Advertisement

Covid-19: The story of a pandemic
A timeline of the coronavirus pandemic, from the first cases in China in December 2019 to 300 million vaccine doses delivered (and counting)
10 March 2021
A year ago this week, the World Health Organization (WHO) declared covid-19 a pandemic. Since the first case of infection with this new coronavirus was reported in China in December 2019, SARS-CoV-2, as we now know it to be called, has killed over 2.5 million people and infected at least 116 million. Beginning as an unexplained, pneumonia-like illness, first detected in China’s Wuhan province , it has since spread to almost every country, bringing life across most of the world to a near-standstill for the last year. World leaders became ill, entire countries were locked down to prevent the spread of infection and international travel ceased.
As most governments struggled to contain the virus, scientists were rushing to identify and find treatments that worked against covid-19 . As infections surged worldwide, new, highly transmissible variants of the virus were identified and are circulating ever further.
With many vaccines now approved, over 300 million doses have been administered, and over 65 million people are now fully vaccinated. But this represents less than 1 per cent of the world’s population, and while the vaccine offers a glimmer of hope for a return to normal, there is still a long way to go. As countries, including the UK, are preparing to lift restrictions, we look back at a year that changed the world forever.
January 2020
New scientist reports on mysterious illness.
New Scientist reports for the first time about 59 cases of a mysterious pneumonia-like illness in China, linked to a wet market in Wuhan. The affected individuals became ill between 12 and 29 December 2019.
A novel coronavirus is identified
WHO reports Chinese authorities have identified a completely novel coronavirus as the cause of the illness and sequenced its genome, less than a month since the first person became ill.
The world records its first coronavirus death
China reports that a 61-year-old man has become the first known victim of the novel coronavirus. He was a regular customer at Wuhan’s wet market.

An empty roadway is seen on February 3, 2020 in Wuhan, Hubei province, China.
Getty Images
Lockdowns begin
Wuhan is put under a strict lockdown by the Chinese government. All travel in and out of the city is prohibited.
25 January
The coronavirus makes it to Europe
The first case of coronavirus in Europe is confirmed in France. The UK reports its first case on 31 January.
February 2020
The disease is named.
WHO names the disease caused by the coronavirus “covid-19” or” coronavirus disease 2019”, after the year the first cases were reported.
15 February
First death recorded outside Asia
In France, a Chinese tourist dies from covid-19 in Paris.
20 February
The Middle East begins to bear the brunt
Iran records its first covid-19 deaths and imposes emergency measures in the affected province. These are the first deaths reported in the Middle East.
21 February
Europe’s lockdowns begin
Italy records its first coronavirus death and 50,000 people from 10 towns in the north of the country enter lockdown.
29 February
The US records its first death
The first death in the US is reported. There have been 22 cases detected in the country so far.
UK’s first coronavirus death
The UK records its first death, a woman in her 70s. 115 cases have now been confirmed in the UK.
The start of nationwide lockdowns
Italy becomes the first European country to impose a nationwide lockdown . Sports events are postponed, schools and universities closed and over 60 million people ordered to stay at home.
WHO declares covid-19 a pandemic
Tedros Ghebreyesus, director general of the WHO , says “WHO has been assessing this outbreak around the clock and we are deeply concerned both by the alarming levels of spread and severity. We have therefore made the assessment that covid-19 can be characterised as a pandemic.”
US declares a state of emergency
President Trump declares a national emergency in the US.
A potential vaccine offers hope
Europe closes its borders. The world’s first human trial of a covid-19 vaccine, an mRNA vaccine developed by US biotechnology company Moderna, begins.
The UK enters its first lockdown
Following other European nations , the UK enters a nationwide lockdown. Shortly afterwards, UK prime minister Boris Johnson tests positive for the coronavirus.

Relatives wearing protective gear prepare to bury the body of a man who died from the coronavirus disease, at a graveyard in New Delhi, India.
REUTERS/Danish Siddiqui
One million cases
Global cases reach one million as the US records the most daily deaths from covid-19 of any country so far. New York City is particularly hard-hit , with hospitals in the city at capacity
China begins to return to normal
Lockdown is lifted in Wuhan, China, where the first coronavirus cases were detected.
Europe begins to ease up
After nearly two months, Italy starts to ease its coronavirus restrictions. As infection rates slow, measures begin to relax in other parts of Europe, too.
The situation in the Americas gets worse
In Latin America , and especially in Brazil, cases continue to grow. By the end of the month, daily infections in the region overtake those in both Europe and the US as more than 2 million cases are reported.
US deaths reach 100,000
Covid-19 deaths in the US pass 100,000, making America the country with the highest number of coronavirus deaths recorded so far.
Cases begin to rise again
WHO warns cases are starting to rise again in Europe, as a result of the easing of restrictions in many countries.
Masks become mandatory in England
With WHO acknowledging evidence that the coronavirus can spread indoors via air particles, it becomes mandatory to wear masks in shops in England, bringing it in line with Scotland and other European nations including Italy and Germany.
August 2020
Russia approves sputnik v vaccine.
Russia announces approval of its Sputnik V covid-19 vaccine before it has undergone large-scale human trials, causing concern among international researchers.
September 2020
29 September
Deaths reach one million
The world reaches a tragic milestone: 1 million deaths caused by covid-19.
October 2020
Lockdowns return.
Ireland becomes the first European country to impose a second nationwide lockdown. England follows two weeks later.
November 2020
Vaccine trials prove successful.
Pfizer and BioNTech announce that results from phase III trials show their mRNA vaccine is more than 90 per cent effective at preventing symptomatic covid-19.
16 November
Moderna’s mRNA vaccine is shown to be effective.
23 November
The University of Oxford and AstraZeneca’s viral vector-based vaccine is also said to have done well in trials.
December 2020
2 December

Vaccines get their first approvals
The UK government becomes the first in the world to authorise the Pfizer/BioNTech vaccine .

Margaret Keenan, 90, is applauded by staff as she returns to her ward after becoming the first person in Britain to receive the Pfizer/BioNTech COVID-19 vaccine at University Hospital, Coventry, at the start of the largest ever immunisation programme in British history.
Jacob King/Pool via REUTERS/Alamy
Mass vaccination begins
The UK’s mass-vaccination programme begins as over 50 hospitals in the UK start administering the Pfizer/BioNTech vaccine to people aged over 80.
14 December
New variants
A new variant of the coronavirus, possibly associated with a faster spread, is identified in the county of Kent in the UK.
31 December
Parts of Africa may have to wait years
A WHO report suggests large parts of Africa may not receive covid-19 vaccines for several years.
January 2021
Uk cases surge.
UK hospitals risk being overwhelmed by surging cases , with evidence suggesting this is partly due to the variant first detected in Kent, which spreads faster.
2 million deaths
2 million people are reported to have died from covid-19 since the pandemic began.
UK deaths reach 100,000
The UK joins America, India, Brazil and Mexico in reaching more than 100,000 deaths from covid-19. It is the first European country to do so.
Vaccinations ramp up (unequally)
Over 7 million vaccine doses have been administered in the UK, compared to just 25 doses in the west African state of Guinea .
February 2021
16 February
Worldwide vaccination
More than 216 million people have now received their first dose worldwide.
Staying ahead of the virus
6 people in the UK test positive for the P.1 coronavirus variant first detected in Brazil. Five of those six had either returned or had close contact with people returning from Brazil. One of several variants, along with the B.1.1.7 and B.1.351 that may be more transmissible, vaccine developers are already modifying existing vaccines to stay ahead of the virus.
- coronavirus /
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
More from New Scientist
Explore the latest news, articles and features

More people are living with pain today than before covid emerged
Subscriber-only

What to know about the new covid-19 XEC variant

Evidence points to Wuhan market as source of covid-19 outbreak

Why do covid cases rise in summer, unlike other respiratory viruses?
Popular articles.
Trending New Scientist articles
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
A Narrative Review of COVID-19: The New Pandemic Disease
Kiana shirani md, erfan sheikhbahaei md, zahra torkpour md, mazyar ghadiri nejad phd, bahareh kamyab moghadas phd, matina ghasemi phd, hossein akbari aghdam md, athena ehsani phd, saeed saber-samandari phd, amirsalar khandan phd.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Amirsalar Khandan, PhD; Technology Incubator Center, Isfahan (Khorasgan) Branch, Islamic Azad University, Postal code: 81595158, Isfahan, Iran. Tel: +983135354069, Fax: +983135354069, Email: [email protected]
Revised 2020 May 6; Accepted 2020 May 18; Received 2020 Apr 4.
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 4.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Nearly every 100 years, humans collectively face a pandemic crisis. After the Spanish flu, now the world is in the grip of coronavirus disease 2019 (COVID-19). First detected in 2019 in the Chinese city of Wuhan, COVID-19 causes severe acute respiratory distress syndrome. Despite the initial evidence indicating a zoonotic origin, the contagion is now known to primarily spread from person to person through respiratory droplets. The precautionary measures recommended by the scientific community to halt the fast transmission of the disease failed to prevent this contagious disease from becoming a pandemic for a whole host of reasons. After an incubation period of about two days to two weeks, a spectrum of clinical manifestations can be seen in individuals afflicted by COVID-19: from an asymptomatic condition that can spread the virus in the environment, to a mild/moderate disease with cold/flu-like symptoms, to deteriorated conditions that need hospitalization and intensive care unit management, and then a fatal respiratory distress syndrome that becomes refractory to oxygenation. Several diagnostic modalities have been advocated and evaluated; however, in some cases, diagnosis is made on the clinical picture in order not to lose time. A consensus on what constitutes special treatment for COVID-19 has yet to emerge. Alongside conservative and supportive care, some potential drugs have been recommended and a considerable number of investigations are ongoing in this regard
Keywords: SARS virus, COVID-19, Epidemiology, Pandemics, Coronavirus
What’s Known
Substantial numbers of articles on COVID-19 have been published, yet there is controversy among clinicians and confusion among the general population in this regard. Furthermore, it is unreasonable to expect physicians to read all the available literature on this subject.
This article reviews high-quality articles on COVID-19 and effectively summarizes them for healthcare providers and the general population.
Introduction
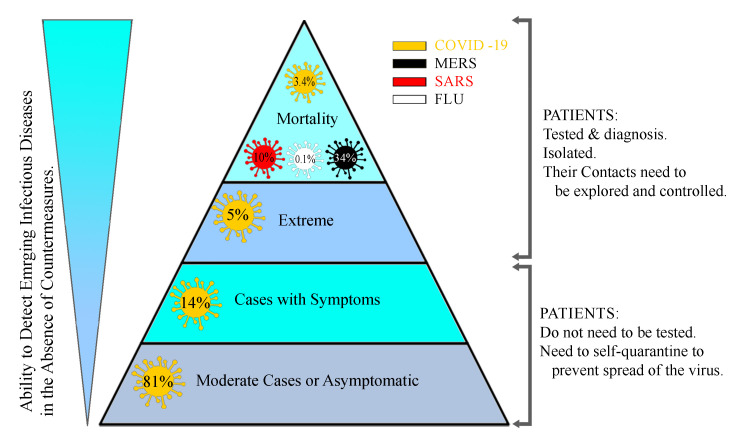
A pathogen from a human-animal virus family, the coronavirus (CoV), which was identified as the main cause of respiratory tract infections, evolved to a novel and wild kind in Wuhan, a city in Hubei Province of China, and spread throughout the world, such that it created a pandemic crisis according to the World Health Organization (WHO). CoV is a large family of viruses that were first discovered in 1960. These viruses cause such diseases as common colds in humans and animals. Sometimes they attack the respiratory system, and sometimes their signs appear in the gastrointestinal tract. There have been different types of human CoV including CoV-229E, CoV-OC43, CoV-NL63, and CoV-HKU1, with the latter two having been discovered in 2004 and 2005, respectively. These types of CoV regularly cause respiratory infections in children and adults. 1 There are also other types of these viruses that are associated with more severe symptoms. The new CoV, scientifically known as “SARS-CoV-2”, causes severe acute respiratory syndrome (SARS). 2 A newer type of the virus was discovered in September 2012 in a 60-year-old man in Saudi Arabia who died of the disease; the man had traveled to Dubai a few days earlier. The second case was a 49-year-old man in Qatar who also passed away. The discovery was first confirmed at the Health Protection Agency’s Laboratory in Colindale, London. The outbreak of this CoV is known as the Middle East Respiratory Syndrome (MERS), commonly referred to as “MERS-CoV”. The virus has infected 2260 people and has killed 912, most of them in the Middle East. 3 - 5 Finally, in December 2019, for the first time in Wuhan, in Hubei Province of China, a new type of CoV was identified that caused pneumonia in humans. 6 SARS-CoV-2 has affected 5404512 people and killed more than 343514 around the world according to the WHO situation report-127 (May 26, 2020). 3 , 7 - 10 The WHO has officially termed the disease “COVID-19”, which refers to corona, the virus, the disease, the year 2019, and its etiology (SARS-CoV-2). This type of CoV had never been seen in humans before. The initial estimates showed a mortality rate ranging from between 1% and 3% in most countries to 5% in the worst-hit areas ( Figure 1 ). 9 Some Chinese researchers succeeded in determining how SARS-CoV-2 affects human cells, which could help to develop techniques of viral detection and had antiviral therapy potential. Via a process termed “cryogenic electron microscopy (cryo-EM)”, these scientists discovered that CoV enters human cells utilizing a kind of cell membrane glycoprotein: angiotensin-converting enzyme 2 (ACE2). Then, the S protein is split into two sub-units: S1 and S2. S1 keeps a receptor-binding domain (RBD); accordingly, SARS-CoV-2 can bind to the peptidase domain of ACE2 directly. It appears that S2 subsequently plays a role in cellular fusion. Chinese researchers used the cryo-EM technique to provide ACE2 when it is linked to an amino acid transporter called “B0AT1”. They also discovered how to connect SARS-CoV-2 to ACE2-B0AT1, which is another complex structure. Given that none of these molecular structures was previously known, the researchers hoped that these studies would lead to the development of an antiviral or vaccine that would help to prevent CoV. Along the way, scientists found that ACE2 has to undergo a molecular process in which it binds to another molecule to be activated. The resulting molecule can bind two SARS-CoV-2 protein molecules simultaneously. The scientists also studied different SARS-CoV-2 RBD binding methods compared with other SARS-CoV-RBDs, which showed how subtle changes in the molecular binding sequence make the coronal structure of the virus stronger.
Most cases with SARS-CoV-2 are asymptomatic or have mild clinical pictures such as influenza and colds. This group of patients should be detected and isolated in their homes to break the transmission chain of the disease and adhere to the precautionary recommendations in order not to infect other people. The screening process will help this group and suppress the outbreak in the community. Patients with the confirmed disease who are admitted to hospitals can contaminate this environment, which should be borne in mind by healthcare providers and policymakers.
Transmission
While the first mode of the transmission of COVID-19 to humans is still unknown, a seafood market where live animals were sold was identified as a potential source at the beginning of the outbreak in the epidemiologic investigations that found some infected patients who had visited or worked in that place. The other viruses in this family, namely MERS and SARS, were both confirmed to be zoonotic viruses. Afterward, the person-to-person spread was established as the main mode of transmission and the reason for the progression of the outbreak. 11 Similar to the influenza virus, SARS-CoV-2 spreads through the population via respiratory droplets. When an infected person coughs, sneezes, or talks, the respiratory secretions, which contain the virus, enter the environment as droplets. These droplets can reach the mucous membranes of individuals directly or indirectly when they touch an infected surface or any other source; the virus, thereafter, finds its ways to the eyes, nose, or mouth as the first incubation places. 11 - 15 It has been reported that droplets cannot travel more than two meters in the air, nor can they remain in the air owing to their high density. Nonetheless, given the other hitherto unknown modes of transmission, routine airborne transmission precautions should be considered in high-risk countries and during high-risk procedures such as manual ventilation with bags and masks, endotracheal intubation, open endotracheal suctioning, bronchoscopy, cardiopulmonary resuscitation, sputum induction, lung surgery, nebulizer therapy, noninvasive positive pressure ventilation (eg, bilevel positive airway pressure and continuous positive airway pressure ), and lung autopsy. In the early stages of the disease, the chances of the spread of the virus to other persons are high because the viral load in the body may be high despite the absence of any symptoms ( Figure 2 ). 11 - 13 The person-to-person transmission rates can be different depending on the location and the infection control intervention; still, according to the latest reports, the secondary COVID-19 infection rate ranges from 1% to 5%. 13 - 23 Although the RNA of the virus has been detected in blood and stool, fecal-oral and blood-borne transmissions are not regarded as significant modes of transmission yet. 19 - 26 There have been no reports of mother-to-fetus transmission in pregnant women. 27
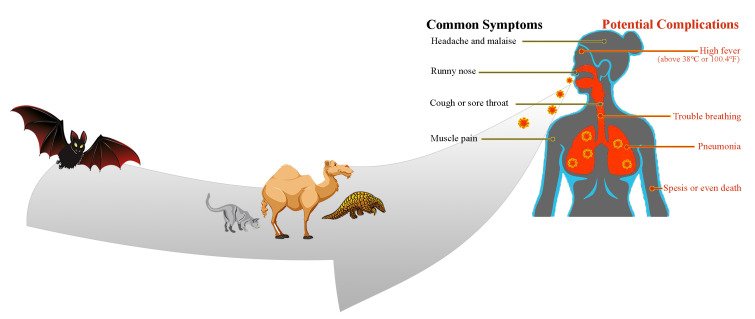
SARS-CoV-2 mode of transmission and clinical manifestations are illustrated in this figure. The potential source of this outbreak was identified to be from animals, similar to MERS and SARS, in epidemiologic studies; nonetheless, person-to-person transmission through droplets is currently the important mode. After reaching mucous membranes by direct or indirect close contact, the virus replicates in the cells and the immune system attacks the body due to its nature. Afterward, the clinical pictures appear, which are much more similar to influenza. However, different patients will have a spectrum of signs and symptoms.
Source Investigation
Recently, the appearance of SARS-CoV-2 in society shocked the healthcare system. 28 - 32 Veterinary corona virologists reported that COVID-19 was isolated from wildlife. Several studies have shown that bats are receptors of the CoV new version in 2019 with variants and changes in the environment featuring various biological characteristics. 33 - 36 The aforementioned mammals are a major source of CoV, which causes mild-to-severe respiratory illness and can even be deadly. In recent years, the virus has killed several thousands of people of all ages. 37 - 39 The mutated alternative of the virus can be transmitted to humans and cause acute respiratory distress. 40 , 41 One of the main causes of the spread of the virus is the exotic and unusual Chinese food in Wuhan: CoV is a direct result of the Chinese food cycle. The virus is found in the body of animals such as bats, 42 and snake or bat soup is a favorite Chinese food. Therefore, this sequence is replicated continuously. Almost everyone who was infected for the first time was directly in the local Wuhan market or had indirectly tried snake or bat soup in a Chinese restaurant. An investigation stated that the Malayan pangolin (Manis javanica) was a possible host for SARS-CoV-2 and recommended that it be removed from the wet market to prevent zoonotic transmissions in the future. 43 , 44
Pathogenesis
The important mechanisms of the severe pathogenesis of SARS-CoV-2 are not fully understood. Extensive lung injury in SARS-CoV-2 has been related to increased virus titers; monocyte, macrophage, and neutrophil infiltrations into the lungs; and elevated levels of pro-inflammatory cytokines and chemokines. Thus, the clinical exacerbation of SARS-CoV-2 infection may be in consequence of a combination of direct virus-induced cytopathic and immunopathological effects due to excessive cytokinesis. Changes in the cytokine/chemokine profile during SARS infection showed increased levels of circulating cytokines such as tumor necrosis factor-α (TNF-α), C–X–C motif chemokine 10 (CXCL10), interleukin (IL)-6, and IL-8 levels, in conjunction with elevated levels of serum pro-inflammatory cytokines such as IL-1, IL-6, IL-12, interferon-gamma (IFN-γ), and transforming growth factor-β (TGF-β). Nevertheless, constant stimulation by the virus creates a cytokine storm that has been related to acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndromes (MODS) in patients with COVID-19, which may ultimately lead to diminished immunity by lowering the number of CD4+ and CD8+ T cells and natural killer cells (crucial in antiviral immunity) and decreasing cytokine production and functional ability (exhaustion). It has been shown that IL-10, an inhibitory cytokine, is a major player and a potential target for therapeutic aims. 45 - 51 Severe cases of COVID-19 have respiratory distress and failure, which has been linked to the altered metabolism of heme by SARS-CoV-2. Some virus proteins can dissociate iron from porphyrins by attacking the 1-β chain of hemoglobin, which decreases the oxygen-transferring ability of hemoglobin. Research has also indicated that chloroquine and favipiravir might inhibit this process. 52
Clinical Manifestations
SARS-CoV-2, which attacks the respiratory system, has a spectrum of manifestations; nonetheless, it has three main primary symptoms after an incubation period of about two days to two weeks: fever and its associated symptoms such as malaise/fatigue/weakness; cough, which is nonproductive in most of the cases but can be productive indeed; and shortness of breath (dyspnea) due to low blood oxygenation. Although these symptoms appear in the body of the affected person over two to 14 days, patients may refer to the clinic with gastrointestinal symptoms (nausea/vomiting-diarrhea) or decreased sense of smell and/or taste. More devastatingly, however, patients may refer to the emergency room with such coagulopathies as pulmonary thromboembolism, cerebral venous thrombosis, and other related manifestations. The WHO has stated that dry throat and dry cough are other symptoms detected in the early stages of the infection. 53 , 54 The estimations of the severity of the disease are as follows: mild (no or mild pneumonia) in 81%, severe (eg, with dyspnea, hypoxia, or >50% lung involvement on imaging within 24 to 48 hours) in 14%, and critical (eg, with respiratory failure, shock, or multiorgan dysfunction) in 5%. In the early stages, the overall mortality rate was 2.3% and no deaths were observed in non-severe patients. Patients with advanced age or underlying medical comorbidities have more mortality and morbidity. 55 Although adults of middle age and older are most commonly affected by SARS-CoV-2, individuals at any age can be infected. A few studies have reported symptomatic infection in children; still, when it occurs, it has mild symptoms. The vast majority of cases have the infection with no signs and symptoms or mild clinical pictures; they are called “the asymptomatic group”. These patients do not seek medical care and if they come into close contact with others, they can spread the virus. Therefore, quarantine in their home is the best option for the population to break the transmission of the virus. It should be considered that some of these asymptomatic patients have clinical signs such as chest computed tomography scan (CT-Scan) infiltrations. Similar to bacterial pneumonia, lower respiratory signs and symptoms are the most frequent manifestations in serious cases of COVID-19, characterized by fever, cough, dyspnea, and bilateral infiltrates on chest imaging. In a study describing pneumonia in Wuhan, the most common clinical signs and symptoms at the onset of the illness were fever in 99% (although fever might not be a universal finding), fatigue in 70%, dry cough in 59%, anorexia in 40%, myalgia in 35%, dyspnea in 31%, and sputum production in 27%. Headache, sore throat, and rhinorrhea are less common, and gastrointestinal symptoms (eg, nausea and diarrhea) are relatively rare. 7 , 42 , 43 , 45 - 48 , 56 , 57 According to our clinical experience in Iran, anosmia, atypical chest pain, diarrhea, nausea/vomiting, and hemoptysis are other presenting symptoms in the clinic. It should be noted that COVID-19 has some unexplained potential complications such as secondary bacterial infections, myocarditis, central nervous system injury, cerebral edema, MODS, acute demyelinating encephalomyelitis (ADEM), kidney injury, liver injury, new-onset seizure, coagulopathy, and arrhythmias.
