The PRISMA 2020 statement: An updated guideline for reporting systematic reviews
Affiliations.
- 1 School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
- 2 Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands.
- 3 Université de Paris, Centre of Epidemiology and Statistics (CRESS), Inserm, Paris, France.
- 4 Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia.
- 5 University of Texas Health Science Center at San Antonio, San Antonio, Texas, United States of America; Annals of Internal Medicine.
- 6 Knowledge Translation Program, Li Ka Shing Knowledge Institute, