to ethical issues and to honing ethical decision-making skills.
- Strictly speaking, casuistry is more properly understood as a method for doing ethics rather than as itself an ethical theory. However, casuistry is not wholly unconnected to ethical theory. The need for a basis upon which to evaluate competing principles, e.g., the importance of the well-being of an individual patient vs. a concern for just allocation of scarce medical resources, makes ethical theory relevant even with case study approaches.
- Applied ethics is a branch of normative ethics. It deals with practical questions particularly in relation to the professions. Perhaps the best known area of applied ethics is bioethics, which deals with ethical questions arising in medicine and the biological sciences, e.g., questions concerning the application of new areas of technology (stem cells, cloning, genetic screening, nanotechnology, etc.), end of life issues, organ transplants, and just distribution of healthcare. Training in responsible conduct of research or "research ethics" is merely one among various forms of professional ethics that have come to prominence since the 1960s. Worth noting, however, is that concern with professional ethics is not new, as ancient codes such as the Hippocratic Oath and guild standards attest (Singer, 1986).
- Adams D, Pimple KD (2005): Research Misconduct and Crime: Lessons from Criminal Science on Preventing Misconduct and Promoting Integrity. Accountability in Research 12(3):225-240.
- Anderson MS, Horn AS, Risbey KR, Ronning EA, De Vries R, Martinson BC (2007): What Do Mentoring and Training in the Responsible Conduct of Research Have To Do with Scientists' Misbehavior? Findings from a National Survey of NIH-Funded Scientists . Academic Medicine 82(9):853-860.
- Bulger RE, Heitman E (2007): Expanding Responsible Conduct of Research Instruction across the University. Academic Medicine. 82(9):876-878.
- Kalichman MW (2006): Ethics and Science: A 0.1% solution. Issues in Science and Technology 23:34-36.
- Kalichman MW (2007): Responding to Challenges in Educating for the Responsible Conduct of Research, Academic Medicine. 82(9):870-875.
- Kalichman MW, Plemmons DK (2007): Reported Goals for Responsible Conduct of Research Courses. Academic Medicine. 82(9):846-852.
- Kalichman MW (2009): Evidence-based research ethics. The American Journal of Bioethics 9(6&7): 85-87.
- Pimple KD (2002): Six Domains of Research Ethics: A Heuristic Framework for the Responsible Conduct of Research. Science and Engineering Ethics 8(2):191-205.
- Steneck NH (2006): Fostering Integrity in Research: Definitions, Current Knowledge, and Future Directions. Science and Engineering Ethics 12:53-74.
- Steneck NH, Bulger RE (2007): The History, Purpose, and Future of Instruction in the Responsible Conduct of Research. Academic Medicine. 82(9):829-834.
- Vasgird DR (2007): Prevention over Cure: The Administrative Rationale for Education in the Responsible Conduct of Research. Academic Medicine. 82(9):835-837.
- Aristotle. The Nichomachean Ethics.
- Beauchamp RL, Childress JF (2001): Principles of Biomedical Ethics, 5th edition, NY: Oxford University Press.
- Bentham, J (1781): An Introduction to the Principles of Morals and Legislation.
- Gilligan C (1993): In a Different Voice: Psychological Theory and Women's Development. Cambridge: Harvard University Press.
- Glover, Jonathan (1977): Penguin Books.
- Honderich T, ed. (1995): The Oxford Companion to Philosophy, Oxford and New York: Oxford University Press.
- Kagan S (1998): Normative Ethics. Westview Press.
- Kant I (1785): Groundwork of the Metaphysics of Morals.
- Kant I (1788): Critique of Practical Reason.
- Kant I (1797): The Metaphysics of Morals.
- Kant I (1797): On a Supposed right to Lie from Benevolent Motives.
- Kuhse H, Singer P (1999): Bioethics: An Anthology. Blackwell Publishers.
- Mill JS (1861): Utilitarianism.
- Rachels J (1999): The Elements of Moral Philosophy, 3rd edition, Boston: McGraw-Hill.
- Regan T (1993): Matters of Life and Death: New Introductory Essays in Moral Philosophy, 3rd edition. New York: McGraw-Hill. The history of ethics.
- Singer P (1993): Practical Ethics, 2nd ed. Cambridge University Press.
The Resources for Research Ethics Education site was originally developed and maintained by Dr. Michael Kalichman, Director of the Research Ethics Program at the University of California San Diego. The site was transferred to the Online Ethics Center in 2021 with the permission of the author.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
15k Accesses
11 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.
Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.

Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
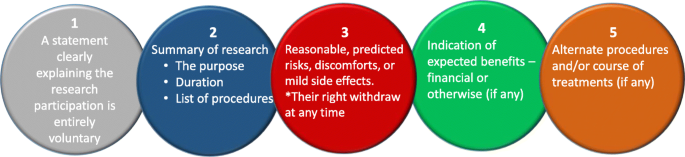
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
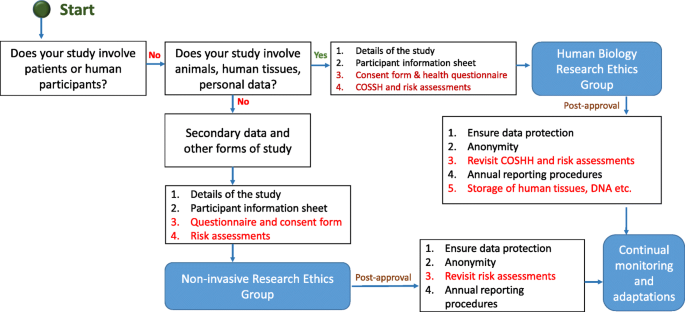
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
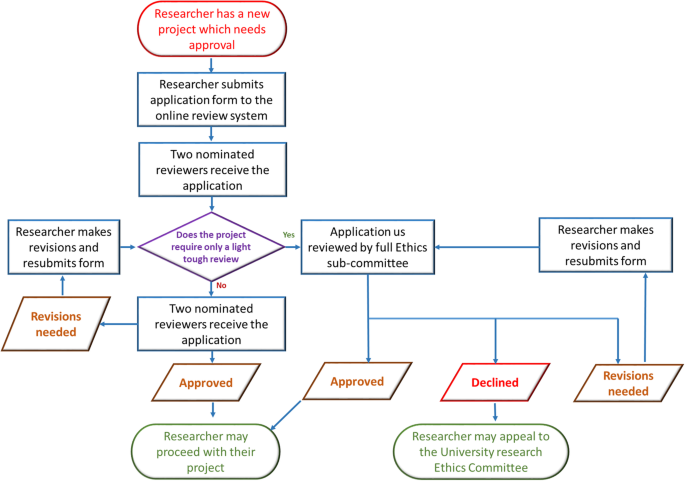
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Browse All Articles
- Newsletter Sign-Up

- 26 Mar 2024
- Cold Call Podcast
How Do Great Leaders Overcome Adversity?
In the spring of 2021, Raymond Jefferson (MBA 2000) applied for a job in President Joseph Biden’s administration. Ten years earlier, false allegations were used to force him to resign from his prior US government position as assistant secretary of labor for veterans’ employment and training in the Department of Labor. Two employees had accused him of ethical violations in hiring and procurement decisions, including pressuring subordinates into extending contracts to his alleged personal associates. The Deputy Secretary of Labor gave Jefferson four hours to resign or be terminated. Jefferson filed a federal lawsuit against the US government to clear his name, which he pursued for eight years at the expense of his entire life savings. Why, after such a traumatic and debilitating experience, would Jefferson want to pursue a career in government again? Harvard Business School Senior Lecturer Anthony Mayo explores Jefferson’s personal and professional journey from upstate New York to West Point to the Obama administration, how he faced adversity at several junctures in his life, and how resilience and vulnerability shaped his leadership style in the case, "Raymond Jefferson: Trial by Fire."

- 02 Jan 2024
Should Businesses Take a Stand on Societal Issues?
Should businesses take a stand for or against particular societal issues? And how should leaders determine when and how to engage on these sensitive matters? Harvard Business School Senior Lecturer Hubert Joly, who led the electronics retailer Best Buy for almost a decade, discusses examples of corporate leaders who had to determine whether and how to engage with humanitarian crises, geopolitical conflict, racial justice, climate change, and more in the case, “Deciding When to Engage on Societal Issues.”

- 12 Dec 2023
Can Sustainability Drive Innovation at Ferrari?
When Ferrari, the Italian luxury sports car manufacturer, committed to achieving carbon neutrality and to electrifying a large part of its car fleet, investors and employees applauded the new strategy. But among the company’s suppliers, the reaction was mixed. Many were nervous about how this shift would affect their bottom lines. Professor Raffaella Sadun and Ferrari CEO Benedetto Vigna discuss how Ferrari collaborated with suppliers to work toward achieving the company’s goal. They also explore how sustainability can be a catalyst for innovation in the case, “Ferrari: Shifting to Carbon Neutrality.” This episode was recorded live December 4, 2023 in front of a remote studio audience in the Live Online Classroom at Harvard Business School.

- 11 Dec 2023
- Research & Ideas
Doing Well by Doing Good? One Industry’s Struggle to Balance Values and Profits
Few companies wrestle with their moral mission and financial goals like those in journalism. Research by Lakshmi Ramarajan explores how a disrupted industry upholds its values even as the bottom line is at stake.

- 27 Nov 2023
Voting Democrat or Republican? The Critical Childhood Influence That's Tough to Shake
Candidates might fixate on red, blue, or swing states, but the neighborhoods where voters spend their teen years play a key role in shaping their political outlook, says research by Vincent Pons. What do the findings mean for the upcoming US elections?

- 21 Nov 2023
The Beauty Industry: Products for a Healthy Glow or a Compact for Harm?
Many cosmetics and skincare companies present an image of social consciousness and transformative potential, while profiting from insecurity and excluding broad swaths of people. Geoffrey Jones examines the unsightly reality of the beauty industry.

- 09 Nov 2023
What Will It Take to Confront the Invisible Mental Health Crisis in Business?
The pressure to do more, to be more, is fueling its own silent epidemic. Lauren Cohen discusses the common misperceptions that get in the way of supporting employees' well-being, drawing on case studies about people who have been deeply affected by mental illness.
- 07 Nov 2023
How Should Meta Be Governed for the Good of Society?
Julie Owono is executive director of Internet Sans Frontières and a member of the Oversight Board, an outside entity with the authority to make binding decisions on tricky moderation questions for Meta’s companies, including Facebook and Instagram. Harvard Business School visiting professor Jesse Shapiro and Owono break down how the Board governs Meta’s social and political power to ensure that it’s used responsibly, and discuss the Board’s impact, as an alternative to government regulation, in the case, “Independent Governance of Meta’s Social Spaces: The Oversight Board.”

- 24 Oct 2023
From P.T. Barnum to Mary Kay: Lessons From 5 Leaders Who Changed the World
What do Steve Jobs and Sarah Breedlove have in common? Through a series of case studies, Robert Simons explores the unique qualities of visionary leaders and what today's managers can learn from their journeys.

- 03 Oct 2023
- Research Event
Build the Life You Want: Arthur Brooks and Oprah Winfrey Share Happiness Tips
"Happiness is not a destination. It's a direction." In this video, Arthur C. Brooks and Oprah Winfrey reflect on mistakes, emotions, and contentment, sharing lessons from their new book.

- 12 Sep 2023
Successful, But Still Feel Empty? A Happiness Scholar and Oprah Have Advice for You
So many executives spend decades reaching the pinnacles of their careers only to find themselves unfulfilled at the top. In the book Build the Life You Want, Arthur Brooks and Oprah Winfrey offer high achievers a guide to becoming better leaders—of their lives.

- 10 Jul 2023
- In Practice
The Harvard Business School Faculty Summer Reader 2023
Need a book recommendation for your summer vacation? HBS faculty members share their reading lists, which include titles that explore spirituality, design, suspense, and more.

- 01 Jun 2023
A Nike Executive Hid His Criminal Past to Turn His Life Around. What If He Didn't Have To?
Larry Miller committed murder as a teenager, but earned a college degree while serving time and set out to start a new life. Still, he had to conceal his record to get a job that would ultimately take him to the heights of sports marketing. A case study by Francesca Gino, Hise Gibson, and Frances Frei shows the barriers that formerly incarcerated Black men are up against and the potential talent they could bring to business.

- 04 Apr 2023
Two Centuries of Business Leaders Who Took a Stand on Social Issues
Executives going back to George Cadbury and J. N. Tata have been trying to improve life for their workers and communities, according to the book Deeply Responsible Business: A Global History of Values-Driven Leadership by Geoffrey Jones. He highlights three practices that deeply responsible companies share.

- 14 Mar 2023
Can AI and Machine Learning Help Park Rangers Prevent Poaching?
Globally there are too few park rangers to prevent the illegal trade of wildlife across borders, or poaching. In response, Spatial Monitoring and Reporting Tool (SMART) was created by a coalition of conservation organizations to take historical data and create geospatial mapping tools that enable more efficient deployment of rangers. SMART had demonstrated significant improvements in patrol coverage, with some observed reductions in poaching. Then a new predictive analytic tool, the Protection Assistant for Wildlife Security (PAWS), was created to use artificial intelligence (AI) and machine learning (ML) to try to predict where poachers would be likely to strike. Jonathan Palmer, Executive Director of Conservation Technology for the Wildlife Conservation Society, already had a good data analytics tool to help park rangers manage their patrols. Would adding an AI- and ML-based tool improve outcomes or introduce new problems? Harvard Business School senior lecturer Brian Trelstad discusses the importance of focusing on the use case when determining the value of adding a complex technology solution in his case, “SMART: AI and Machine Learning for Wildlife Conservation.”

- 14 Feb 2023
Does It Pay to Be a Whistleblower?
In 2013, soon after the US Securities and Exchange Commission (SEC) had started a massive whistleblowing program with the potential for large monetary rewards, two employees of a US bank’s asset management business debated whether to blow the whistle on their employer after completing an internal review that revealed undisclosed conflicts of interest. The bank’s asset management business disproportionately invested clients’ money in its own mutual funds over funds managed by other banks, letting it collect additional fees—and the bank had not disclosed this conflict of interest to clients. Both employees agreed that failing to disclose the conflict was a problem, but beyond that, they saw the situation very differently. One employee, Neel, perceived the internal review as a good-faith effort by senior management to identify and address the problem. The other, Akash, thought that the entire business model was problematic, even with a disclosure, and believed that the bank may have even broken the law. Should they escalate the issue internally or report their findings to the US Securities and Exchange Commission? Harvard Business School associate professor Jonas Heese discusses the potential risks and rewards of whistleblowing in his case, “Conflicts of Interest at Uptown Bank.”

- 17 Jan 2023
Good Companies Commit Crimes, But Great Leaders Can Prevent Them
It's time for leaders to go beyond "check the box" compliance programs. Through corporate cases involving Walmart, Wells Fargo, and others, Eugene Soltes explores the thorny legal issues executives today must navigate in his book Corporate Criminal Investigations and Prosecutions.

- 29 Nov 2022
How Will Gamers and Investors Respond to Microsoft’s Acquisition of Activision Blizzard?
In January 2022, Microsoft announced its acquisition of the video game company Activision Blizzard for $68.7 billion. The deal would make Microsoft the world’s third largest video game company, but it also exposes the company to several risks. First, the all-cash deal would require Microsoft to use a large portion of its cash reserves. Second, the acquisition was announced as Activision Blizzard faced gender pay disparity and sexual harassment allegations. That opened Microsoft up to potential reputational damage, employee turnover, and lost sales. Do the potential benefits of the acquisition outweigh the risks for Microsoft and its shareholders? Harvard Business School associate professor Joseph Pacelli discusses the ongoing controversies around the merger and how gamers and investors have responded in the case, “Call of Fiduciary Duty: Microsoft Acquires Activision Blizzard.”
- 15 Nov 2022
Stop Ignoring Bad Behavior: 6 Tips for Better Ethics at Work
People routinely overlook wrongdoing, even in situations that cause significant harm. In his book Complicit: How We Enable the Unethical and How to Stop, Max Bazerman shares strategies that help people do the right thing even when those around them aren't.

- 08 Nov 2022
How Centuries of Restrictions on Women Shed Light on Today's Abortion Debate
Going back to pre-industrial times, efforts to limit women's sexuality have had a simple motive: to keep them faithful to their spouses. Research by Anke Becker looks at the deep roots of these restrictions and their economic implications.
More From Forbes
Scientists tend to inflate how ethical they are in doing their research.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
We have known for a long time that people tend to paint a rosy picture of how good they are. Now we know that scientists are no exception, at least when it comes to conducting their own research. This is especially surprising since scientists are regularly thought to be objective.
Research and Ethics - clinical trial law and rules, Medical compliance.
This new discovery emerged from a massive survey of 11,050 scientific researchers in Sweden, conducted by Amanda M. Lindkvist, Lina Koppel, and Gustav Tinghög at Linköping University and published in the journal Scientific Reports. The survey was very simple, with only two questions. Here was the first:
Question One : In your role as a researcher, to what extent do you perceive yourself as following good research practices—compared to other researchers in your field?
Rather than allowing the survey participants to each define what ‘good research practice’ is, the researchers gave them these criteria:
(1) Tell the truth about one’s research.
(2) Consciously review and report the basic premises of one’s studies.
(3) Openly account for one’s methods and results.
(4) Openly account for one’s commercial interests and other associations.
One Of The Best Shows Ever Made Lands On Netflix Today For The Very First Time
This popular google app will stop working in 3 days how to migrate your data, google suddenly reveals surprise android update that beats iphone.
(5) Not make unauthorized use of the research results of others.
(6) Keep one’s research organized, for example through documentation and filing.
(7) Stive to conduct one’s research without doing harm to people, animals or the environment.
(8) Be fair in one’s judgement of others’ research.
Note that many of these criteria have to do with honesty, but there are also ones on conscientiousness, non-malevolence, and fairness.
What were the results? Participants used a scale to rate themselves from 1 to 7, with 1 = Much less than other researchers, 4 = As much as other researchers, and 7 = Much more than other researchers. This is what the responses revealed:
44% rated themselves as more ethical in their research practices than other researchers in their field.
55% rated themselves as the same as their peers.
Not even 1% rated themselves as less ethical than their peers.
Of course these results can’t reflect real life, since mathematically there have to be more than 1% of scientists who are less than average in this area of their lives.
The other question that Lindkvist and his colleagues asked these scientific researchers was this:
Question Two : To what extent do you perceive researchers within your field as following good research practices–compared to researchers within other fields?
Here too the results were very skewed. 29% said their field followed good research practices to a greater extent than did scientists in other fields. Only 8% said it was the other way around.
These results should surprise us for a couple of reasons. One is that they go against the popular narrative of scientists as objective and neutral. When it comes to their own ethical behavior in conducting their research, they appear as a whole to be biased and overconfident. Another reason these results are surprising is that many scientists are likely aware of the existence of scientific research on how people in general tend to have an inflated view of their own virtue . So you’d expect that they would be on guard against such a tendency in their own case.
There are dangers that come with scientists having an overly positive view of their own research ethics. Lindkvist helpfully explains one of them: it “may lead researchers to underestimate the ethical implications of the decisions they make and to sometimes be blind to their own ethical failures. For example, researchers may downplay their own questionable practices but exaggerate those of other researchers, perhaps especially researchers outside their field.” Another danger that Lindkvist notes is a greater tendency to ignore warnings and ethical safeguards, if they are dismissed by a scientist as applying to others but not to her since she thinks she is above average.
It would be interesting in future work to see if similar patterns emerge with researchers in other countries besides Sweden. It would also be interesting to look at researchers anonymously rating the research ethics of their colleagues in their own departments and schools.
If these results hold up, it will be important to find ways to encourage scientific researchers to correct their inflated perceptions. As Lindkvist urges, “To restore science’s credibility, we need to create incentive structures, institutions, and communities that foster ethical humility and encourage us to be our most ethical selves in an academic system that otherwise incentivizes us to be bad.”

- Editorial Standards
- Reprints & Permissions
Help | Advanced Search
Computer Science > Human-Computer Interaction
Title: "i'm categorizing llm as a productivity tool": examining ethics of llm use in hci research practices.
Abstract: Large language models are increasingly applied in real-world scenarios, including research and education. These models, however, come with well-known ethical issues, which may manifest in unexpected ways in human-computer interaction research due to the extensive engagement with human subjects. This paper reports on research practices related to LLM use, drawing on 16 semi-structured interviews and a survey conducted with 50 HCI researchers. We discuss the ways in which LLMs are already being utilized throughout the entire HCI research pipeline, from ideation to system development and paper writing. While researchers described nuanced understandings of ethical issues, they were rarely or only partially able to identify and address those ethical concerns in their own projects. This lack of action and reliance on workarounds was explained through the perceived lack of control and distributed responsibility in the LLM supply chain, the conditional nature of engaging with ethics, and competing priorities. Finally, we reflect on the implications of our findings and present opportunities to shape emerging norms of engaging with large language models in HCI research.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Med Princ Pract
- v.30(1); 2021 Feb

Principles of Clinical Ethics and Their Application to Practice
An overview of ethics and clinical ethics is presented in this review. The 4 main ethical principles, that is beneficence, nonmaleficence, autonomy, and justice, are defined and explained. Informed consent, truth-telling, and confidentiality spring from the principle of autonomy, and each of them is discussed. In patient care situations, not infrequently, there are conflicts between ethical principles (especially between beneficence and autonomy). A four-pronged systematic approach to ethical problem-solving and several illustrative cases of conflicts are presented. Comments following the cases highlight the ethical principles involved and clarify the resolution of these conflicts. A model for patient care, with caring as its central element, that integrates ethical aspects (intertwined with professionalism) with clinical and technical expertise desired of a physician is illustrated.
Highlights of the Study
- Main principles of ethics, that is beneficence, nonmaleficence, autonomy, and justice, are discussed.
- Autonomy is the basis for informed consent, truth-telling, and confidentiality.
- A model to resolve conflicts when ethical principles collide is presented.
- Cases that highlight ethical issues and their resolution are presented.
- A patient care model that integrates ethics, professionalism, and cognitive and technical expertise is shown.
Introduction
A defining responsibility of a practicing physician is to make decisions on patient care in different settings. These decisions involve more than selecting the appropriate treatment or intervention.
Ethics is an inherent and inseparable part of clinical medicine [ 1 ] as the physician has an ethical obligation (i) to benefit the patient, (ii) to avoid or minimize harm, and to (iii) respect the values and preferences of the patient. Are physicians equipped to fulfill this ethical obligation and can their ethical skills be improved? A goal-oriented educational program [ 2 ] (Table (Table1) 1 ) has been shown to improve learner awareness, attitudes, knowledge, moral reasoning, and confidence [ 3 , 4 ].
Goals of ethics education
Ethics, Morality, and Professional Standards
Ethics is a broad term that covers the study of the nature of morals and the specific moral choices to be made. Normative ethics attempts to answer the question, “Which general moral norms for the guidance and evaluation of conduct should we accept, and why?” [ 5 ]. Some moral norms for right conduct are common to human kind as they transcend cultures, regions, religions, and other group identities and constitute common morality (e.g., not to kill, or harm, or cause suffering to others, not to steal, not to punish the innocent, to be truthful, to obey the law, to nurture the young and dependent, to help the suffering, and rescue those in danger). Particular morality refers to norms that bind groups because of their culture, religion, profession and include responsibilities, ideals, professional standards, and so on. A pertinent example of particular morality is the physician's “accepted role” to provide competent and trustworthy service to their patients. To reduce the vagueness of “accepted role,” physician organizations (local, state, and national) have codified their standards. However, complying with these standards, it should be understood, may not always fulfill the moral norms as the codes have “often appeared to protect the profession's interests more than to offer a broad and impartial moral viewpoint or to address issues of importance to patients and society” [ 6 ].
Bioethics and Clinical (Medical) Ethics
A number of deplorable abuses of human subjects in research, medical interventions without informed consent, experimentation in concentration camps in World War II, along with salutary advances in medicine and medical technology and societal changes, led to the rapid evolution of bioethics from one concerned about professional conduct and codes to its present status with an extensive scope that includes research ethics, public health ethics, organizational ethics, and clinical ethics.
Hereafter, the abbreviated term, ethics, will be used as I discuss the principles of clinical ethics and their application to clinical practice.
The Fundamental Principles of Ethics
Beneficence, nonmaleficence, autonomy, and justice constitute the 4 principles of ethics. The first 2 can be traced back to the time of Hippocrates “to help and do no harm,” while the latter 2 evolved later. Thus, in Percival's book on ethics in early 1800s, the importance of keeping the patient's best interest as a goal is stressed, while autonomy and justice were not discussed. However, with the passage of time, both autonomy and justice gained acceptance as important principles of ethics. In modern times, Beauchamp and Childress' book on Principles of Biomedical Ethics is a classic for its exposition of these 4 principles [ 5 ] and their application, while also discussing alternative approaches.
Beneficence
The principle of beneficence is the obligation of physician to act for the benefit of the patient and supports a number of moral rules to protect and defend the right of others, prevent harm, remove conditions that will cause harm, help persons with disabilities, and rescue persons in danger. It is worth emphasizing that, in distinction to nonmaleficence, the language here is one of positive requirements. The principle calls for not just avoiding harm, but also to benefit patients and to promote their welfare. While physicians' beneficence conforms to moral rules, and is altruistic, it is also true that in many instances it can be considered a payback for the debt to society for education (often subsidized by governments), ranks and privileges, and to the patients themselves (learning and research).
Nonmaleficence
Nonmaleficence is the obligation of a physician not to harm the patient. This simply stated principle supports several moral rules − do not kill, do not cause pain or suffering, do not incapacitate, do not cause offense, and do not deprive others of the goods of life. The practical application of nonmaleficence is for the physician to weigh the benefits against burdens of all interventions and treatments, to eschew those that are inappropriately burdensome, and to choose the best course of action for the patient. This is particularly important and pertinent in difficult end-of-life care decisions on withholding and withdrawing life-sustaining treatment, medically administered nutrition and hydration, and in pain and other symptom control. A physician's obligation and intention to relieve the suffering (e.g., refractory pain or dyspnea) of a patient by the use of appropriate drugs including opioids override the foreseen but unintended harmful effects or outcome (doctrine of double effect) [ 7 , 8 ].
The philosophical underpinning for autonomy, as interpreted by philosophers Immanuel Kant (1724–1804) and John Stuart Mill (1806–1873), and accepted as an ethical principle, is that all persons have intrinsic and unconditional worth, and therefore, should have the power to make rational decisions and moral choices, and each should be allowed to exercise his or her capacity for self-determination [ 9 ]. This ethical principle was affirmed in a court decision by Justice Cardozo in 1914 with the epigrammatic dictum, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body” [ 10 ].
Autonomy, as is true for all 4 principles, needs to be weighed against competing moral principles, and in some instances may be overridden; an obvious example would be if the autonomous action of a patient causes harm to another person(s). The principle of autonomy does not extend to persons who lack the capacity (competence) to act autonomously; examples include infants and children and incompetence due to developmental, mental or physical disorder. Health-care institutions and state governments in the US have policies and procedures to assess incompetence. However, a rigid distinction between incapacity to make health-care decisions (assessed by health professionals) and incompetence (determined by court of law) is not of practical use, as a clinician's determination of a patient's lack of decision-making capacity based on physical or mental disorder has the same practical consequences as a legal determination of incompetence [ 11 ].
Detractors of the principle of autonomy question the focus on the individual and propose a broader concept of relational autonomy (shaped by social relationships and complex determinants such as gender, ethnicity and culture) [ 12 ]. Even in an advanced western country such as United States, the culture being inhomogeneous, some minority populations hold views different from that of the majority white population in need for full disclosure, and in decisions about life support (preferring a family-centered approach) [ 13 ].
Resistance to the principle of patient autonomy and its derivatives (informed consent, truth-telling) in non-western cultures is not unexpected. In countries with ancient civilizations, rooted beliefs and traditions, the practice of paternalism ( this term will be used in this article, as it is well-entrenched in ethics literature, although parentalism is the proper term ) by physicians emanates mostly from beneficence. However, culture (a composite of the customary beliefs, social forms, and material traits of a racial, religious or social group) is not static and autonomous, and changes with other trends over passing years. It is presumptuous to assume that the patterns and roles in physician-patient relationships that have been in place for a half a century and more still hold true. Therefore, a critical examination of paternalistic medical practice is needed for reasons that include technological and economic progress, improved educational and socioeconomic status of the populace, globalization, and societal movement towards emphasis on the patient as an individual, than as a member of a group. This needed examination can be accomplished by research that includes well-structured surveys on demographics, patient preferences on informed consent, truth-telling, and role in decision-making.
Respecting the principle of autonomy obliges the physician to disclose medical information and treatment options that are necessary for the patient to exercise self-determination and supports informed consent, truth-telling, and confidentiality.
Informed Consent
The requirements of an informed consent for a medical or surgical procedure, or for research, are that the patient or subject (i) must be competent to understand and decide, (ii) receives a full disclosure, (iii) comprehends the disclosure, (iv) acts voluntarily, and (v) consents to the proposed action.
The universal applicability of these requirements, rooted and developed in western culture, has met with some resistance and a suggestion to craft a set of requirements that accommodate the cultural mores of other countries [ 14 ]. In response and in vigorous defense of the 5 requirements of informed consent, Angell wrote, “There must be a core of human rights that we would wish to see honored universally, despite variations in their superficial aspects …The forces of local custom or local law cannot justify abuses of certain fundamental rights, and the right of self-determination on which the doctrine of informed consent is based, is one of them” [ 15 ].
As competence is the first of the requirements for informed consent, one should know how to detect incompetence. Standards (used singly or in combination) that are generally accepted for determining incompetence are based on the patient's inability to state a preference or choice, inability to understand one's situation and its consequences, and inability to reason through a consequential life decision [ 16 ].
In a previously autonomous, but presently incompetent patient, his/her previously expressed preferences (i.e., prior autonomous judgments) are to be respected [ 17 ]. Incompetent (non-autonomous) patients and previously competent (autonomous), but presently incompetent patients would need a surrogate decision-maker. In a non-autonomous patient, the surrogate can use either a substituted judgment standard (i.e., what the patient would wish in this circumstance and not what the surrogate would wish), or a best interests standard (i.e., what would bring the highest net benefit to the patient by weighing risks and benefits). Snyder and Sulmasy [ 18 ], in their thoughtful article, provide a practical and useful option when the surrogate is uncertain of the patient's preference(s), or when patient's preferences have not kept abreast of scientific advances. They suggest the surrogate use “substituted interests,” that is, the patient's authentic values and interests, to base the decision.
Truth-Telling
Truth-telling is a vital component in a physician-patient relationship; without this component, the physician loses the trust of the patient. An autonomous patient has not only the right to know (disclosure) of his/her diagnosis and prognosis, but also has the option to forgo this disclosure. However, the physician must know which of these 2 options the patient prefers.
In the United States, full disclosure to the patient, however grave the disease is, is the norm now, but was not so in the past. Significant resistance to full disclosure was highly prevalent in the US, but a marked shift has occurred in physicians' attitudes on this. In 1961, 88% of physicians surveyed indicated their preference to avoid disclosing a diagnosis [ 19 ]; in 1979, however, 98% of surveyed physicians favored it [ 20 ]. This marked shift is attributable to many factors that include − with no order of importance implied − educational and socioeconomic progress, increased accountability to society, and awareness of previous clinical and research transgressions by the profession.
Importantly, surveys in the US show that patients with cancer and other diseases wish to have been fully informed of their diagnoses and prognoses. Providing full information, with tact and sensitivity, to patients who want to know should be the standard. The sad consequences of not telling the truth regarding a cancer include depriving the patient of an opportunity for completion of important life-tasks: giving advice to, and taking leave of loved ones, putting financial affairs in order, including division of assets, reconciling with estranged family members and friends, attaining spiritual order by reflection, prayer, rituals, and religious sacraments [ 21 , 22 ].
In contrast to the US, full disclosure to the patient is highly variable in other countries [ 23 ]. A continuing pattern in non-western societies is for the physician to disclose the information to the family and not to the patient. The likely reasons for resistance of physicians to convey bad news are concern that it may cause anxiety and loss of hope, some uncertainty on the outcome, or belief that the patient would not be able to understand the information or may not want to know. However, this does not have to be a binary choice, as careful understanding of the principle of autonomy reveals that autonomous choice is a right of a patient, and the patient, in exercising this right, may authorize a family member or members to make decisions for him/her.
Confidentiality
Physicians are obligated not to disclose confidential information given by a patient to another party without the patient's authorization. An obvious exception (with implied patient authorization) is the sharing necessary of medical information for the care of the patient from the primary physician to consultants and other health-care teams. In the present-day modern hospitals with multiple points of tests and consultants, and the use of electronic medical records, there has been an erosion of confidentiality. However, individual physicians must exercise discipline in not discussing patient specifics with their family members or in social gatherings [ 24 ] and social media. There are some noteworthy exceptions to patient confidentiality. These include, among others, legally required reporting of gunshot wounds and sexually transmitted diseases and exceptional situations that may cause major harm to another (e.g., epidemics of infectious diseases, partner notification in HIV disease, relative notification of certain genetic risks, etc.).
Justice is generally interpreted as fair, equitable, and appropriate treatment of persons. Of the several categories of justice, the one that is most pertinent to clinical ethics is distributive justice . Distributive justice refers to the fair, equitable, and appropriate distribution of health-care resources determined by justified norms that structure the terms of social cooperation [ 25 ]. How can this be accomplished? There are different valid principles of distributive justice. These are distribution to each person (i) an equal share, (ii) according to need, (iii) according to effort, (iv) according to contribution, (v) according to merit, and (vi) according to free-market exchanges. Each principle is not exclusive, and can be, and are often combined in application. It is easy to see the difficulty in choosing, balancing, and refining these principles to form a coherent and workable solution to distribute medical resources.
Although this weighty health-care policy discussion exceeds the scope of this review, a few examples on issues of distributive justice encountered in hospital and office practice need to be mentioned. These include allotment of scarce resources (equipment, tests, medications, organ transplants), care of uninsured patients, and allotment of time for outpatient visits (equal time for every patient? based on need or complexity? based on social and or economic status?). Difficult as it may be, and despite the many constraining forces, physicians must accept the requirement of fairness contained in this principle [ 26 ]. Fairness to the patient assumes a role of primary importance when there are conflicts of interests. A flagrant example of violation of this principle would be when a particular option of treatment is chosen over others, or an expensive drug is chosen over an equally effective but less expensive one because it benefits the physician, financially, or otherwise.
Conflicts between Principles
Each one of the 4 principles of ethics is to be taken as a prima facie obligation that must be fulfilled, unless it conflicts, in a specific instance, with another principle. When faced with such a conflict, the physician has to determine the actual obligation to the patient by examining the respective weights of the competing prima facie obligations based on both content and context. Consider an example of a conflict that has an easy resolution: a patient in shock treated with urgent fluid-resuscitation and the placement of an indwelling intravenous catheter caused pain and swelling. Here the principle of beneficence overrides that of nonmaleficence. Many of the conflicts that physicians face, however, are much more complex and difficult. Consider a competent patient's refusal of a potentially life-saving intervention (e.g., instituting mechanical ventilation) or request for a potentially life-ending action (e.g., withdrawing mechanical ventilation). Nowhere in the arena of ethical decision-making is conflict as pronounced as when the principles of beneficence and autonomy collide.
Beneficence has enjoyed a historical role in the traditional practice of medicine. However, giving it primacy over patient autonomy is paternalism that makes a physician-patient relationship analogous to that of a father/mother to a child. A father/mother may refuse a child's wishes, may influence a child by a variety of ways − nondisclosure, manipulation, deception, coercion etc., consistent with his/her thinking of what is best for the child. Paternalism can be further divided into soft and hard .
In soft paternalism, the physician acts on grounds of beneficence (and, at times, nonmaleficence) when the patient is nonautonomous or substantially nonautonomous (e.g., cognitive dysfunction due to severe illness, depression, or drug addiction) [ 27 ]. Soft paternalism is complicated because of the difficulty in determining whether the patient was nonautonomous at the time of decision-making but is ethically defensible as long as the action is in concordance with what the physician believes to be the patient's values. Hard paternalism is action by a physician, intended to benefit a patient, but contrary to the voluntary decision of an autonomous patient who is fully informed and competent, and is ethically indefensible.
On the other end of the scale of hard paternalism is consumerism, a rare and extreme form of patient autonomy, that holds the view that the physician's role is limited to providing all the medical information and the available choices for interventions and treatments while the fully informed patient selects from the available choices. In this model, the physician's role is constrained, and does not permit the full use of his/her knowledge and skills to benefit the patient, and is tantamount to a form of patient abandonment and therefore is ethically indefensible.
Faced with the contrasting paradigms of beneficence and respect for autonomy and the need to reconcile these to find a common ground, Pellegrino and Thomasma [ 28 ] argue that beneficence can be inclusive of patient autonomy as “the best interests of the patients are intimately linked with their preferences” from which “are derived our primary duties to them.”
One of the basic and not infrequent reasons for disagreement between physician and patient on treatment issues is their divergent views on goals of treatment. As goals change in the course of disease (e.g., a chronic neurologic condition worsens to the point of needing ventilator support, or a cancer that has become refractory to treatment), it is imperative that the physician communicates with the patient in clear and straightforward language, without the use of medical jargon, and with the aim of defining the goal(s) of treatment under the changed circumstance. In doing so, the physician should be cognizant of patient factors that compromise decisional capacity, such as anxiety, fear, pain, lack of trust, and different beliefs and values that impair effective communication [ 29 ].
The foregoing theoretical discussion on principles of ethics has practical application in clinical practice in all settings. In the resource book for clinicians, Jonsen et al. [ 30 ] have elucidated a logical and well accepted model (Table (Table2), 2 ), along the lines of the systematic format that practicing physicians have been taught and have practiced for a long time (Chief Complaint, History of Present Illness, Past History, pertinent Family and Social History, Review of Systems, Physical Examination and Laboratory and Imaging studies). This practical approach to problem-solving in ethics involves:
- Clinical assessment (identifying medical problems, treatment options, goals of care)
- Patient (finding and clarifying patient preferences on treatment options and goals of care)
- Quality of life (QOL) (effects of medical problems, interventions and treatments on patient's QOL with awareness of individual biases on what constitutes an acceptable QOL)
- Context (many factors that include family, cultural, spiritual, religious, economic and legal).
Application of principles of ethics in patient care
Using this model, the physician can identify the principles that are in conflict, ascertain by weighing and balancing what should prevail, and when in doubt, turn to ethics literature and expert opinion.
Illustrative Cases
There is a wide gamut of clinical patient encounters with ethical issues, and some, especially those involving end-of-life care decisions, are complex. A few cases (Case 1 is modified from resource book [ 30 ]) are presented below as they highlight the importance of understanding and weighing the ethical principles involved to arrive at an ethically right solution. Case 6 was added during the revision phase of this article as it coincided with the outbreak of Coronavirus Infectious Disease-2019 (COVID-19) that became a pandemic rendering a discussion of its ethical challenges necessary and important.
A 20-year old college student living in the college hostel is brought by a friend to the Emergency Department (ED) because of unrelenting headache and fever. He appeared drowsy but was responsive and had fever (40°C), and neck rigidity on examination. Lumbar puncture was done, and spinal fluid appeared cloudy and showed increased white cells; Gram stain showed Gram-positive diplococci. Based on the diagnosis of bacterial meningitis, appropriate antibiotics were begun, and hospitalization was instituted. Although initial consent for diagnosis was implicit, and consent for lumbar puncture was explicit, at this point, the patient refuses treatment without giving any reason, and insists to return to his hostel. Even after explanation by the physician as to the seriousness of his diagnosis, and the absolute need for prompt treatment (i.e., danger to life without treatment), the patient is adamant in his refusal.
Comment . Because of this refusal, the medical indications and patient preferences (see Table Table2) 2 ) are at odds. Is it ethically right to treat against his will a patient who is making a choice that has dire consequences (disability, death) who gives no reason for this decision, and in whom a clear determination of mental incapacity cannot be made (although altered mental status may be presumed)? Here the principle of beneficence and principle of autonomy are in conflict. The weighing of factors: (1) patient may not be making a reasoned decision in his best interest because of temporary mental incapacity; and (2) the severity of life-threatening illness and the urgency to treat to save his life supports the decision in favor of beneficence (i.e., to treat).
A 56-year old male lawyer and current cigarette smoker with a pack-a-day habit for more than 30 years, is found to have a solitary right upper lobe pulmonary mass 5 cm in size on a chest radiograph done as part of an insurance application. The mass has no calcification, and there are no other pulmonary abnormalities. He has no symptoms, and his examination is normal. Tuberculosis skin test is negative, and he has no history of travel to an endemic area of fungal infection. As lung cancer is the most probable and significant diagnosis to consider, and early surgical resection provides the best prospects for cure, the physician, in consultation with the thoracic surgeon, recommends bronchoscopic biopsy and subsequent resection. The patient understands the treatment plan, and the significance of not delaying the treatment. However, he refuses, and states that he does not think he has cancer; and is fearful that the surgery would kill him. Even after further explanations on the low mortality of surgery and the importance of removing the mass before it spreads, he continues to refuse treatment.
Comment . Even though the physician's prescribed treatment, that is, removal of the mass that is probably cancer, affords the best chance of cure, and delay in its removal increases its chance of metastases and reaching an incurable stage − the choice by this well informed and mentally competent patient should be respected. Here, autonomy prevails over beneficence. The physician, however, may not abandon the patient and is obligated to offer continued outpatient visits with advice against making decision based on fear, examinations, periodic tests, and encouragement to seek a second opinion.
A 71-year-old man with very severe chronic obstructive pulmonary disease (COPD) is admitted to the intensive care unit (ICU) with pneumonia, sepsis, and respiratory failure. He is intubated and mechanically ventilated. For the past 2 years, he has been on continuous oxygen treatment and was short of breath on minimal exertion. In the past 1 year, he had 2 admissions to the ICU; on both occasions he required intubation and mechanical ventilation. Presently, even with multiple antibiotics, intravenous fluid hydration, and vasopressors, his systolic blood pressure remains below 60 mm Hg, and with high flow oxygen supplementation, his oxygen saturation stays below 80%; his arterial blood pH is 7.0. His liver enzymes are elevated. He is anuric, and over next 8 h his creatinine has risen to 5 mg/dL and continues to rise. He has drifted into a comatose state. The intensivist suggests discontinuation of vasopressors and mechanical ventilation as their continued use is futile. The patient has no advance care directives or a designated health-care proxy.
Comment . The term “futility” is open to different definitions [ 31 ] and is often controversial, and therefore, some experts suggest the alternate term, “clinically non-beneficial interventions” [ 32 ]. However, in this case the term futility is appropriate to indicate that there is evidence of physiological futility (multisystem organ failure in the setting of preexisting end stage COPD, and medical interventions would not reverse the decline). It is appropriate then to discuss the patient's condition with his family with the goal of discontinuing life-sustaining interventions. These discussions should be done with sensitivity, compassion and empathy. Palliative care should be provided to alleviate his symptoms and to support the family until his death and beyond in their bereavement.
A 67-year old widow, an immigrant from southern India, is living with her son and his family in Wisconsin, USA. She was experiencing nausea, lack of appetite and weight loss for a few months. During the past week, she also had dark yellow urine, and yellow coloration of her skin. She has basic knowledge of English. She was brought to a multi-specialty teaching hospital by her son, who informed the doctor that his mother has “jaundice,” and instructed that, if any serious life-threatening disease was found, not to inform her. He asked that all information should come to him, and if there is any cancer not to treat it, since she is older and frail. Investigations in the hospital reveals that she has pancreatic cancer, and chemotherapy, while not likely to cure, would prolong her life.
Comment . In some ancient cultures, authority is given to members of the family (especially senior men) to make decisions that involve other members on marriage, job, and health care. The woman in this case is a dependent of her son, and given this cultural perspective, the son can rightfully claim to have the authority to make health-care decisions for her. Thus, the physician is faced with multiple tasks that may not be consonant. To respect cultural values [ 33 ], to directly learn the patient's preferences, to comply with the American norm of full disclosure to the patient, and to refuse the son's demands.
The principle of autonomy provides the patient the option to delegate decision-making authority to another person. Therefore, the appropriate course would be to take the tactful approach of directly informing the patient (with a translator if needed), that the diagnosed disease would require decisions for appropriate treatment. The physician should ascertain whether she would prefer to make these decisions herself, or whether she would prefer all information to be given to her son, and all decisions to be made by him.
A 45-year-old woman had laparotomy and cholecystectomy for abdominal pain and multiple gall stones. Three weeks after discharge from the hospital, she returned with fever, abdominal pain, and tenderness. She was given antibiotics, and as her fever continued, laparotomy and exploration were undertaken; a sponge left behind during the recent cholecystectomy was found. It was removed, the area cleansed, and incision closed. Antibiotics were continued, and she recovered without further incident and was discharged. Should the surgeon inform the patient of his error?
Comment . Truth-telling, a part of patient autonomy is very much applicable in this situation and disclosure to patient is required [ 34 , 35 , 36 ]. The mistake caused harm to the patient (morbidity and readmission, and a second surgery and monetary loss). Although the end result remedied the harm, the surgeon is obligated to inform the patient of the error and its consequences and offer an apology. Such errors are always reported to the Operating Room Committees and Surgical Quality Improvement Committees of US Hospitals. Hospital-based risk reduction mechanisms (e.g., Risk Management Department) present in most US hospitals would investigate the incident and come up with specific recommendations to mitigate the error and eliminate them in the future. Many institutions usually make financial settlements to obviate liability litigation (fees and hospital charges waived, and/or monetary compensation made to the patient). Elsewhere, if such mechanisms do not exist, it should be reported to the hospital. Acknowledgment from the hospital, apologies from the institution and compensation for the patient are called for. Whether in US or elsewhere, a malpractice suit is very possible in this situation, but a climate of honesty substantially reduces the threat of legal claims as most patients trust their physicians and are not vindictive.
The following scenario is at a city hospital during the peak of the COVID-19 pandemic: A 74-year-old woman, residing in an assisted living facility, is brought to the ED with shortness of breath and malaise. Over the past 4 days she had been experiencing dry cough, lack of appetite, and tiredness; 2 days earlier, she stopped eating and started having a low-grade fever. A test for COVID-19 undertaken by the assisted living facility was returned positive on the morning of the ED visit.
She, a retired nurse, is a widow; both of her grown children live out-of-state. She has had hypertension for many years, controlled with daily medications. Following 2 strokes, she was moved to an assisted living facility 3 years ago. She recovered most of her functions after the strokes and required help only for bathing and dressing. She is able to answer questions appropriately but haltingly, because of respiratory distress. She has tachypnea (34/min), tachycardia (120/min), temperature of 101°F, BP 100/60 and 90% O 2 saturation (on supplemental O 2 of 4 L/min). She has dry mouth and tongue and rhonchi on lung auscultation. Her respiratory rate is increasing on observation and she is visibly tiring.
Another patient is now brought in by ambulance; this is a 22-year-old man living in an apartment and has had symptoms of “flu” for a week. Because of the pandemic, he was observing the recommended self-distancing, and had no known exposure to coronavirus. He used saline gargles, acetaminophen, and cough syrup to alleviate his sore throat, cough, and fever. In the past 2 days, his symptoms worsened, and he drove himself to a virus testing station and got tested for COVID-19; he was told that he would be notified of the results. He returned to his apartment and after a sleepless night with fever, sweats, and persistent cough, he woke up and felt drained of all strength. The test result confirmed COVID-19. He then called for an ambulance.
He has been previously healthy. He is a non-smoker and uses alcohol rarely. He is a second-year medical student. He is single, and his parents and sibling live hundreds of miles away.
On examination, he has marked tachypnea (>40/min), shallow breathing, heart rate of 128/min, temperature of 103°F and O 2 saturation of 88 on pulse oximetry. He appears drowsy and is slow to respond to questions. He is propped up to a sitting position as it is uncomfortable for him to be supine. Accessory muscles of neck and intercostals are contracting with each breath, and on auscultation, he has basilar crackles and scattered rhonchi. His O 2 saturation drops to 85 and he is in respiratory distress despite nebulized bronchodilator treatment.
Both of these patients are in respiratory failure, clinically and confirmed by arterial blood gases, and are in urgent need of intubation and mechanical ventilation. However, only one ventilator is available; who gets it?
Comment . The decision to allocate a scarce and potentially life-saving equipment (ventilator) is very difficult as it directly addresses the question “Who shall live when not everyone can live? [ 5 ]. This decision cannot be emotion-driven or arbitrary; nor should it be based on a person's wealth or social standing. Priorities need to be established ethically and must be applied consistently in the same institution and ideally throughout the state and the country. The general social norm to treat all equally or to treat on a first come, first saved basis is not the appropriate choice here. There is a consensus among clinical ethics scholars, that in this situation, maximizing benefits is the dominant value in making a decision [ 37 ]. Maximizing benefits can be viewed in 2 different ways; in lives saved or in life-years saved; they differ in that the first is non-utilitarian while the second is utilitarian. A subordinate consideration is giving priority to patients who have a better chance of survival and a reasonable life expectancy. The other 2 considerations are promoting and rewarding instrumental value (benefit to others) and the acuity of illness. Health-care workers (physicians, nurses, therapists etc.) and research participants have instrumental value as their work benefits others; among them those actively contributing are of more value than those who have made their contributions. The need to prioritize the sickest and the youngest is also a recognized value when these are aligned with the dominant value of maximizing benefits. In the context of COVID-19 pandemic, Emanuel et al. [ 37 ] weighed and analyzed these values and offered some recommendations. Some ethics scholars opine that in times of a pandemic, the burden of making a decision as to who gets a ventilator and who does not (often a life or death choice) should not be on the front-line physicians, as it may cause a severe and life-long emotional toll on them [ 35 , 36 ]. The toll can be severe for nurses and other front-line health-care providers as well. As a safeguard, they propose that the decision should rest on a select committee that excludes doctors, nurses and others who are caring for the patient(s) under consideration [ 38 ].
Both patients described in the case summaries have comparable acuity of illness and both are in need of mechanical ventilator support. However, in the dominant value of maximizing benefits the two patients differ; in terms of life-years saved, the second patient (22-year-old man) is ahead as his life expectancy is longer. Additionally, he is more likely than the older woman, to survive mechanical ventilation, infection, and possible complications. Another supporting factor in favor of the second patient is his potential instrumental value (benefit to others) as a future physician.
Unlike the other illustrative cases, the scenario of these 2 cases, does not lend itself to a peaceful and fully satisfactory resolution. The fairness of allocating a scarce and potentially life-saving resource based on maximizing benefits and preference to instrumental value (benefit to others) is open to question. The American College of Physicians has stated that allocation decisions during resource scarcity should be made “based on patient need, prognosis (determined by objective scientific measure and informed clinical judgment) and effectiveness (i.e., likelihood that the therapy will help the patient to recover), … to maximize the number of patients who will recover” [ 39 ].
This review has covered basics of ethics founded on morality and ethical principles with illustrative examples. In the following segment, professionalism is defined, its alignment with ethics depicted, and virtues desired of a physician (inclusive term for medical doctor regardless of type of practice) are elucidated. It concludes with my vision of an integrated model for patient care.
The core of professionalism is a therapeutic relationship built on competent and compassionate care by a physician that meets the expectation and benefits a patient. In this relationship, which is rooted in the ethical principles of beneficence and nonmaleficence, the physician fulfills the elements shown in Table Table3. 3 . Professionalism “demands placing the interest of patients above those of the physician, setting and maintaining standards of competence and integrity, and providing expert advice to society on matters of health” [ 26 , 40 ].
Physicians obligations
Drawing on several decades of experience in teaching and mentoring, I envisage physicians with qualities of both “heart” and “head.” Ethical and humanistic values shape the former, while knowledge (e.g., by study, research, practice) and technical skills (e.g., medical and surgical procedures) form the latter. Figure Figure1 1 is a representation of this model. Morality that forms the base of the model and ethical principles that rest on it were previously explained. Virtues are linked, some more tightly than others, to the principles of ethics. Compassion, a prelude to caring, presupposes sympathy, is expressed in beneficence. Discernment is especially valuable in decision-making when principles of ethics collide. Trustworthiness leads to trust, and is a needed virtue when patients, at their most vulnerable time, place themselves in the hands of physicians. Integrity involves the coherent integration of emotions, knowledge and aspirations while maintaining moral values. Physicians need both professional integrity and personal integrity, as the former may not cover all scenarios (e.g., prescribing ineffective drugs or expensive drugs when effective inexpensive drugs are available, performing invasive treatments or experimental research modalities without fully informed consent, any situation where personal monetary gain is placed over patient's welfare). Conscientiousness is required to determine what is right by critical reflection on good versus bad, better versus good, logical versus emotional, and right versus wrong.