Laboratory data : Complete blood counts, which constitute a routine laboratory test, have shown different results in terms of the white blood cell count: from leukopenia and lymphopenia to leukocytosis, although lymphopenia appears to be the most common. Fatal cases have exhibited severe lymphopenia accompanied by an increased level of D-dimer. Liver function enzymes can be increased; however, it is not sufficient to diagnose a disease. The serum procalcitonin level is a marker of infection, especially in bacterial diseases. Patients with COVID-19 who require intensive care unit (ICU) management may have elevated procalcitonin. Increased urea and creatinine, creatinine-phosphokinase, lactate dehydrogenase, and C-reactive protein are other findings in some cases. 7 , 56 , 57
Imaging studies : Routine chest X-ray (CXR) is widely deemed the first-step management to evaluate any respiratory involvement. Although negative findings in CXR do not rule out the viral disease, patients without common findings do not have severe disease and can, consequently, be managed in the outpatient setting. 58 , 59 Another modality is chest CT-Scan. It can be ordered in suspected cases with typical symptoms at the first step, or it can be performed after the detection of any abnormalities in CXR. The most common demonstrations in CT-Scan images are ground-glass opacification, round opacities, and crazy paving with or without bilateral consolidative abnormalities (multilobar involvement) in contrast to most cases of bacterial pneumonia, which have locally limited involvement. Pleural thickening, pleural effusion, and lymphadenopathy are less common. 58 - 61 Tree-in-bud, peribronchial distribution, nodules, and cavity are not in favor of common COVID-19 findings. Although reverse transcriptase-polymerase chain reaction (RT-PCR) is used to confirm the diagnosis, it is a time-consuming procedure and has high false-negative/false-positive findings; hence, in the emergency clinical setting, CT-Scan findings can be a good approach to make the diagnosis. It is deserving of note, however, that false-positive/false-negative cases were reported by one study to be high and other differential diagnoses should be in mind in order not to miss any other cases such as acute pulmonary edema in patients with heart disease.
Suspected cases should be diagnosed as soon as possible to isolate and control the infection immediately. COVID-19 should be considered in any patient with fever and/or lower respiratory tract symptoms with any of the following risk factors in the previous 2 weeks: close contact with confirmed or suspected cases in any environment, especially at work in healthcare places without sufficient protective equipment or long-time standing in those places, and living in or traveling from well-known places where the disease is an epidemic. 61 - 66 Patients with severe lower respiratory tract disease without alternative etiologies and a clear history of exposure should be considered having COVID-19 unless confirmed otherwise. According to the Centers for Disease Control and Prevention (CDC), sending tests to check SARS-CoV-2 in suspected cases is based on physicians’ clinical judgment. Although there are some positive cases without clinical manifestations (ie, fever and/or symptoms of acute respiratory illness such as cough and dyspnea), infectious disease and control centers should take action in society to limit the exposure of such patients to other healthy individuals. The CDC prioritizes the use of the specific test for hospitalized patients, symptomatic patients who are at risk of fatal conditions (eg, age ≥65 y, chronic medical conditions, and immunocompromising conditions) and those who have exposure risks (recent travel, contact with patients with COVID-19, and healthcare workers). 61 - 66 Although treatment should be started after the confirmation of the disease, RT-PCR for highly suspected cases is a time-consuming test; accordingly, a considerable number of clinicians favor the use of a combination of clinical manifestations with imaging modalities (eg, CT-Scan findings) and their clinical judgment regarding the probability of the disease in order not to lose more time. 61 - 66
Treatment of COVID-19
There is no confirmed recommended treatment or vaccine for SARS-CoV-2; prevention is, therefore, better than treatment. Nevertheless, the high contagiousness of COVID-19, combined with the fact that some individuals fail to adhere to precautionary measures or they have significant risk factors, means that this infectious disease is inevitable in some people. Beside supportive treatments, many types of medications have been introduced. These medications come from previous experimental studies on SARS, MERS, influenza, or human immunodeficiency virus (HIV); hence, their efficacy needs further experimental and clinical approval. Patients with mild symptoms who do not have significant risk factors should be managed in their home like a self-made quarantine (in an isolated room); still, prompt hospital admission is required if patients exhibit signs of disease deterioration. 25 , 67 , 68 Isolation from other family members is an important prevention tip. Patients should wear face masks, eat healthy and warm foods similar to when struggling with influenza or colds, do the handwashing process, dispose of the contaminated materials cautiously, and disinfect suspicious surfaces with standard disinfectants. 69 Patients with severe symptoms or admission criteria should be hospitalized with other patients who have the same disease in an isolated department. When the disease is progressed, ICU care is mandatory. 25 , 67 , 68 SARS-CoV-2 attacks the respiratory system, diminishing the oxygenation process and forcing patients with low blood oxygen saturation to take extra oxygen from different modalities. Nasal cannulae, face masks with or without a reservoir, intubation in severe cases, and then extracorporeal membrane oxygenation in refractory hypoxia have been used; however, the safety and efficacy of these measures should be evaluated. As was mentioned above, impaired coagulation is one of the major complications of the disease; consequently, alongside all recommended supportive care and drugs, anticoagulants such as heparin should be administered prophylactically ( Table 1 ). Although it is said that all the clinical signs and symptoms of COVID-19 are induced by the immune system, as other research on influenza and MERS has revealed, glucocorticoids are not recommended in COVID-19 pneumonia unless other indications are present (eg, exacerbation of chronic obstructive pulmonary disease and refractory septic shock) due to the high risk of mortality and delayed viral clearance. Earlier in the national and international guidelines, nonsteroidal anti-inflammatory drugs such as naproxen were recommended on the strength of their antipyretic and anti-inflammatory components; however, the guideline has been revised recently and acetaminophen with or without codeine is currently the favored drug in patients with COVID-19. 25 , 67 , 68 According to the pathogenesis of the disease, whereby cytokine storm and immune-cell exhaustion can be seen in severe cases, selective antibodies against harmful interleukins such as IL-6 and IL-10 or other possible agents can be therapeutic for fatal complications. Tocilizumab, an IL-6 inhibitor, albeit with limited clinical efficacy, has been introduced in China’s National Health Commission treatment guideline for severe infection with profound pulmonary involvement (ie, white lung). 70 , 87
Summary of possible anti-COVID-19 drugs
mg, Milligrams; BD, Every 12 hours; RdRP, RNA-dependent RNA polymerase; TDS, Every 8 hours; IV, Intravenous; IL, Interleukin; μg, Micrograms
RNA synthesis inhibitors (eg, tenofovir disoproxil fumarate and 2’-deoxy-3’-thiacytidine [3TC]), neuraminidase inhibitors (NAIs), nucleoside analogs, lopinavir/ritonavir, atazanavir, remdesivir, favipiravir, INF-β, and Chinese traditional medicine (eg, Shufeng Jiedu and Lianhuaqingwen capsules) are the major candidates for COVID-19. 26 , 70 , 85 , 88 - 96 Antiviral drugs have been investigated for various diseases, but their efficacy in the treatment of COVID-19 is under investigation and several randomized clinical trials are ongoing to release a consensus result on the treatment of this infectious disease. Moderate-to-severe SARS-CoV-2 disease needs drug therapy. Favipiravir, a previously validated drug for influenza, is a drug that has shown promising results for COVID-19 in experimental and clinical studies, but it is under further evaluation. 70 , 79 , 80 Remdesivir, which was developed for Ebola, is an antiviral drug that is under evaluation for moderate-to-severe COVID-19 owing to its promising results in in vitro investigations. 70 , 73 - 75 , 81 Remdesivir was shown to have reduced the virus titer in infected mice with MERS-CoV and improved lung tissue damage with more efficiency compared with a group treated with lopinavir/ritonavir/INF-β. 67 , 70 Another investigation studied the potential efficacy of INF-β-1 in the early stages of COVID-19 as a potential antiviral drug. 86 Although there is some hope, an evidence-based consensus requires further clinical trials. 70 , 77 A combined protease inhibitor, lopinavir/ritonavir, is used for HIV infection and has shown interesting results for SARS and MERS in in vitro studies. 73 - 75 The clinical effectiveness of lopinavir/ritonavir for SARS-CoV-2 was also reported in a case report. 70 , 71 , 74 , 76 Atazanavir, another protease inhibitor, with or without ritonavir is another possible anti-COVID-19 treatment. 77 , 78 NAIs, including oseltamivir, zanamivir, and peramivir, are recommended as antiviral treatment in influenza. 68 Oral oseltamivir was tried for COVID-19 in China and was first recommended in the Iranian guideline for COVID-19 treatment; nevertheless, because of the absence of strong evidence indicating its efficacy for SARS-CoV-2, it was eliminated from the subsequent updates of the guideline. 85 RNA-dependent RNA polymerase inhibitors with anti-hepatitis C effects such as ribavirin have shown satisfactory results against SARS-CoV-2 RNA polymerase; however, they have limited clinical approval. 82 - 84 The well-known drugs for rheumatoid arthritis, systemic lupus erythematosus, and an antimalarial drug, chloroquine 71 and hydroxychloroquine 21 are other potential drugs for moderate-to-severe COVID-19 but with limited or no clinical appraisal. Hydroxychloroquine has exhibited better safety and fewer side effects than chloroquine, which makes it the preferred choice. 70 Furthermore, the immunomodulatory effects of hydroxychloroquine can be used to control the cytokine precipitation in the late phases of SARS-CoV-2 infections. There are numerous mechanisms for the antiviral activity of hydroxychloroquine. A weak base drug, hydroxychloroquine concentrates on such intracellular sections as endosomes and lysosomes, thereby halting viral replication in the phase of fusion and uncoating. Additionally, this immunosuppressive and antiparasitic drug is capable of altering the glycosylation of ACE2 and inhibiting both S-protein binding and phagocytosis. 72 A recent multicenter study showed that regarding the risks of cardiovascular adverse effects and mortality rates, hydroxychloroquine or chloroquine with or without a macrolide (eg, azithromycin) was not beneficial for hospitalized patients, although further research is needed to end such controversies. 97
Disease Duration
It is not easy to quarantine the patients who have fully recovered because there is evidence that they are highly infectious. 81 The recovery time for confirmed cases based on the National Health Commission reports of China’s government was estimated to range between 18 and 22 days. 73 As indicated by the WHO, the healing time seems to be around two weeks for moderate infections and 3 to 6 weeks for the severe/ serious disease. 75 Pan Feng and others studied 21 confirmed cases with COVID-19 pneumonia with about 82 CT-Scan images with a mean interval of four days. Lung abnormalities on chest CT showed the highest severity approximately 10 days after the initial onset of symptoms. All patients became clear after 11 to 26 days of hospitalization. From day zero to day 26, four stages of lung CT were defined as follows: Stage 1 (first 4 days): ground-glass opacities; Stage 2 (second 4 days): crazy-paving patterns; Stage 3 (days 9–13): maximum total CT scores in the consolidations; and Stage 4 (≥14 d): steady improvements in the consolidations with a reduction in the total CT score without any crazy-paving pattern. 74 Nevertheless, there are also rare cases reported from some studies that show the recurrence of COVID-19 after negative preliminary RT-PCR results. For example, Lan and othersstudied one hospitalized and three home-quarantined patients with COVID-19 and evaluated them with RT-PCR tests of the nucleic acid. All the patients with positive RT-PCR test results had CT imaging with ground-glass opacification or mixed ground-glass opacification and consolidation with mild-to-moderate disease. After antiviral treatments, all four patients had two consecutive negative RT-PCR test results within 12 to 32 days. Five to 13 days after hospital discharge or the discontinuation of the quarantine, RT-PCR tests were repeated, and all were positive. An additional RT-PCR test was performed using a kit from a different manufacturer, and the results were also positive. Their findings propose that a minimum percentage of recovered patients may still be infection carriers. 76
Supplements for COVID-19
Since the appearance of SARS-CoV-2 in Wuhan, China, there have been reports of the unreliable and unpredictable use of mysterious therapies. Some recommendations such as the use of certain herbs and extracts including oregano oil, mulberry leaf, garlic, and black sesame may be safe as long as people do not utilize their hands for instance. 98 According to data released by the CDC, vitamin C (VitC) supplements can decrease the risk of colds in people besides preventing CoV from spreading. The aforementioned organization states that frequent consumption of VitC supplements can also decrease the duration of the cold; however, if used only after the cold has risen, its consumption does not influence the disease course. VitC also plays an important role in the body. One of the main reasons for taking VitC is to strengthen the immune system because this vitamin plays a significant part in the immune system. Firstly, VitC can increase the production of white blood cells (lymphocytes and phagocytes) in the bone marrow, which can support and protect the body against infections. Secondly, VitC helps immune cells to function better while preserving white blood cells from damaging molecules such as free oxidative radicals and ions. Thirdly, VitC is an essential part of the skin’s immune system. This vitamin is actively transported to the skin surface, where it serves as an antioxidant and helps to strengthen the skin barrier by optimizing the collagen synthesis process. Patients with pneumonia have lower levels of VitC and have been revealed to have a longer recovery time. 69 , 99 In a randomized investigation, 200 mg/d of VitC was applied to older patients and resulted in improvements in the respiratory symptoms. Another investigation reported 80% fewer mortalities in a controlled group of VitC takers. 73 However, for effective immune system improvement, VitC should be consumed alongside adequate doses of several other supplements. Although VitC plays an important role in the body, often a balanced diet and the consumption of fresh fruits and vegetables can quickly fill the blanks. While taking high amounts of VitC is less risky because it is water-soluble and its waste is eliminated in the urine, it can induce diarrhea, nausea, and abdominal spasms at higher concentrations. Too much VitC may cause calcium-oxalate kidney stones. People with genetic hemochromatosis, an iron deficiency disorder, should consult a physician before taking any VitC supplements as high levels of VitC can lead to tissue damage. Some studies have evaluated the different doses of oral or intravenous VitC for patients admitted to the hospital for COVID-19. Although they used different regimens, all of them demonstrated satisfactory results regarding the resolution of the compilations of the disease, decreased mortality, and shortened lengths of stay in the ICU and/or the hospital. 100 , 101 Immunologists have also recommended 6 000 units of vitamin A (VitA) per day for two weeks, more than twice the recommended limit for VitA, which can create a poisoning environment over time. According to the guidance of the National Institutes of Health (NIH), middle-aged men and women should take 1 and 2 mg of VitA every day, respectively. The safe upper limit of this vitamin is 6000 mg or 5000 units, and overdose can have serious outcomes such as dizziness, nausea, headache, coma, and even death. Extreme consumption of VitA throughout pregnancy can lead to birth anomalies.
Similar to VitC, vitamin D (VitD) has antioxidant, anti-inflammatory, and immune-modulatory effects in our body such as reducing pro-inflammatory cytokines and inhibiting viral replication according to experimental studies. 83 The VitD state of our body is checked through 25 (OH) VitD in the serum. VitD deficiency is pandemic around the world due to multifactorial reasons. It has been shown that VitD deficient patients are prone to SARS-CoV-2 and, accordingly, treating VitD deficiency is not without benefits. Grant and others recommended 10 000 units per day for two weeks and then 5 000 units per day as the maintenance dose to keep the level between 40 and 100 ng/mL. 102 VitD toxicity causes gastrointestinal discomfort (dyspepsia), congestion, hypercalcemia, confusion, positional disorders, dysrhythmia, and kidney dysfunction.