Integrated model of patient care.
In my conceptualized model of patient care (Fig. (Fig.1), 1 ), medical knowledge, skills to apply that knowledge, technical skills, practice-based learning, and communication skills are partnered with ethical principles and professional virtues. The virtues of compassion, discernment, trustworthiness, integrity, and conscientiousness are the necessary building blocks for the virtue of caring. Caring is the defining virtue for all health-care professions. In all interactions with patients, besides the technical expertise of a physician, the human element of caring (one human to another) is needed. In different situations, caring can be expressed verbally and non-verbally (e.g., the manner of communication with both physician and patient closely seated, and with unhurried, softly spoken words); a gentle touch especially when conveying “bad news”; a firmer touch or grip to convey reassurance to a patient facing a difficult treatment choice; to hold the hand of a patient dying alone). Thus, “caring” is in the center of the depicted integrated model, and as Peabody succinctly expressed it nearly a hundred years ago, “The secret of the care of the patient is caring for the patient” [ 41 ].
Conflict of Interest Statement
The author declares that he has no conflicts of interest.
- Share full article

The Psychedelic Evangelist
A Johns Hopkins scientist was known for rigorous studies of psychedelics. Was he a true believer?
Credit... Caitlin Teal Price
Supported by
By Brendan Borrell
- Published March 21, 2024 Updated March 27, 2024
Before he died last year, Roland Griffiths was arguably the world’s most famous psychedelics researcher. Since 2006, his work has suggested that psilocybin, found in magic mushrooms, can induce mystical experiences, and that those experiences, in turn, can help treat anxiety, depression, addiction and the terror of death.
Listen to this article with reporter commentary
Open this article in the New York Times Audio app on iOS.
Dr. Griffiths and his colleagues at Johns Hopkins University received widespread recognition among scientists and the popular press, helping to pull the psychedelic field from the deep backwater of the 1960s hippie movement. This second wave of research on the hallucinogenic compounds bolstered political campaigns to decriminalize them and spurred biotech investment.
Dr. Griffiths was known to friends and colleagues as an analytical thinker and a religious agnostic, and he warned fellow researchers against hype . But he also saw psychedelics as more than mere medicines: Understanding them could be “critical to the survival of the human species,” he said in one talk . Late in life, he admitted to taking psychedelics himself, and said he wanted science to help unlock their transformative power for humanity.
Perhaps unsurprisingly, he held a vaunted, even prophetic role among psychonauts, the growing community of psychedelic believers who want to bring the drugs into mainstream society. For years, critics have denounced the outsize financial and philosophical influence of these advocates on the insular research field. And some researchers have quietly questioned whether Dr. Griffiths, in his focus on the mystical realm , made some of the same mistakes that doomed the previous era of psychedelic science.
Now, one of his longtime collaborators is airing a more forceful critique. “Dr. Griffiths has run his psychedelic studies more like a ‘new-age’ retreat center, for lack of a better term, than a clinical research laboratory,” reads an ethics complaint filed to Johns Hopkins last fall by Matthew Johnson, who worked with Dr. Griffiths for nearly 20 years but resigned after a charged dispute with colleagues.
Dr. Griffiths acted like a “spiritual leader,” the complaint said, infusing the research with religious symbolism and steering volunteers toward the outcome he wanted. And he allowed some of his longstanding donors — supporters of drug legalization — to assist in studies, raising ethical questions.
“These are serious allegations that need to be investigated,” said Joanna Kempner, a medical sociologist at Rutgers University who reviewed the complaint for The New York Times. The clashes at Hopkins, she added, mirror a broader debate in the field over “blurring the lines between empirical research and spiritual practice.”
Many researchers see medical promise in the mind-opening power of psilocybin. But so far, it has not performed better than traditional drugs for depression in the only head-to-head comparison conducted to date . Its potential for treating other conditions, such as addiction and anorexia, is also uncertain. And the jury is still out on whether mystical experiences are key to the drug’s effectiveness.
“The inferences drawn in the literature at large certainly don’t follow from the evidence,” said Eiko Fried, a psychologist at Leiden University in the Netherlands who recently published a critical review of the field. The drugs also come with unpredictable risks, such as psychotic episodes , increased suicidality or extended emotional difficulties , which are most likely underreported .
In an email, Johns Hopkins told Dr. Johnson that it was investigating his allegations. A university spokeswoman did not respond to detailed questions for this article, but said that the research “is expected to meet the highest standards for research integrity and participant safety.”
Skeptical Beginnings
In the 1950s and ’60s, a spate of studies reported near-miraculous results using hallucinogens to treat alcoholism and depression. Then came the backlash.
Harvard made headlines for firing professors who doled out LSD and psilocybin to students. During the 1971 murder trial of the cult leader Charles Manson, a psychiatrist testified that LSD could have made Mr. Manson’s followers more likely to commit murder.
Psychiatric researchers, meanwhile, began adopting the randomized clinical trials that had revolutionized other fields. Seven controlled clinical trials in the 1960s and ’70s tested LSD’s utility for alcohol addiction. Six came back negative.
Dr. Griffiths, who grew up near Berkeley, Calif., experimented with LSD during college, he later told interviewers, but was skeptical of the claims around it. He was finishing up his doctoral research in psychopharmacology in 1970 when LSD and psilocybin became illegal, making them harder to study.
He set up a lab at Johns Hopkins that for decades published well-regarded studies on caffeine , heroin and other drugs. He didn’t think much about psychedelics until the 1990s, when he began practicing meditation and reading about mystical traditions.
Around that time, a friend introduced him to Bob Jesse, a former technology executive who founded a nonprofit called the Council on Spiritual Practices. Through legal briefs, scholarly research and a book-publishing venture, Mr. Jesse advocated the use of hallucinogenic chemicals and plants for the greater good of humanity. Now he wanted to give them the imprimatur of science, as he later said in a talk .
In 1999, with funding from Mr. Jesse’s nonprofit, Dr. Griffiths began recruiting healthy volunteers for an experiment. Mind-altering mushrooms had been used in religious rituals of various cultures for centuries. Could the same kind of meaningful experiences be induced in a lab?
His team distributed fliers around Baltimore: “Seeking Persons Committed to Spiritual Development for a Study of States of Consciousness.”
Buddha in the Mind
Dr. Griffiths’s laboratory looked like a living room, with a couch, a selection of spiritual and art books and a shelf holding a Buddha statue. The idea was to make volunteers “appreciative of the spiritual states that can awaken,” according to Bill Richards, a psychotherapist and former Methodist minister who worked on multiple trials.
Dr. Richards delivered the psilocybin pill or a placebo to participants in a chalice-shaped incense burner from Mexico that Mr. Jesse had given the team. Neither the researchers nor the participants knew which pill was in the burner.
Donning an eye mask and headphones, volunteers were encouraged to lie down on the couch for the peak effects of the drug, which last around five hours. At the end of the session, Dr. Griffiths came in to document their experiences. “He was just amazed,” Dr. Richards said. “He wanted to hear their story over and over.”
Dr. Griffiths used a “Mystical Experience Questionnaire,” which has roots in a philosophy espoused by the novelist and psychedelic enthusiast Aldous Huxley. It asks volunteers to rate, for example, their sense of having “profound humility before the majesty of what was felt to be sacred or holy.”
More than half of the 36 participants in the first Hopkins study had a “complete” mystical experience. Many ranked it among the most meaningful of their lives. When the study was published in 2006, four commentaries from drug researchers ran alongside it, praising its rigor.
In his studies of other drugs, Dr. Griffiths later said , he had “never seen anything so unique and powerful and enduring.” The results, he said, suggested that “we’re wired for these kinds of experiences.” The Council on Spiritual Practices sent out a fund-raising letter claiming that the study “uses science, which modernity trusts, to undermine modernity’s secularism.”
The volunteers were not a random cross-section of the population. In his 2018 book, “How to Change Your Mind,” the author Michael Pollan observed that there were no “stone-cold atheists” among the participants, which included an energy healer, a former Franciscan friar and an herbalist. Dr. Griffiths was open about this drawback of the study. “We were interested in a spiritual effect and were biasing the condition initially,” he told Mr. Pollan.
Some researchers suspected that the drug elicited mystical experiences because the unusual laboratory and questionnaire had primed the volunteers for that result. Dr. Richards also carried out some lengthy preparatory sessions with volunteers at his home office, he said, in order to develop trust.
“Roland did not do the kind of study I was both expecting and hoping he would do,” said Dr. Rick Strassman, a psychiatrist at the University of New Mexico. “He just jumped with both feet into the mystical experience world.”
Years earlier, Dr. Strassman had given psilocybin and intravenous DMT, a compound in ayahuasca tea, to more than 50 volunteers inside an austere room. Only one individual, a religious studies major, had a mystical experience. An architect with an interest in computers, by contrast, reported seeing “the raw bits of reality.” Others thought they had been abducted by aliens.
The drugs “had no inherent spiritual properties,” Dr. Strassman said.
Psychedelic researchers have long recognized that a volunteer’s mind-set and the setting where the session takes place — “ set and setting ,” they call it — are crucial to a subject’s response.
Such expectancy effects influence clinical trials of all kinds. Because of volunteers’ hopes around a trial, even those who receive a placebo will often show more improvement than those who receive nothing. Some experts have suggested that psychedelics function as “super placebos” because they increase suggestibility .
Natasha Mason, a psychopharmacologist at Maastricht University in the Netherlands, said that while she understood the Hopkins researchers’ goals, the experimental design had put a thumb on the spiritual scale. “Their mystical experiences results are very high compared to other groups,” she said.
Dr. Richards rejected such criticism. Psychedelic drugs, he said, open a state of consciousness that allows for religious experiences.
“The Buddha, if you will, is in the human mind,” he said. “Whether there is a statue in the room or not doesn’t matter.”