James Robb, 103 a researcher who detected CoV for the first time as a consultant pathologist with the National Cancer Institute of America, suggested the influence of zinc consumption. Oral zinc supplements can be dissolved in the nback of the throat. Short-term therapy with oral zinc can decrease the duration of viral colds in adults. Zinc intake is also associated with the faster resolution of nasal congestion, nasal drainage, sore throats, and coughs. Researchers 104 , 105 have warned that the consumption of more than 1 mg of zinc a day can lead to zinc poisoning and have side effects such as lowered immune function. Children and old people with zinc insufficiency in developing nations are extremely vulnerable to pneumonia and other viral infections. It has also been determined that zinc has a major role in the production and activation of T-cell lymphocytes. 106 , 107
And finally, for high-risk people or those who work in high-risk places such as healthcare providers, hydroxychloroquine has been mentioned to be effective as a prophylactic regimen ( Table 2 ). Although different doses have been investigated so far, Pourdowlat and others recommended 200 mg daily before exposure, and for the post-exposure scenario, a loading dose of 600-800 mg followed by a maintenance dose of 200 mg daily. 74
Possible prophylactic regimens against SARS-CoV-2 infection
IU, International unit; mg, Milligrams; kg, Kilograms; ICU, Intensive care unit; g, Grams; IV, Intravenous; Vit, Vitamin; ng, Nanograms; mL, Milliliter
COVID-19 Kits and Deep Learning
COVID-19 has threatened public health, and its fast global spread has caught the scientific community by surprise. 108 Hence, developing a technique capable of swiftly and reliably detecting the virus in patients is vital to prevent the spreading of the virus. 109 , 110 One of the ways to diagnose this new virus is through RT-PCR, a test that has previously demonstrated its efficacy in detecting such CoV infections as MERS-CoV and SARS-CoV. Consequently, increasing the availability of RT-PCR kits is a worldwide concern. The timing of the RT-PCR test and the type of strain collected are of vital importance in the diagnosis of COVID-19. One of the characteristics of this new virus is that the serum is negative in the early stage, while respiratory specimens are positive. The level of the virus at the early stage of the illness is also high, even though the infected individual experiences mild symptoms. 111 For the management of the emerging situation of COVID-19 in Wuhan, various effective diagnostic kits were urgently made available to markets. While a few different diagnostics kits are used merely for research endeavors, only a single kit developed by the Beijing Genome Institute (BGI) called “Real-Time Fluorescent PCR” has been authenticated for clinical diagnostics. Fluorescent RT-PCR is reliable and able to offer fast results probably within a few hours (usually within two hours). Besides RT-PCR, China has successfully developed a metagenomic-sequencing kit based on combinatorial probe-anchor synthesis that can identify virus-related bacteria, allowing observation and evaluation during the transmission of the virus. Furthermore, the metagenomic-sequencing kit based on combinatorial probe-anchor synthesis is far faster than the abovementioned fluorescent RT-PCR kit. Apart from China, a Singapore-based laboratory, Veredus, developed a virus detection kit (Vere-CoV) in late January. It is a portable Lab-On-Chip used to detect MERS-CoV, SARS-CoV, and SARS-CoV-2, in a single examination. This kit works based on the VereChip™ technology, the lines of code (LOC) program incorporating two different influential molecular biological functions (microarray and PCR) precisely. Several studies have focused on SARS-CoV diagnostic testing. These papers have presented investigative approaches to the identification of the virus using molecular testing (ie, RT-PCR). Researchers probed into the use of a nested PCR technique that contains a pre-amplification step or integrating the N gene as an extra subtle molecular marker to improve on the sensitivity. 112 - 115 CT-Scan is very useful for diagnosing, evaluating, and screening infections caused by COVID-19. One recommendation for scanning the disease is to take a scan every three to five days. According to researchers, most CT-Scan images from patients with COVID-19 are bilateral or peripheral ground-glass opacification, with or without stabilization. Nowadays, because of a paucity of computerized quantification tools, only qualitative reports and sometimes inaccurate analyses of contaminated areas are drawn upon in radiology reports. A categorization system based on the deep learning approach was proposed by a study to automatically measure infected parts and their volumetric ratios in the lung. The functionality of this system was evaluated by making some comparisons between the infected portions and the manually-delineated ones on the CT-Scan images of 300 patients with COVID-19. To increase the manual drawing of training samples and the non-interference in the automated results, researchers adopted a human-based approach in collaboration with radiologists so as to segment the infected region. This approach shortens the time to about four minutes after 3-time updating. The mean Dice similarity coefficient illustrated that the automatically detected infected parts were 91.6% similar to the manually detected ones, and the average of the percentage estimated error was 0.3% for the whole lung. 116 , 117
Prevention Considerations
In the healthcare setting, any individual with the manifestations of COVID-19 (eg, fever, cough, and dyspnea) should wear a face mask, have a separate waiting area, and keep the distance of at least two meters. Symptomatic patients should be asked about recent travel or close contact with a patient in the preceding two weeks to find other possible infected patients. The CDC and WHO have announced special precautions for healthcare providers in the hospital and during different procedures. Wearing tight-fitting face masks with special filters and impermeable face shields is necessary for all of them. 11 , 18 , 65 , 66 , 76 , 118 - 124 Other people should pay attention to the CDC and WHO preventive strategies, which recommend that individuals not touch their eyes, mouth, and nose before washing or disinfecting their hands; wash their hands regularly according to the standard protocol; use effective disinfection solutions (ie, containing at least 60% ethylic alcohol) for contaminated surfaces; cover their mouth when coughing and sneezing; avoid waiting or walking in crowded areas, and observe isolation protocols in their home. Postponing elective work and decreasing non-urgent visits and traveling to areas in the grip of COVID-19 may be useful to lessen the risk of exposure. If suspected individuals with mild symptoms are managed in outpatient settings, an isolated room with minimal exposure to others should be designed. Patients and their caregivers should wear tight-fitting face masks. 11 , 18 , 65 , 66 , 76 , 118 - 124 Substantial numbers of individuals with COVID-19 are asymptomatic with potential exposure; accordingly, a screening tool should be employed to evaluate these cases. In addition to passport checks, corona checks have been incorporated into the protocols in airports and other crowded places. The use of a remote thermometer to measure body temperature leads to an increase in the number of false-negative cases. It is, thus, essential that everyone pay sufficient heed to the WHO and CDC recommendations in their daily life. Traveling is not prohibited, but it should be restricted and passengers from any country should be monitored. 11 , 18 , 65 , 66 , 76 , 118 - 124
SARS-CoV-2 is the new highly contagious CoV, which was first reported in China. While it had a zoonotic origin in the beginning, it subsequently spread throughout the world by human contact. COVID-19 has a spectrum of manifestations, which is not lethal most of the time. To diagnose this condition, physicians can avail themselves of laboratory and imaging findings besides signs and symptoms. RT-PCR is the gold standard, but it lacks sufficient sensitivity and specificity. Although there are some potential drugs for COVID-19 and some vitamins or minerals for prophylaxis, the best preventive strategies are quarantine (staying at home) and the use of personal protective equipment and disinfectants.
Acknowledgement
The authors express their gratitude toward the Supporting Organizations for Foreign Iranian Students, Islamic Azad University Isfahan (Khorasgan) Branch, and Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
- 1. Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163–88. doi: 10.1016/bs.aivir.2018.01.001. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Smith RD. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Soc Sci Med. 2006;63:3113–23. doi: 10.1016/j.socscimed.2006.08.004. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–24. doi: 10.1097/CM9.0000000000000722. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Memish ZA, Al-Tawfiq JA, Assiri A, AlRabiah FA, Al Hajjar S, Albarrak A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904–6. doi: 10.1097/INF.0000000000000325. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Al-Tawfiq JA, Kattan RF, Memish ZA. Middle East respiratory syndrome coronavirus disease is rare in children: An update from Saudi Arabia. World J Clin Pediatr. 2016;5:391–6. doi: 10.5409/wjcp.v5.i4.391. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Nishiura H, Jung SM, Linton NM, Kinoshita R, Yang Y, Hayashi K, et al. The Extent of Transmission of Novel Coronavirus in Wuhan, China, 2020. J Clin Med. 2020;9 doi: 10.3390/jcm9020330. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Sarkar B, Ullah MA, Johora FT, Taniya MA, Araf Y. The Essential Facts of Wuhan Novel Coronavirus Outbreak in China and Epitope-based Vaccine Designing against 2019-nCoV. BioRxiv. 2020 doi: 10.1101/2020.02.05.935072. [ DOI ] [ Google Scholar ]
- 10. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) . 2020;133:1025–31. doi: 10.1097/CM9.0000000000000744. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Control. Interim Clinical Guidance for Management of Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) Infection. Updated February 12, 2020 [ Google Scholar ]
- 12. Organization WH. Novel Coronavirus (2019-nCoV) technical guidance: Patient management. 2020 [ Google Scholar ]
- 13. Organization WH. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. 2020 [ Google Scholar ]
- 14. Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–50. doi: 10.1016/j.jhin.2015.08.027. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. McIntosh K. Novel coronavirus (2019-nCoV) 2020 [ Google Scholar ]
- 16. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Groneberg DA, Poutanen SM, Low DE, Lode H, Welte T, Zabel P. Treatment and vaccines for severe acute respiratory syndrome. Lancet Infect Dis. 2005;5:147–55. doi: 10.1016/S1473-3099(05)01307-1. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, et al. Treatment With Lopinavir/Ritonavir or Interferon-beta1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212:1904–13. doi: 10.1093/infdis/jiv392. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. The Author’s Response: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35:e89. doi: 10.3346/jkms.2020.35.e89. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–8. doi: 10.5582/bst.2020.01030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. McIntosh K, Hirsch MS, Bloom A. Coronavirus disease 2019 (COVID-19) UpToDate Hirsch MS Bloom. 2020;5 [ Google Scholar ]
- 27. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–26. doi: 10.1016/j.ajog.2020.02.017. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Tabari P, Amini M, Moghadami M, Moosavi M. International Public Health Responses to COVID-19 Outbreak: A Rapid Review. Iran J Med Sci. 2020;45:157–69. doi: 10.30476/ijms.2020.85810.1537. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Negahdaripour M. The Battle Against COVID-19: Where Do We Stand Now? Iran J Med Sci. 2020;45:81–2. doi: 10.30476/ijms.2020.46357. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Bahadori M, Yaghoubi M, Haghgoshyie E, Ghasemi M, Hasanpoor E. Patients’ and physicians’ perspectives and experiences on the quality of medical consultations: a qualitative study. Int J Evid Based Healthc. 2020;18:247–55. doi: 10.1097/XEB.0000000000000210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Ghasemi M, Ghadiri Nejad M, Bagzibagli K. Knowledge Management Orientation: An Innovative Perspective to Hospital Management. Iran J Public Health. 2017;46:1639–45. [ PMC Free Article ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Ghadirinejad M, Atasoylu E, Izbirak G, Gha-Semi M. A Stochastic Model for the Ethanol Pharmacokinetics. Iran J Public Health. 2016;45:1170–8. [ PMC Free Article ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Raj VS, Osterhaus AD, Fouchier RA, Haagmans BL. MERS: emergence of a novel human coronavirus. Curr Opin Virol. 2014;5:58–62. doi: 10.1016/j.coviro.2014.01.010. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Banik GR, Khandaker G, Rashid H. Middle East respiratory syndrome coronavirus “MERS-CoV”: current knowledge gaps. Paediatr Respir Rev. 2015;16:197–202. doi: 10.1016/j.prrv.2015.04.002. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, et al. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18:20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Ismail MM, Cho KO, Ward LA, Saif LJ, Saif YM. Experimental bovine coronavirus in turkey poults and young chickens. Avian Dis. 2001;45:157–63. [ PubMed ] [ Google Scholar ]
- 37. Moon HW, Bemrick WJ. Fecal transmission of calf cryptosporidia between calves and pigs. Vet Pathol. 1981;18:248–55. doi: 10.1177/030098588101800213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Bhatt PN, Percy DH, Jonas AM. Characterization of the virus of sialodacryoadenitis of rats: a member of the coronavirus group. J Infect Dis. 1972;126:123–30. doi: 10.1093/infdis/126.2.123. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Mackay IM, Arden KE. MERS coronavirus: diagnostics, epidemiology and transmission. Virol J. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–23. doi: 10.3201/eid1911.131172. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Cotten M, Watson SJ, Kellam P, Al-Rabeeah AA, Makhdoom HQ, Assiri A, et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet. 2013;382:1993–2002. doi: 10.1016/S0140-6736(13)61887-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–8. doi: 10.1126/science.1087139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Wu P, Hao X, Lau EHY, Wong JY, Leung KSM, Wu JT, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Lam TT, Jia N, Zhang YW, Shum MH, Jiang JF, Zhu HC, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–63. doi: 10.1007/s15010-020-01401-y. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970–1. doi: 10.1056/NEJMc2001468. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–4. doi: 10.1002/jmv.25709. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–64. doi: 10.1128/MMBR.69.4.635-664.2005. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Pickett BE, Greer DS, Zhang Y, Stewart L, Zhou L, Sun G, et al. Virus pathogen database and analysis resource (ViPR): a comprehensive bioinformatics database and analysis resource for the coronavirus research community. Viruses. 2012;4:3209–26. doi: 10.3390/v4113209. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–5. doi: 10.1038/s41423-020-0402-2. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Liu W, Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Preprint revised on. 2020;10 doi: 10.26434/chemrxiv.11938173.v4. [ DOI ] [ Google Scholar ]
- 53. Li S, Yue J, Dong BR, Yang M, Lin X, Wu T. Acetaminophen (paracetamol) for the common cold in adults. Cochrane Database Syst Rev. 2013:CD008800. doi: 10.1002/14651858.CD008800.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Winther B. Pathogenesis of viral induced rhinitis. The Nose. 1998;8:135. [ Google Scholar ]
- 55. Haynes L, Szaba FM, Eaton SM, Kummer LW, Lanthier PA, Petell AH, et al. Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections. J Immunol. 2012;189:4921–9. doi: 10.4049/jimmunol.1201916. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–34. doi: 10.1016/S1473-3099(20)30086-4. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214:1072–7. doi: 10.2214/AJR.20.22976. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020:200823. doi: 10.1148/radiol.2020200823. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Patel A, Jernigan DB, nCo VCDCRT. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–6. doi: 10.15585/mmwr.mm6905e1. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Organization WH. Coronavirus disease (COVID-19) technical guidance: Surveillance and case definitions. Geneva: World Health Organization; 2020. [ Google Scholar ]
- 66. Organization WH. Updated WHO advice for international traffic in relation to the outbreak of the novel coronavirus 2019-nCoV, 24 January 2020. Geneva: World Health Organization; 2020. [ Google Scholar ]