Disappointment Effects
After his splashy first paper, Dr. Griffiths began investigating whether psilocybin could help people cope with cancer.
That study followed 51 cancer patients. About 80 percent of the volunteers were less depressed and anxious after receiving a high dose of psilocybin, an effect that persisted for at least six months. And the stronger their reports of mystical experiences, the better their outcomes.
The study, published in 2016, became the most cited paper in the field. But Dr. Johnson, who was a co-author, was troubled by parts of it.
He learned from the study’s therapist that Dr. Griffiths had asked a volunteer to reconsider her written rating of the spiritual significance of a psilocybin session because it was not as positive as what she had told him verbally. She then increased the rating, according to Dr. Johnson’s complaint. (The therapist declined to comment.)
Another volunteer, a businessman with terminal cancer, grew frustrated that he wasn’t feeling anything during his first session and stormed out, according to Dr. Richards and Dr. Johnson. The volunteer had received the placebo, a trivial dose of psilocybin.
He asked to return to the trial, but the team did not allow it. A week later, he died by suicide.
The researchers reported the incident to regulators and the study was allowed to continue. It’s not especially uncommon for trial volunteers to feel disappointment when they do not experience the benefits they hope for.
Dr. Griffiths suggested to collaborators that they better prepare participants for possible letdown. He also stressed that the man’s case should not be made public. “Distribution should be on a NEED TO KNOW basis,” he wrote in a 2012 email.
In November 2016, a week before publication, the journal gave journalists an early look at the paper. The man’s death was not mentioned, recalled Bob Tedeschi, a reporter who covered the research for Stat.
He only learned about it after sending the initial version of the paper to Katherine MacLean, who was a professor at Hopkins when the suicide took place. “Go back to Roland and ask if there were any deaths,” she recalled telling him.
In Mr. Tedeschi’s article , Dr. Griffiths said the suicide was unrelated to the research. A brief description of the case, which was appended to the study by the time it was published, reiterated this point.
Few took notice of the suicide until they heard the full story from Dr. MacLean, who subsequently made it her mission to share it with other researchers and practitioners.
Dr. Strassman, the DMT researcher, said at a scientific conference in 2022 that the suicide illustrated the grave risks of raising patients’ hopes.
“It’s a shame that this case isn’t being dissected under the microscope,” he said in an interview. “What mistakes were made? How could they have been prevented?”
Such risks are beginning to be more widely discussed. In a recent trial of treatment-resistant depression sponsored by Compass Pathways, a biotechnology company, three volunteers who did not respond to psilocybin reported either an aborted suicide attempt or acquiring materials for a suicide.
The results reflected a “relatively high rate of adverse events,” according to a commentary by Dr. Natalie Gukasyan, a former mentee of Dr. Griffiths.
“With recent media portrayals of psychedelics as cure-all drugs, patients who have failed to find relief from other treatments may come to see psychedelics as a last resort,” she wrote.
Rising Tensions
In 2019, with $17 million in private funding, Dr. Griffiths opened the Center for Psychedelic and Consciousness Research at Johns Hopkins. Dr. Johnson, the assistant director, frequently argued with his boss, he said, over how to talk to study volunteers and the public at large.
Dr. Griffiths regularly recommended spiritual literature, documentaries and meditation classes to volunteers, according to Dr. Johnson’s complaint. And during participant interviews, it said, he reinforced volunteers’ claims of experiencing an “ultimate reality” by “staring into their eyes, smiling and nodding knowingly.”
Dr. Johnson wasn’t the only researcher who was uncomfortable with the mystical atmosphere. “I’ve seen people do some ‘namastes’ in there,” said Manoj Doss, a former postdoctoral researcher at the center. Dr. Doss was also taken aback by the incense burner and the preparatory sessions done at Dr. Richards’s home. “That’s pretty weird, I’m not going to lie,” Dr. Doss said.
In 2021, Tehseen Noorani, a medical humanities researcher who embedded with the Hopkins team from 2013 to 2015, published a paper based on dozens of interviews with psychedelic enthusiasts in Baltimore, many of whom were participants in the Hopkins trials. “Despite posturing to the contrary, the clinical research team was not separate from underground communities of psychonauts,” the paper said .
Around the same time, Dr. Johnson published a paper warning that, without realizing it, psychedelic researchers might “fall into the trap of playing guru or priest.”
Dr. Griffiths was diagnosed with colon cancer in November 2021, and Dr. Johnson became the center’s acting director. He wasn’t widely liked. Some employees thought he could be curt and preoccupied with personal media appearances.
The center’s research manager filed a grievance with human resources claiming that Dr. Johnson had made “passive aggressive remarks,” chided her about a Facebook interaction and yelled at her during an incident years earlier. The manager, who declined to comment, posted on social media about “workplace trauma.” In a subsequent email chain, 11 of her co-workers said they were taking a day off in solidarity.
Dr. Griffiths was not happy. If the media got wind of the controversy, he told Dr. Johnson, it could jeopardize the psychedelic renaissance.
In an interview, Dr. Johnson denied that he acted unprofessionally. In a letter reviewed by The Times, a department leader told him the allegations did not rise to the level of an investigation at the medical school, but that he should refrain from belittling others. He was removed from his role, and Dr. Griffiths returned as director.
Funding conflicts
Dr. Griffiths’s interest in psychedelics went beyond the clinic: He saw them as a tool to awaken altered states of consciousness. His pro-psychedelic funders allowed him to test this notion in the lab.
Since 2008, the RiverStyx Foundation, started by a philanthropist named T. Cody Swift, has donated more than $1.4 million to support psychedelics research at Johns Hopkins, including the cancer study and five other trials. Mr. Swift has also given more than $4 million toward drug policy reform, including campaigns to decriminalize psychedelics in Oregon and Colorado.
Mr. Swift enjoyed an unusually close relationship with the Hopkins team. In 2013, he served as an “assistant guide” on the cancer study, sitting in the room during sessions with volunteers and providing them support. He also co-wrote three studies with Dr. Griffiths between 2018 and 2022.
In 2015, he helped pay for one of Dr. Griffiths’s final studies , which gave psilocybin to 20 religious leaders to see whether it changed the way they practiced their ministry. Mr. Swift got to know many of the participants during follow-up interviews, he said.
For some, the drug had been a revelation. “It took my worldview and busted it open,” said James Lindberg, a Lutheran pastor who leads a church in suburban Omaha.
The experience spurred Zac Kamenetz, a rabbi from Berkeley, to start an organization that integrates psychedelics into Judaism. Hunt Priest, an Episcopalian priest from Savannah, founded a Christian psychedelic society.
Both organizations received financial backing from Mr. Swift. In June 2022, he also sponsored a retreat that brought former subjects and researchers together at a Tibetan center in the Catskill Mountains.
Dr. Griffiths, who was too ill to attend, requested that the retreat take place after all the data had been collected. Over video conferences inside an airy cabin, he spoke to each participant one by one and shared the study’s preliminary results, which have not yet been published.
Dr. Johnson, who collaborated on the study, did not know about the Catskills gathering. In the summer of 2023, he accepted a new job at the nearby Sheppard Pratt Hospital, and then submitted his complaint to Johns Hopkins. Dr. Griffiths died in October.
The next month, after learning about the retreat, Dr. Johnson submitted a second complaint. He was alarmed by the mingling of researchers, funders and volunteers, and did not believe it had been approved by the university’s board of research ethics.
Carl Elliott, a bioethicist at the University of Minnesota who reviewed Dr. Johnson’s complaint for The Times, agreed that the retreat was troubling.
“This reminds me of pharmaceutical companies making allies of patients,” Dr. Elliott said. “Here, it’s going through religious channels instead.”
Mr. Swift said that his foundation provided financial disclosures in published papers and on its website. “I always try to hold awareness of how my personal views might influence the people I work with,” he said.
‘This Marvelous Experience’
Around 2012, a dozen years into his research program, Dr. Griffiths tried a psychedelic for the first time since college, according to an interview on Tim Ferriss’s podcast in late 2022. A few trips since then, he said, had given him a powerful feeling of mindfulness, much like meditation.
“What we want, those of us who have done spiritual practices and have experience with psychedelics, is we want the world, humankind, to awaken to what this marvelous experience is,” he said in the interview.
The public’s interest in that experience is indeed rising. In 2022, more than 4 percent of middle-aged adults reported psychedelic use, up from 0.5 percent a decade earlier. “The scientific research has destigmatized these substances,” said Amy McGuire, a bioethicist at Baylor College of Medicine in Houston.
At times, Dr. Griffiths seemed to be conflicted about his role as a psychedelic evangelist. Last summer, he exchanged emails with a former trial volunteer, Travis Kitchens, who had written a critical article about the “occult roots” of the Hopkins research.
“I have ambivalence that this narrative might be interpreted as me wanting to promote a psychedelic religion,” Dr. Griffiths wrote.
And yet, he also embraced those who saw him as a prophet. Last June, he attended a dinner in his honor at the Psychedelic Science meeting in Denver. An artist unveiled a portrait of him with a psilocybin molecule hovering above his outstretched hands.
Inscribed on the gold frame were words drawn from the Mystical Experience Questionnaire: holy, spirit, ecstasy, awe and paradox. Dr. Griffiths had signed the back, writing, “May you remain aware of awareness.”
The painting is on view at the Chapel of Sacred Mirrors in upstate New York.
Caitlin Teal Price is an artist based in Washington, D.C. Her works, “Dune” and “Fortress” from 2023, combine X-acto blade etching on photographic prints.
Produced by Antonio de Luca , Matt McCann and Elijah Walker .
Read by Brendan Borrell
Audio produced by Sarah Diamond .
The Great Read
Here are more fascinating tales you can’t help reading all the way to the end..
Deathbed Visions: Researchers are documenting deathbed visions , a phenomenon that seems to help the dying, as well as those they leave behind.
The Pants Pendulum: Around 2020, the “right” pants began to swing from skinny to wide. But is there even a consensus around trends anymore ?
The Psychic Peril of Mars: NASA is conducting tests on what might be the greatest challenge of a human mission to the red planet: the trauma of isolation .
Saved by a Rescue Dog: He spent 13 years addicted to cocaine. Running a shelter for abused and neglected dogs in New York has kept him sober, but it hasn’t been easy .
An Art Mogul's Fall: After a dramatic rise in business and society, Louise Blouin finds herself unloading a Hamptons dream home in bankruptcy court .
Advertisement
- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
Research Roundup: How the Pandemic Changed Management
- Mark C. Bolino,
- Jacob M. Whitney,
- Sarah E. Henry

Lessons from 69 articles published in top management and applied psychology journals.
Researchers recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic that were published between March 2020 and July 2023 in top journals in management and applied psychology. The review highlights the numerous ways in which employees, teams, leaders, organizations, and societies were impacted and offers lessons for managing through future pandemics or other events of mass disruption.
The recent pandemic disrupted life as we know it, including for employees and organizations around the world. To understand such changes, we recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic. These papers were published between March 2020 and July 2023 in top journals in management and applied psychology.
- Mark C. Bolino is the David L. Boren Professor and the Michael F. Price Chair in International Business at the University of Oklahoma’s Price College of Business. His research focuses on understanding how an organization can inspire its employees to go the extra mile without compromising their personal well-being.
- JW Jacob M. Whitney is a doctoral candidate in management at the University of Oklahoma’s Price College of Business and an incoming assistant professor at Kennesaw State University. His research interests include leadership, teams, and organizational citizenship behavior.
- SH Sarah E. Henry is a doctoral candidate in management at the University of Oklahoma’s Price College of Business and an incoming assistant professor at the University of South Florida. Her research interests include organizational citizenship behaviors, workplace interpersonal dynamics, and international management.
Partner Center
- Open access
- Published: 29 March 2024
A collaborative endeavour to integrate leadership and person-centred ethics: a focus group study on experiences from developing and realising an educational programme to support the transition towards person-centred care
- Qarin Lood 1 , 2 , 3 , 4 ,
- Eric Carlström 2 , 5 , 6 ,
- Charlotte Klinga 2 , 7 , 8 &
- Emmelie Barenfeld 1 , 2 , 3
BMC Health Services Research volume 24 , Article number: 395 ( 2024 ) Cite this article
39 Accesses
Metrics details
Ensuring the transition towards person-centred care is a growing focus in health and social care systems globally. Presented as an ethical framework for health and social care professionals, such a transition requires strong leadership and organisational changes. However, there is limited guidance available on how to assist health and social care leaders in promoting person-centred practices. In response to this, the Swedish Association of Health Professionals and the University of Gothenburg Centre for Person-Centred Care collaborated to develop an educational programme on person-centred leadership targeting health and social care leaders to support the transition towards person-centred care in Sweden. The aim with this study was to explore programme management members’ experiences from the development and realisation of the programme.
Focus group discussions were conducted, involving 12 members of the programme management team. Data from the discussions were analysed using a structured approach with emphasis the collaborative generation of knowledge through participant interaction.
The analysis visualises the preparations and actions involved in programme development and realisation as a collaborative endeavour, aimed at integrating leadership and person-centred ethics in a joint learning process. Participants described the programme as an ongoing exploration, extending beyond its formal duration. Leadership was thoughtfully interwoven with person-centred ethics throughout the programme, encompassing both the pedagogical approach and programme curriculum, to provide leaders with tangible tools for their daily use.
Conclusions
According to our analysis, we conclude that a person-centred approach to both development and realisation of educational initiatives to support person-centred leadership is essential for programme enhancement and daily implementation of person-centred leadership. Our main message is that educational initiatives on the application of person-centred ethics is an ongoing and collaborative process, characterised by an exchange of ideas and collective efforts.
Peer Review reports
There is a growing emphasis on endeavours to establish health and social care systems, procedures, and practices that prioritise the importance of persons [ 1 ]. This indicates a need to delve into how to promote the principles of person-centred care (PCC). Conceptualised as an ethical framework that directs healthcare professionals in their daily responsibilities, PCC serves as a core care philosophy necessitating strong leadership and substantial structural and organisational adjustments [ 2 ]. As such, the implementation of PCC has been described as complex and challenging [ 3 , 4 ], requiring collective efforts and partnerships between health and social care stakeholders [ 5 ]. Health and social care leaders have been described as key stakeholders in the implementation of PCC [ 6 , 7 , 8 ], but there is little guidance on how to educate leaders to take on the role of leading towards PCC.
According to Swedish law [ 9 ], the design and execution of health and social care interventions should be person-centred in terms of being determined in partnership with the person in need of care as far as possible. Nevertheless, there are challenges in determining how person-centred ethics can be seamlessly incorporated into routine care practices [ 10 ]. Even in countries known to practice PCC, like the United Kingdom and Sweden, there seem to be barriers for the implementation of PCC. For instance, Moore et al. [ 10 ] describe adverse consequences of organisational norms and role expectations, recommending the need for robust leadership and adaptive strategies to support the implementation process. It is therefore argued that a person-centred approach should permeate leaders’ and, managers’ actions and their way of being when leading towards PCC to achieve the change in organisational culture required for implementation of PCC [ 7 , 11 , 12 ]. Hereafter, this leadership approach is referred to as person-centred leadership, described previously as an intricate, relational, and dynamic context-based approach to leadership, aspiring to empower both co-workers and leaders [ 13 ]. Person-centred leadership is portrayed as translating the ethics of PCC into everyday leadership practice, promoting a person-centred atmosphere by establishing trust and responsibility, encouraging innovation, and potentiating cultural bearers among co-workers [ 12 ]. Moreover, person-centred leadership includes making use of relational competencies to facilitate a workplace culture based on partnerships in decision-making and collaboration between leaders, co-workers, and persons in need of care [ 8 ]. Establishing prerequisites to enable PCC is also raised as an essential element of person-centred leadership [ 12 , 14 ]. Still, health and social care leaders have limited resources when it comes to leading in a person-centred way, and past research has recommended the development of educational curricula that emphasise person-centred leadership [ 6 , 7 , 10 ]. Previous educational programmes on PCC have mainly targeted health and social care professionals [ 4 ], and little is known on how educational curricula should be developed to promote a person-centred culture throughout health and social care organisations.
The University of Gothenburg Centre for Person-centred Care (GPCC) has developed three routines to facilitate the translation of person-centred ethics to healthcare practice: (1) Initiating a partnership—patient narrative, (2) working in partnership—shared decision-making, (3) safeguarding the partnership—documenting the narrative [ 15 ]. These routines have been evaluated in healthcare research, indicating effectiveness on individual as well as organisational levels [ 7 ]. What is not yet known is how these routines can be applied on leadership to facilitate the transition towards PCC in different health and social care contexts. In 2015, the Swedish Association of Health Professionals (SAHP, a trade union for registered nurses, midwives, radiographers, and biomedical scientists) and GPCC therefore decided to initiate the development of an educational programme on person-centred leadership, targeting health and social care leaders who are members of the SAHP. The programme has later been revised to adhere to societal changes and the needs and preferences of health and social care leaders. As one part of the scientific exploration of this programme (assessments of effects and significance will be reported in separate studies), the aim with the study was thus to explore programme management members’ experiences from the development and realisation of an educational programme on person-centred leadership. More specifically, we sought answers to the following research question: what preparations and actions were involved in developing and realising an educational programme to support leadership directed towards PCC?
With the aim to explore programme management members’ experiences, this study had a social constructivist design, applying focus group methodology [ 16 , 17 ]. This means that the study was built on a view that knowledge is co-created in interaction between participants who share their views and experiences in focus group discussions. More specifically, this meant that the participants in the study were encouraged to stimulate each other in discussions, to explore their shared experiences from developing and realising the educational programme. This approach to focus group research is suitable to uncover knowledge that is concealed but understood by participants [ 18 ] (e.g., tacit knowledge on pedagogical approach and leadership skills in programme development and realisation). Moreover, as described in the literature [ 16 , 17 ], shared experiences are a powerful tool for expressing both positive and negative aspects of what is being studied, which is why focus groups were considered an appropriate method for the study, rather than individual interviews. The Swedish Ethical Review Authority approved the study (dnr. 2022-04052-01) and the Consolidated criteria for reporting qualitative research (COREQ) [ 19 ] were utilised when writing this report.
The curriculum for the educational programme under exploration has been developed and revised in collaboration between researchers from GPCC and educators from SAHP, forming the programme management, to support the realisation of person-centred ethics in leadership across different health and social care organisations in Sweden. Admitting 40 health and social care leaders per year, the programme was initially provided between 2015 and 2019. After being put on hold between 2020 and 2021 due to the COVID-19 pandemic, the programme was revised to a more digital format in 2022, admitting 80 leaders per year. To be admitted to the programme, leaders had to be responsible for units or care activities targeting people in need of health or social care. Most leaders in the programme have been middle managers, but there have also been leaders with other leading positions within the Swedish health and social care system.
Incorporating blended learning, the programme illuminates person-centred ethics and leadership from various perspectives over a six-month period to support leaders in achieving the following learning outcomes:
Knowledge and understanding
Summarise key foundational principles relevant to person-centred care and person-centred leadership.
Explain what characterises a person-centred leadership and employee perspective within the own organisation, supported by course literature and proven experience.
Competence and skills
Discover and define opportunities and areas of development regarding how person-centred ethics is expressed in current practice.
Create a proposal for an action plan for a change process towards person-centred care or person-centred leadership.
Apply person-centred principles during the implementation of a change process.
Judgement and approach
Critically discuss how organisation, culture/structure within different contexts influence the conditions for person-centred care.
From a leadership perspective, assess the implementation, results of the change process, as well as the need for further actions.
Discuss central assumptions within person-centred ethics in relation to sustainable development.
The programme corresponds to 7.5 higher education credits, divided into five digital modules (module 1, 3–6) and one physical module (module 2). Each module focuses on different aspects of person-centred leadership and person-centred ethics as follows: (1) Foundations for PCC and person-centred leadership, (2) communication and narration, you in relation to others, (3) person-centred implementation strategies, (4) to be and to lead in a person-centred way towards PCC, (5) ethical dilemmas and jurisdiction of importance for PCC, (6) leading future care—presentation of developmental work. Practical home-assignments to practice work in partnership were performed between the learning modules in both the original and revised programme. For an overview of the educational curriculum, please see Fig. 1 .

Overview of the educational curriculum
Participants
The participants were researchers with experience from studying PCC ( n = 4) or leadership ( n = 1) and educators from the SAHP ( n = 7), all with experience of either developing and/or realising the programme. They were 11 females and one male, and they had been involved in different stages of the programme development and realisation between year 2015–2022. A total of four focus groups with three to four participants per group were conducted digitally with the participants taking part from their homes or offices during working hours. In line with the focus group methodology [ 16 , 17 ], both homogeneity and heterogeneity were considered when selecting participants and putting together the groups. Homogeneity concerns having similar experiences and is important to generate discussion. In this study, homogeneity within each group was ensured by inviting persons with shared experiences of programme development phase and assignment. Heterogeneity concerns diversity within the target group and in this study, diversity was ensured by mixing participants with different work experience and roles in the development and realisation of the programme. Due to their roles in the development and realisation of the programme, two of the participants have been involved as co-authors (CK and EB), providing an insider perspective of the programme teaching methods and curriculum content that could not have been captured without their involvement. To ensure credibility of the findings they have not been involved in the primary analysis. Four persons in the programme management participated in two focus group discussions, with the aim to capture experiences from both the original development and realisation of the programme, and from the revision of the programme to a digital format. See Table 1 for details on participant roles. The names of participants referred to in this context are fictious to safeguard personal integrity and adhere to Swedish data protection regulations.
Potential participants were invited via email, with a participant information statement and consent form attached. The statement comprised information on the aim of the study and what participation would require of participants should they choose to participate. All persons except one (who did not reply) consented to participate and were scheduled in for a digital focus group discussion using their preferred software (Microsoft Teams or Zoom). All focus groups were moderated by the second author and observed by a research amanuensis (group 1) or the first author (group 2–4) who took notes on the interaction between participants as well as each person’s engagement in the discussions.
Each focus group started with a confirmation of consent and a reminder to send the informed consent form to the researchers, followed by a short presentation of the participants and the researchers, including the participants’ current work role and their role in the development of the programme. Then, the moderator (second author) initiated the discussion by posing key questions developed by the research team involved in this study (see Supplementary file 1 ), starting with a question on why a leadership programme with focus on person-centred care was developed. Follow-up questions were posed to deepen the understanding of the participants’ experiences from the development and realisation of the programme. An important role for the moderator and the observer was to ensure that all participants were given an opportunity to speak, and to identify common elements in the discussions. The focus groups were audio recorded and transcribed verbatim by a professional transcription firm. Video was only used to stimulate interaction between participants and was not used in the analysis. Interaction was further facilitated by the moderator who encouraged participants to discuss their experiences with each other. The focus groups lasted between 57 and 100 min and were performed during 2022 and 2023.
Data analysis
Krueger and Casey’s [ 20 ] systematic method for data analysis was used to analyse the audio recordings and transcriptions iteratively. This meant that the first author started the analysis procedure by listening to all focus group recordings and reading the transcripts and field notes carefully, making notes on content in relation to the study aim, to identify preliminary themes that were discussed with the other authors. Then, the first author started coding the transcribed data by sorting it according to the study aim and coding each response. To describe the content of the focus groups, the first author then prepared a summary statement that was discussed with all authors. The next step involved a formulation of themes. The summary statement was compared with the transcribed data and the field notes to identify internal consistency and the participants’ expressed experiences of importance of each question in terms of frequency, extensiveness, intensity, and specificity. This step resulted in revised themes and sub-themes that were discussed with all authors to reach a final interpretation of the meaning of the focus group discussions. The analysis was conducted in Swedish until the final formulation of themes and sub-themes was reached. The results were then translated to English.
The participants’ experiences from the development and realisation of the educational programme are described in the overarching theme “A collaborative endeavour to integrate leadership and person-centred ethics”, visualising a person-centred approach as essential in both preparations and actions involved in the development and realisation of the programme. These experiences are further described in four themes: (1) Taking the lead in a larger movement, (2) Practicing what you preach, (3) Using narrative, partnership, and documentation as pedagogical tools , and (4) Creating preconditions for continuous development , as visualised in Fig. 2 and described in detail below.

Overview of the thematical structure
A collaborative endeavour to integrate leadership and person-centred ethics
The overarching theme visualises the development and realisation of the educational programme as a dynamic and continuous process marked by collective efforts and an atmosphere of free exchange of ideas to allow for refinements of programme structure and content. Drawing from their experiences from academia, healthcare practice and leadership, the participants described how they developed the programme in partnership, to integrate knowledge on leadership and person-centred ethics. Used as both goals and means, person-centred ethics were thus experienced to have permeated the whole programme from development to realisation, with focus on joint learning among programme management as well as participating leaders. Dialogues between programme management and participating leaders were described as a pivotal element, allowing for joint discussions and reflections as a contrast to one-way communication through lectures. This was further described to foster partnerships between programme management and the participating leaders, which meant that the unique competencies and experiences of each person were harnessed to generate synergy effects during programme development, realisation and beyond.
Taking the lead in a larger movement
With the goal to contribute to a deeper understanding of both what person-centred ethics entail, and how they can be integrated into leadership to change everyday care practice, the development of the programme was experienced to lead the way in the larger movement towards PCC in Sweden. The participants strove to be at the forefront of the larger movement, for example by admitting more leaders from different parts of the country in the revised, more digital version of the programme. Although there were some technical issues with the digital format, the benefits of reaching out geographically were experienced as facilitators for making it a joint course to lead Swedish health and social care organisations towards becoming more person-centred. Continuous reflection and openness to different persons’ perspectives during programme development and realisation were experienced as a support for both the participants’ own, as well as the leaders’, learning on how to facilitate the transition towards PCC. The participants also described how they came to realise that theoretical knowledge was not enough, practical knowledge on how to lead in a person-centred way was also needed. Practical exercises on who the leaders are and how they view themselves in relation to others were therefore created to deepen the participating leaders’ understanding of the significance of mutual respect and clarity around roles. These exercises were developed to assist leaders in pioneering person-centred practices for a broad spectrum of health and social care professionals, as envisioned in the quote from focus group one:
Moderator: Well, the first question must be: Why a leadership education in person-centredness? Maria:…the initial standpoint is that the Swedish Association of Health Professionals wants to take a leading role in the development of person-centred care… We want to contribute to the development of managers and leaders in healthcare, enabling them to work with person-centred approaches and also with more person-centred leadership… By investing in managers, a tremendous number of people will have the opportunity to benefit from this and will also be involved in the development and transformation towards more person-centred care. Emma:…I think that we have seen that managers need support in this. There is a great interest in person-centred care, but how should it be implemented? How can one feel confident in person-centred care? What steps should be taken to make it a reality in the organisations? So, I believe it was a natural step to start with our managers and leaders. Selma: What I find so exciting when looking at this question from sort of another perspective is that, from a research standpoint, we know that leadership is crucial for achieving implementation and sustainability in person-centred care. So, it feels, yes, so exciting and important that you have had and continue to have this education.
Practicing what you preach
During programme realisation, the participants described how they became aware of the need to practice what they preached in terms of having a person-centred approach towards leaders participating in the programme. Both knowing what PCC is, and being able to practice it through the programme’s pedagogical approach were described as important to facilitate each person’s learning process, illustrated by a quotation from focus group four:
Lena: The actual pedagogy in the education was also about viewing them as persons. As you said Rakel,” What resources do you have?”. To also be very genuine in the person-ce(ntred) approach, it was not just knowledge that needed to be conveyed, it was also an… truly an attitude that needed to be embraced by those leading the programme. Frida: Exactly. And I think that’s very… the pedagogical aspect of embodying— of also conveying…not necessarily living as one preaches, but also, what is a person-centred approach when you apply it to your own… where you stand? I believe… that was probably the challenging aspect to convey later in this bridge because it wasn’t explicitly stated that this would also be the task for those who became the carriers of culture from GPCC afterward.
Practicing what you preach was further described in relation to the experienced need for transparency from programme management regarding programme organisation and pedagogical approach, i.e., putting words on what was done and how and why they were done, to clarify the thoughts and reasons behind it. Still, there were challenges described with the pedagogical approach, in striving to see each leader as a person and support their learning by listening to their narratives and acknowledging their individual resources and needs. As such, flexibility was a virtue emphasised as essential during programme realisation, allowing for different pedagogical methods, for plans to shift, goals to evolve, and perspectives to change.
Using narrative, partnership, and documentation as pedagogical tools
This theme describes how the core concepts of GPCC’s model for PCC [ 15 ], i.e., narrative, partnership , and documentation , became key pedagogical tools to bridge experienced challenges with conveying how person-centred ethics could be applied in day-to-day leadership. The participants described how using narratives seemed to have sparked the leaders’ awareness of how they lead, which role they have within their organisation, and who they are in relation to other people. Partnership was experienced as a tool for leaders to understand how their relationships with co-workers had evolved by listening to, and acknowledging, them as persons. Practical exercises and examples were experienced to support the leaders in how narratives can be used to build partnerships with co-workers and come to shared decisions that are jointly documented. Documentation was also experienced as a pedagogical tool for joint reflection and learning among the participating leaders, through presenting and discussing their documented plans for how to integrate person-centred ethics with their leadership within their organisations. We have chosen a quotation from focus group two to visualise how GPCC’s model was used as a pedagogical tool during the programme.
Selma: We have discussed partnerships based on the model you (the moderator) just described. We have provided practical examples throughout the entire education on how to work in partnership and how to use narratives and dialogue. How we arrive at shared decision-making, documentation. This is something we have integrated into the entire education that is present during each session. And to emphasise that it should be a mutuality in this partnership, where we see each other as persons with unique resources and abilities. But where we, at the same time, know that humans have a vulnerability, and that’s what allows us to open up to each other in a partnership. Maria: That’s one part, I think. But it’s also… just as you said (moderator’s name), it is… being a manager also means having certain expectations placed on oneself that you should… So it’s… What should I say? This respect we have, the mutual respect we have for each other, understanding each other’s roles as well, I believe, is an important part of this person-centred leadership and partnership, making it clear to everyone what roles we have. And then it’s this with the competence and the person one is, with one’s entire life history in some way, that can be valuable to the organisation one is part of. And so I agree with what you are saying, Selma. But we also incorporate… Because Lisa has also talked about the fact that one needs… One needs a role where one needs to take on a different responsibility than what the employees may need as a manager.
Creating preconditions for continuous development
Supporting the collaborative endeavour towards PCC, the participants experienced a need for creating preconditions for continuous development of person-centred leadership after the programme has ended. One way of doing this was to cultivate a sense of shared purpose to leave an indelible mark on the leaders when it comes to person-centred ethics and becoming person-centred in their leadership. Even so, there was an experienced risk of leaders going back to working as before, without sustainable changes. To support leaders to go from knowledge to action, practical homework exercises were developed to provide the leaders with tools on a day-to-day basis, as described in previous themes. Fulfilling the expectation of participating leaders to take a leading role in the movement towards PCC, national networking also became part of the programme, to contribute to a sense of community and opportunities for continuous reflection and development. National networks of leaders and managers who have completed the programme were developed to provide an arena for sharing positive examples and mutual learning on how leadership and person-centred ethics can be integrated. Nevertheless, as many health and social care leaders have other professions than SAHP’s members, the participants expressed a need for continuous development of the programme to include all healthcare professions in the programme, to allow for a sharing of knowledge across professional boundaries. The importance of networks is described in the quotation from focus group three below.
Tanja: The third (purpose of the programme) was to form networks within it. I mean, to create networks within this group and for ongoing work. So, you, you got, well, let’s put it this way, a national network of colleagues… whom I know continue to exchange thoughts and ideas with each other. I’ve met several of these participants now in… over the years and in my new assignments. And since there are 200 people, I can’t remember exactly which course. But they remember precisely. And they talk about how they have continued. And also that it has inspired them to take leadership positions in the transition towards integrated care. Eva-Britt: Yes, I know that… think that last part is really important to emphasise as well, that you form networks and that it’s not just… just as you said, that it is the components, Tanja. You gain knowledge and abilities to find your motivations and so on. And it’s also part of that… that “I set the ball in motion both at home and the contacts I have across the country and so on.” It’s truly an active education that aims to bring about change in healthcare towards person-centredness.
This study aimed to explore programme management members’ experiences from the development and realisation of an educational programme on person-centred leadership. Our main finding is the illustration of how person-centred ethics permeated the whole programme, from development to realisation. The participants highlighted the importance of dialogues and continuous reflection during programme development, to allow for innovative collaboration, mutual support, and a commitment to support leaders to go from knowledge to action. Overcoming challenges with communicating person-centred ethics, the participants further provided examples of how to apply GPCC’s routines for PCC (narratives, partnership and documentation) [ 15 ] as pedagogical tools to support a person-centred leadership. This knowledge can be used to develop educational curricula to support health and social care leaders in leading towards PCC.
To the best of our knowledge, there is a limited presence of educational curricula that genuinely embrace a person-centred pedagogical approach or are designed to educate health and social care leaders with a person-centred focus [ 21 ]. Previous research has highlighted that educational curricula should be characterised by innovation, not only in their preparation of practitioners but also in their proactive development of healthcare practice environments and cultures that promote PCC [ 22 ]. Björkman et al. [ 23 ] further describe the implementation of PCC in Swedish higher education of healthcare professionals as an ongoing and fragmented process, primarily led by persons with particular interests. Highlighting uncertainty and ambiguity concerning the significance and worth of PCC, as well as the methods for effective implementation, they suggest further research on the fundamental essence of PCC as an educational subject, alongside the development of suitable didactic strategies aimed at guiding students to become proficient in person-centred practice [ 23 ]. Our findings answer to this call for research and are especially relevant in the light of the paucity of research concerning the practical implementation of values within health and social care organisations, particularly in the realm of person-centred leadership [ 8 ]. A novel discovery from our study involves outlining factors to consider when creating person-centred curricula for leaders in health and social care. We recommend adopting a person-centred pedagogical approach, which utilises narratives, partnership, and documentation as tools for leaders to employ a person-centred approach in their leadership roles. Visualising person-centred ethics both as goals and means, the participants described the employment of a person-centred approach as an iterative learning process, facilitated by partnerships between programme management, participating leaders, and co-workers within the leaders’ organisations. This finding is supported by a recent international education initiative [ 24 ], illustrating the need for person-centred curricula to be both philosophically and methodologically aligned with person-centred principles. In agreement with this initiative, our findings suggest that person-centred curricula are needed to capture the intricacies of implementing PCC in contemporary healthcare organisations.
The interconnectedness between PCC and person-centred leadership has been described in previous literature [ 8 ], and implementation of PCC can be seen as a strategic healthcare system change. Such system changes can be very difficult [ 25 ] with contextual aspects shaping the change process in complex ways [ 26 , 27 ]. For instance, change may be affected by the complex integration of local cultures, professional attitudes, communication patterns and leadership styles [ 28 ]. The studied programme was specifically developed to take the lead in the ongoing transition towards PCC in Sweden. Practical homework assignments were combined with theoretical lectures, literature studies and reflective practice to support the leaders’ learning process and provide them with everyday tools for person-centred leadership. The homework assignments were developed to allow leaders to experience person-centredness in their leadership roles. Put in relation to the existing literature on learning [ 29 ], this could be understood as second-degree learning, denoting a profound form of learning where collective values undergo transformation to the extent that they impact the person’s actual work. As described by Binns [ 30 ], leadership at the level of everyday practice is fundamentally relational and somewhat removed from management and hierarchical position [ 30 ]. Viewed from this perspective, second-degree learning is a crucial requirement for successful integration of person-centred ethics and leadership. Allowing leaders to experience person-centredness in actual encounters with co-workers, the homework exercises in the studied programme provided a sense of authenticity. However, planning suitable and effective homework exercises poses a significant challenge for educators, especially when the goal is to enhance preparedness for collectively addressing the complexity of implementing PCC. Consequently, the development of person-centred curricula for health and social care leaders has a key-role in bridging challenges to implementation and supporting the realisation of PCC in everyday practice.
The power of healthcare systems has been demonstrated in shaping the implementation of new working methods [ 25 ], such as PCC. For example, health and social care professionals might exhibit resistance to reforms that are seen as altering established work routines. This mirrors the influence of professionals and the strategies employed by them. As reflected in our findings, educational programmes on person-centred ethics could provide guidance for leaders to enact essential changes among co-workers within their organisations. McCormack et al. [ 31 ] further describe the need for ongoing support of a learning culture within healthcare systems to facilitate PCC [ 31 ]. The continuous development described in our findings could be seen as such a support, incorporating learning environments for healthcare leaders to cultivate collaborative practices. Person-centred leadership can be regarded a collaborative practice, rooted in caring for co-workers. The modules in the educational programme under exploration were developed to support leaders in their learning process, to recognise and acknowledge the resources of both them and co-workers. In combination with the fostering of national networks of peers, the educational programme was described as a safe environment for reflection on person-centred ethics in the hierarchical environments which healthcare leaders often find themselves in. This was believed to enable leaders to act in a person-centred way and maintain authenticity in their leadership roles, but the nature of this study does not allow for such conclusions. We therefore suggest and plan for further evaluations of the programme’s impact on participating leaders.
Finally, regarding the practical implications of our findings, we suggest continuous development and refinements of educational curricula to fully embrace person-centred ethics as both the goals and means. Notably, dialogues played a crucial role in promoting such development. Inclusive discussions and joint reflection are needed for both programme development and realisation, to support second-degree learning on how to integrate person-centred ethics and leadership on a day-to-day basis. The person-centred approach to both pedagogics and leadership in the educational programme fostered a strong partnership between programme management and leaders and was described as a foundation for partnerships between leaders and co-workers. In management research, there are indications that organisations are characterised by workplace partnerships that may impact practice. For instance, Ferris et al. [ 32 ] delineate leaders as persons with proficiency in effectively comprehending co-workers and leveraging this understanding to motivate them to align with organisational goals. This is corroborated by our findings, which illustrate the need for educational curricula to recognise the practicalities of how change is put to action, namely, through collaborative efforts in everyday practice.
Methodological considerations
Social constructivist focus groups are particularly useful for generating rich, context-specific data. They allow participants to interact with one another, building upon each other’s responses and providing nuanced insights that might be missed in individual interviews or surveys [ 17 ]. This epistemological foundation for the study was thoroughly considered in relation to the involvement of two of the participants as co-authors of this manuscript (CK and EB). The risk of biased interpretations of the findings was carefully reflected upon in relation to the benefits of utilising their insider perspective from being part of the programme development and realisation for both describing the programme and for translating the findings to practical implications. Furthermore, as Krueger and Casey’s [ 20 ] method is built upon understanding collective understanding, rather than focusing on individual participants’ voices, the risk of biased interpretation was minimised.
To avoid the development of excessive uniformity within the groups, we took great care in composing the groups, aiming to strike a balance between heterogeneity and homogeneity [ 20 ]. What bound the participants in our study together (homogeneity) was their mutual involvement in the programme development. Considering group dynamics, we also attuned to power imbalances and divided participants according to their roles in programme development. Past research has demonstrated that when people with shared experiences come together, they can engage in discussions with a sense of companionship, knowing that others can relate to their experiences, thus promoting a spirit of sharing [ 16 ]. There is, however, always a risk in focus group studies, that one or a few participants dominate the discussion, while others remain silent. This may skew the results and prevent a full exploration of diverse viewpoints. During the focus groups we therefore attuned to dominant voices and encouraged everyone to interact to positively impact the authenticity of the responses.
Since our focus groups were conducted digitally to enable participants from all over Sweden to participate, we were careful to compose the focus groups with people who already knew each other to establish rapport and trust among participants. In contrast to limitations highlighted in the literature [ 33 ] we encountered no technical problems during conduct, and the digital context was not experienced as a disruption in the participants’ sharing of experiences. Nevertheless, digital platforms may limit the ability to observe non-verbal cues such as body language, which can provide valuable context and depth to responses in traditional face-to-face focus groups [ 34 ]. What we observed in our study was a limited fluidity of conversation, possibly due to the digital context. The moderator strove to create a comfortable and non-judgemental atmosphere to encourage interaction, but due to the digital platform, participants were not able to engage in spontaneous exchanges and took turns rather than sharing their experiences freely.
It is important to note that each focus group in our study had a limited number of participants.
However, methodological literature [ 17 , 20 ] indicates that small groups of three to six participants are typically very dynamic, and that the quality of discussions are more influenced by participant engagement that the sheer number of participants. Despite issues with spontaneous exchanges described above, the participants in our study seemed to value the opportunity to participate in focus groups, leading to rich discussions where they openly shared their perspectives. It is also important to remember that findings from focus groups are context-specific, and even if we involved all eligible persons but one, the participants may not represent the diversity of perspectives within a larger population. With the aim to understand the participants’ shared experiences and provide insight into their articulation of knowledge [ 17 ], our findings thus provide insight into the perspectives of the specific participants involved.
In the context of implementing PCC through leadership, our findings advocate for an integration of person-centred ethics and leadership through a person-centred approach throughout programme development and realisation. The person-centred approach nurtured strong partnerships between programme management and leaders, forming the basis for leaders to build partnerships with co-workers within their organisations. Our findings further support continuous development and refinement of educational curricula, with meaningful dialogues being described as essential for both programme enhancement and the daily realisation of person-centred leadership. By recognising and harnessing the distinct competencies and experiences of each person involved, the development and realisation of the programme were experienced to yield synergistic outcomes, both during and after its completion. In essence, the person-centred approach aimed to create a dynamic and supportive environment for both programme management and participating leaders, to successfully integrate person-centred ethics with leadership in an educational curriculum for person-centred leadership.
Data availability
The data generated and analysed during this study are not publicly available due to the information provided to the involved persons when obtaining their informed consent, stating that all attempts would be made to maintain their confidentiality. De-identified data are available are available from the corresponding author upon reasonable request to enable review and will be stored for 10 years from publication at the University of Gothenburg, Sweden. All data are covered by the Swedish Public Access to Information and Secrecy Act and a confidentiality assessment will be performed at each individual request.
Abbreviations
- Person-centred care
University of Gothenburg Centre for Person-Centred Care
The Swedish Association of Health Professionals
Phelan A, McCormack B, Dewing J, Brown D, Cardiff S, Cook N, et al. Review of developments in person-centred healthcare. Int Pract Dev J. 2020;10:1–29.
Article Google Scholar
Naldemirci Ö, Wolf A, Elam M, Lydahl D, Moore L, Britten N. Deliberate and emergent strategies for implementing person-centred care: a qualitative interview study with researchers, professionals and patients. BMC Health Serv Res. 2017;17:527.
Article PubMed PubMed Central Google Scholar
Gyllensten H, Björkman I, Jakobsson Ung E, Ekman I, Jakobsson S. A national research centre for the evaluation and implementation of person-centred care: content from the first interventional studies. Health Expect. 2020;23(5):1362–75.
Dellenborg L, Wikström E, Andersson Erichsen A. Factors that may promote the learning of person-centred care: an ethnographic study of an implementation programme for healthcare professionals in a medical emergency ward in Sweden. Adv Health Sci Education: Theory Pract. 2019;24(2):353–81.
Article CAS Google Scholar
Fridberg H, Wallin L, Tistad M. Tracking, naming, specifying, and comparing implementation strategies for person-centred care in a real-world setting: a case study with seven embedded units. BMC Health Serv Res. 2022;22:1409.
Backman A, Lövheim H, Lindkvist M, Sjögren K, Edvardsson D. The significance of nursing home managers’ leadership - longitudinal changes, characteristics and qualifications for perceived leadership, person-centredness and climate. J Clin Nurs. 2022;31(9–10):1377–88.
Article PubMed Google Scholar
Britten N, Ekman I, Naldemirci Ö, Javinger M, Hedman H, Wolf A. Learning from Gothenburg model of person centred healthcare. BMJ. 2020;370.
Eide T, Cardiff S. Leadership research: a person-centred agenda. In: McCormack B, van Dulmen S, Eide H, Skovdahl K, Eide T, editors. Person-centred Health care research. Hoboken; NJ.: Wiley; 2017.
Google Scholar
Ministry of Health and Social Affairs (Socialdepartementet). Patient law (2014:821). (Patientlag 2014:821. https://www.riksdagen.se/sv/dokument-och-lagar/dokument/svensk-forfattningssamling/patientlag-2014821_sfs-2014-821/ #K5 Accessed 16 Feb 2024.
Moore L, Britten N, Lydahl D, Naldemirci Ö, Elam M, Wolf A. Barriers and facilitators to the implementation of person-centred care in different healthcare contexts. Scand J Caring Sci. 2017;31(4):662–73.
Ekman I. Practising the ethics of person-centred care balancing ethical conviction and moral obligations. Nurs Philos. 2022;23:e12382.
Backman A, Ahnlund P, Sjögren K, Lövheim H, McGilton K, Edvardsson D. Embodying person-centred being and doing: leading towards person-centred care in nursing homes as narrated by managers. J Clin Nurs. 2019;29(1–2):172–83.
PubMed Google Scholar
Cardiff S, Phil D, McCormack B, McCance T. Person-centred leadership: a relational approach to leadership derived through action research. J Clin Nurs. 2018;27(15–16):3056–69.
Rosengren K, Buttigieg S, Badanta B, Carlstrom E. Diffusion of person-centred care within 27 European countries—interviews with managers, officials, and researchers at the micro, meso, and macro levels. J Health Organ Manag. 2022;37(1):17–34.
Ekman I, Swedberg K, Taft C, Lindseth A, Norberg A, Brink E, et al. Person-centered care–ready for prime time. Eur J Cardiovasc Nurs. 2011;10(4):248–51.
Dahlin-Ivanoff S, Hultberg J. Understanding the multiple realities of everyday life: basic assumptions in focus-group methodology. Scand J Occup Ther. 2006;13(2):125–32.
Kitzinger J. The methodology of focus groups: the importance of interaction between research participants. Sociol Health Illn. 1994;16:103–21.
Ryan KE, Gandha T, Culbertson J, Carlson C. Focus group evidence: implications for design and analysis. Am J Evaluation. 2014;35(3):328–45.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Krueger R, Casey M. Focus groups: a practical guide for applied research. 5th ed. Thousand Oaks: Sage Publications Inc.; 2015.
Fields L, Trostian B, Moroney T, Dean B. Active learning pedagogy transformation: a whole-of-school approach to person-centred teaching and nursing graduates. Nurse Educ Pract. 2021;53:103051.
Dickson C, van Lieshout F, Kmetec S, McCormack B, Skovdahl K, Phelan A, et al. Developing philosophical and pedagogical principles for a pan-european person-centred curriculum framework. Int Pract Dev J. 2020;10:1–20.
Björkman I, Feldthusen C, Forsgren E, Jonnergård A, Lindström Kjellberg I, Wallengren Gustafsson C, et al. Person-centred care on the move - an interview study with programme directors in Swedish higher education. BMC Med Educ. 2022;22:589.
O’Donnell D, Dickson C, Phelan A, Brown D, Byrne G, Cardiff S, et al. A mixed methods approach to the development of a person-centred curriculum framework: surfacing person-centred principles and practices. Int Pract Dev J. 2022;12:1–14.
Best A, Greenhalgh T, Lewis SJ, Saul J, Carroll S, Bitz J. Large-system transformation in health care: a realist review. Milbank Q. 2012;90(3):421–56.
Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
Turner S, Ramsay A, Perry C, Boaden R, McKevitt C, Morris S, et al. Lessons for major system change: centralization of stroke services in two metropolitan areas of England. J Health Serv Res Policy. 2016;21(3):156–65.
Braithwaite J, Churruca K, Long J, Ellis L, Herkes J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med. 2018;16(1):63.
Stein J. How institutions learn:a socio-cognitive perspective. J Econ Issues. 1997;31(3):729–40.
Binns J. The ethics of relational leading: gender matters. Gend Work Organ. 2008;15:600–20.
McCormack B, van Dulmen S, Eide H, Skovdahl K, Eide T. In: McCormack S, van Dulmen S, Eide H, Skovdahl K, Eide T, editors. Person-centredness in healthcare policy, practice and research. Person-centred healthcare research: John Wiley & Sons Ltd.; 2017.
Chapter Google Scholar
Ferris G, Treadway D, Perrewé P, Brouer R, Douglas C, Lux S. Political skill in organizations. J Manag. 2007;33(3):290–320.
Stewart D, Shamdasani P. Online focus groups. J Advertising. 2017;46(1):48–60.
Gamhewage G, Mahmoud M, Tokar A, Attias M, Mylonas C, Canna S, et al. Digital transformation of face-to-face focus group methodology: engaging a globally dispersed audience to manage institutional change at the World Health Organization. J Med Internet Res. 2022;24(5):e28911.
Download references
Acknowledgements
First and foremost, we would like to thank the persons who agreed to participate in the focus group discussions. We would also like to thank the research amanuensis Noah Löfqvist for acting as the observer of focus group one, and the Swedish Association of Health Professionals and the University of Gothenburg Centre for Person-Centred Care for developing and organising the programme.
The study was financed through funding from the University of Gothenburg Centre for Person-Centred Care (Dnr. GU 2021/995). The funding body had no role in study design, data collection, analysis, or interpretation of results, or in writing the manuscript. Open access funding provided by University of Gothenburg.
Author information
Authors and affiliations.
Department of Health and Rehabilitation, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Box 455, SE-40530, Gothenburg, Sweden
Qarin Lood & Emmelie Barenfeld
Centre for Person‑Centred Care (GPCC), University of Gothenburg, Gothenburg, Sweden
Qarin Lood, Eric Carlström, Charlotte Klinga & Emmelie Barenfeld
Centre for Ageing and Health—AgeCap, University of Gothenburg, Gothenburg, Sweden
School of Nursing and Midwifery, La Trobe University, Melbourne, Australia
Institute of Health and Care Sciences, Sahlgrenska Academy, University of Gothenburg, Box 457, SE-40530, Gothenburg, Sweden
Eric Carlström
School of Business, Campus Vestfold, University of South-Eastern Norway, Kongsberg, Norway
Department of Learning, Informatics, Management and Ethics, Medical Management Centre, Karolinska Institutet, Stockholm, Sweden
Charlotte Klinga
Research and Development Unit for Older Persons (FOU nu), Stockholm Health Care Services, Stockholm, Sweden
You can also search for this author in PubMed Google Scholar
Contributions
EC and EB were responsible for the conception and design of the study. EC and QL were responsible for the data collection. QL was responsible for data analysis and interpretation, and all authors were involved in the final interpretations and formulation of themes. QL and EB drafted the manuscript, which was revised critically by EC and CK. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Qarin Lood .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (dnr. 2022-04052-01). All methods were carried out in accordance with relevant guidelines and regulations. Informed written consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lood, Q., Carlström, E., Klinga, C. et al. A collaborative endeavour to integrate leadership and person-centred ethics: a focus group study on experiences from developing and realising an educational programme to support the transition towards person-centred care. BMC Health Serv Res 24 , 395 (2024). https://doi.org/10.1186/s12913-024-10793-8
Download citation
Received : 16 November 2023
Accepted : 27 February 2024
Published : 29 March 2024
DOI : https://doi.org/10.1186/s12913-024-10793-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Person-centredness
- Person-centred ethics
- Social care
- Curriculum development
- Implementation
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction. Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted).University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity ...
Others argue that local development of research ethics is crucial to ensuring buy-in and avoiding bioethical imperialism [11,12]. Acknowledging the past abuses that took place under the guise of research, Western ethical thought now insists on voluntary consent, with special protections for traditionally vulnerable populations such as children ...
Research Ethics is aimed at all readers and authors interested in ethical issues in the conduct of research, the regulation of research, the procedures and process of ethical review as well as broader ethical issues related to research such as scientific … | View full journal description. This journal is a member of the Committee on ...
Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For ...
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across a range of disciplines that can include ethicists, social workers, physicians, nurses, other scientific researchers, counselors and mental health professionals, and advocates ...
Morality, ethics and ethical practice. Ethics, or moral philosophy, is a branch of philosophy that seeks to address questions of morality. Morality refers to beliefs or standards about concepts like good and bad, right and wrong (Jennings Citation 2003).When used as the basis for guiding individual and collective behaviour, ethics takes on a normative function, helping individuals consider how ...
To discuss principles of research ethics in Social Sciences: Case study—ethnography: The study shows the urgent need to expand the horizons of the debate around research ethics beyond biomedical fundaments of this field: Fuentes MP. Spanish. 2017: III: To analyze the ethical dilemmas during anthropologic investigation: Case study of ethnography
The areas of Research Ethics (RE) and Research Integrity (RI) are rapidly evolving. Cases of research misconduct, other transgressions related to RE and RI, and forms of ethically questionable behaviors have been frequently published. The objective of this scoping review was to collect RE and RI cases, analyze their main characteristics, and discuss how these cases are represented in the ...
Research ethics are moral principles that need to be adhered to when conducting a research study as well as when writing a scientific article, with the prime aim of avoiding deception or intent to harm study's participants, the scientific community, and society. Practicing and adhering to research ethics is essential for personal integrity as ...
4.5 Research ethics and patient approvals. It is the policy of Research Ethics to require a declaration about research ethics approval enabling a statement to be carried within the paginated pages of all published articles. To ensure anonymous peer-review, please include these details on the title page of your submission.
Research is a duty for health professionals and in the best interest of patients in times of a pandemic: Empirical exploration and ethical implications of the Research Ethics in Times of Pandemic ...
Abstract. Ethics, as an integral component of human decision-making, undeniably shape the landscape of scientific research. This article delves deeply into the nuanced realm of ethical ...
According to Resnik (2011), many people think of ethics as a set of rules distinguishing right from wrong, but actually the term "ethics" refers to norms of conduct or of action and in disciplines of study. Research ethics or norms promote the "knowledge, truth, and avoidance of error" (p. 1) and protect against "fabricating ...
Revised on June 22, 2023. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective ...
Articles on Research ethics. Displaying 1 - 20 of 83 articles. People in the Living Museum of the Ju/'hoansi San, Grashoek, Namibia. Oleksandr Rupeta/NurPhoto via Getty Images February 12, ...
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include: Protections of human and animal subjects. However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research ...
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own ...
Abstract. Published articles in scientific journals are a key method for knowledge-sharing. Researchers can face the pressures to publish and this can sometimes lead to a breach of ethical values, whether consciously or unconsciously. The prevention of such practices is achieved by the application of strict ethical guidelines applicable to ...
This article critiques research ethics committees (RECs) for stifling social sciences research through rigid, one-size-fits-all ethics protocols. It highlights how these protocols, rooted in medical science perspectives, ignore the complexities of fieldwork, and prioritize institutional protection over knowledge advancement.
The impact on research ethics. To explore how the landscape of the contemporary social sciences is changing and impacting upon ethical considerations, let me return here to two dominant issues raised in the previous section: the increase of participatory, egalitarian, co-constructed research approaches and the rise of non-university-affiliated ...
by Avery Forman. So many executives spend decades reaching the pinnacles of their careers only to find themselves unfulfilled at the top. In the book Build the Life You Want, Arthur Brooks and Oprah Winfrey offer high achievers a guide to becoming better leaders—of their lives. 10 Jul 2023.
Research and Ethics - clinical trial law and rules, Medical compliance. getty. This new discovery emerged from a massive survey of 11,050 scientific researchers in Sweden, conducted by Amanda M. ...
In the early years of the journal concerns about ethics were largely framed as issues of reputation and trust. Ensuring compliance with an established set of ethics codes was key to the effective establishment of research as a profession (Wilson, 1962).Even at this stage—long before formal data protection legislation—it was recognized that it was not only research clients and managerial ...
Large language models are increasingly applied in real-world scenarios, including research and education. These models, however, come with well-known ethical issues, which may manifest in unexpected ways in human-computer interaction research due to the extensive engagement with human subjects. This paper reports on research practices related to LLM use, drawing on 16 semi-structured ...
The concept of vulnerability is a cornerstone of the theoretical basis and practical application of ethics in human subjects research. Risks to humans participating in research must be minimized; that is, subjects must be offered protection from risks. Vulnerable subjects require additional protections. However, despite the common usage of the ...
The 4 main ethical principles, that is beneficence, nonmaleficence, autonomy, and justice, are defined and explained. Informed consent, truth-telling, and confidentiality spring from the principle of autonomy, and each of them is discussed. In patient care situations, not infrequently, there are conflicts between ethical principles (especially ...
Griffiths has run his psychedelic studies more like a 'new-age' retreat center, for lack of a better term, than a clinical research laboratory," reads an ethics complaint filed to Johns ...
Researchers recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic that were published between March 2020 and July 2023 in top journals in management and ...
Ensuring the transition towards person-centred care is a growing focus in health and social care systems globally. Presented as an ethical framework for health and social care professionals, such a transition requires strong leadership and organisational changes. However, there is limited guidance available on how to assist health and social care leaders in promoting person-centred practices.
Biomedical research ethics, already codified for half a century, had led the field in ethics and had heavily influenced those ethical review processes in place in universities and other institutions that conducted research, including social science research (Sikes and Piper, 2010). Consequently, it was felt that whilst social science had its ...