- 67. Cho KO, Hasoksuz M, Nielsen PR, Chang KO, Lathrop S, Saif LJ. Cross-protection studies between respiratory and calf diarrhea and winter dysentery coronavirus strains in calves and RT-PCR and nested PCR for their detection. Arch Virol. 2001;146:2401–19. doi: 10.1007/s007050170011. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Chow KC, Hsiao CH, Lin TY, Chen CL, Chiou SH. Detection of severe acute respiratory syndrome-associated coronavirus in pneumocytes of the lung. Am J Clin Pathol. 2004;121:574–80. doi: 10.1309/C0ED-U0RA-QBTX-BHCE. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Saul AW. Nutritional treatment of coronavirus. Orthomolecular Medicine News Service. 2020;16:22. [ Google Scholar ]
- 70. Lu CC, Chen MY, Lee WS, Chang YL. Potential therapeutic agents against COVID-19: What we know so far. J Chin Med Assoc. 2020;83:534–6. doi: 10.1097/JCMA.0000000000000318. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Qin X, Qiu S, Yuan Y, Zong Y, Tuo Z, Li J, et al. Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China. China (February 18, 2020) 2020 doi: 10.2139/ssrn.3541147. [ DOI ] [ Google Scholar ]
- 72. Pourdowlat G, Panahi P, Pooransari P, Ghorbani F. Prophylactic Recommendation for Healthcare Workers in COVID-19 Pandemic. Advanced Journal of Emergency Medicine. 2020;4:e39. [ Google Scholar ]
- 73. Anastassopoulou C, Russo L, Tsakris A, Siettos C. Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PLoS One. 2020;15:e0230405. doi: 10.1371/journal.pone.0230405. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295:715–21. doi: 10.1148/radiol.2020200370. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Winter G. COVID-19 and emergency planning. Br J Community Nurs. 2020;25:184–6. doi: 10.12968/bjcn.2020.25.4.184. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Fintelman-Rodrigues N, Sacramento CQ, Lima CR, da Silva FS, Ferreira A, Mattos M, et al. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. bioRxiv. 2020 doi: 10.1128/AAC.00825-20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J Int AIDS Soc. 2020;23:e25489. doi: 10.1002/jia2.25489. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [ DOI ] [ Google Scholar ]
- 81. Shi P, Cao S, Feng P. SEIR Transmission dynamics model of 2019 nCoV coronavirus with considering the weak infectious ability and changes in latency duration. MedRxiv. 2020 doi: 10.1101/2020.02.16.20023655. [ DOI ] [ Google Scholar ]
- 82. Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92:740–6. doi: 10.1002/jmv.25798. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Anderson PO. Breastfeeding and Respiratory Antivirals: Coronavirus and Influenza. Breastfeed Med. 2020;15:128. doi: 10.1089/bfm.2020.29149.poa. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Saif LJ. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev Sci Tech. 2004;23:643–60. doi: 10.20506/rst.23.2.1513. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Tsunemitsu H, Smith DR, Saif LJ. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch Virol. 1999;144:167–75. doi: 10.1007/s007050050493. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Giomarelli P, Scolletta S, Borrelli E, Biagioli B. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Ann Thorac Surg. 2003;76:117–23. doi: 10.1016/s0003-4975(03)00194-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Vanr K, Nauwynck H, Pensaert M. A potential role for tumour necrosis factor-alpha in synergy between porcine respiratory coronavirus and bacterial lipopolysaccharide in the induction of respiratory disease in pigs. J Med Microbiol. 2000;49:613–20. doi: 10.1099/0022-1317-49-7-613. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38:379–81. doi: 10.1038/d41587-020-00003-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Cavanagh D, Naqi S. Infectious bronchitis. Diseases of poultry. 2003;11:101–19. [ Google Scholar ]
- 94. Centers for Disease C, Prevention. Prevalence of IgG antibody to SARS-associated coronavirus in animal traders--Guangdong Province, China, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:986–7. [ PubMed ] [ Google Scholar ]
- 95. Chim SS, Tsui SK, Chan KC, Au TC, Hung EC, Tong YK, et al. Genomic characterisation of the severe acute respiratory syndrome coronavirus of Amoy Gardens outbreak in Hong Kong. Lancet. 2003;362:1807–8. doi: 10.1016/s0140-6736(03)14901-x. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Cho KO, Hoet AE, Loerch SC, Wittum TE, Saif LJ. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route in feedlot cattle. Am J Vet Res. 2001;62:1436–41. doi: 10.2460/ajvr.2001.62.1436. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Mehra MR, Desai SS, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] [ Retracted ]
- 98. Peduto Hand F, Choudhury RA, Gubler WD. First Report of Cytospora punicae Causing Wood Canker and Branch Dieback of Pomegranate (Punica granatum) in the United States. Plant Dis. 2014;98:853. doi: 10.1094/PDIS-11-13-1133-PDN. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Saul AW. Vitamin C Protects Against Coronavirus. 2020 [ Google Scholar ]
- 100. Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133. doi: 10.1186/s13054-020-02851-4. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Cheng RZ. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? . Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Robb JA, Bond CW. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses Virology 1979;94:352–70. doi: 10.1016/0042-6822(79)90467-7. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Feldmann HR, Williams DR, Champagne JD, Lehenbauer TW, Aly SS. Effectiveness of zinc supplementation on diarrhea and average daily gain in pre-weaned dairy calves: A double-blind, block-randomized, placebo-controlled clinical trial. PLoS One. 2019;14:e0219321. doi: 10.1371/journal.pone.0219321. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Senathilake K, Samarakoon S, Tennekoon K. Virtual screening of inhibitors against spike glycoprotein of 2019 novel corona virus: A drug repurposing approach. 2020 [ Google Scholar ]
- 106. John E, Laskow TC, Buchser WJ, Pitt BR, Basse PH, Butterfield LH, et al. Zinc in innate and adaptive tumor immunity. J Transl Med. 2010;8:118. doi: 10.1186/1479-5876-8-118. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521–34. doi: 10.1007/s00204-011-0775-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem. 2020;66:549–55. doi: 10.1093/clinchem/hvaa029. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020;9 doi: 10.3390/jcm9030623. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Lau LT, Fung YW, Wong FP, Lin SS, Wang CR, Li HL, et al. A real-time PCR for SARS-coronavirus incorporating target gene pre-amplification. Biochem Biophys Res Commun. 2003;312:1290–6. doi: 10.1016/j.bbrc.2003.11.064. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Hui RK, Zeng F, Chan CM, Yuen KY, Peiris JS, Leung FC. Reverse transcriptase PCR diagnostic assay for the coronavirus associated with severe acute respiratory syndrome. J Clin Microbiol. 2004;42:1994–9. doi: 10.1128/jcm.42.5.1994-1999.2004. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Poon LL, Chan KH, Wong OK, Yam WC, Yuen KY, Guan Y, et al. Early diagnosis of SARS coronavirus infection by real time RT-PCR. J Clin Virol. 2003;28:233–8. doi: 10.1016/j.jcv.2003.08.004. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Jiang SS, Chen TC, Yang JY, Hsiung CA, Su IJ, Liu YL, et al. Sensitive and quantitative detection of severe acute respiratory syndrome coronavirus infection by real-time nested polymerase chain reaction. Clin Infect Dis. 2004;38:293–6. doi: 10.1086/380841. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Beck B, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. bioRxiv. 2020 doi: 10.1016/j.csbj.2020.03.025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Xu X, Jiang X, Ma C, Du P, Li X, Lv S, et al. Deep learning system to screen coronavirus disease 2019 pneumonia. arXiv 2020. doi: 10.1016/j.eng.2020.04.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 118. Control CfD, Prevention. Interim Considerations for Disposition of Hospitalized Patients with 2019-nCoV Infection. 2020 [ Google Scholar ]
- 119. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–51. doi: 10.1016/j.jhin.2020.01.022. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Organization WH [Internet] Advice on the use of masks the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak. c2020 [Cited 5 Feb 2020] . Avilble from: https://apps.who.int/iris/bitstream/handle/10665/331493/WHO-2019-nCoVIPC_Masks-2020.2-eng.pdf .
- 123. Organization WH. Key considerations for repatriation and quarantine of travellers in relation to the outbreak of novel coronavirus 2019-nCoV: 11 February 2020. 2020d [ Google Scholar ]
- 124. Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D, et al. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N Engl J Med. 2020;382:1278–80. doi: 10.1056/NEJMc2001899. [ PMC Free Article ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO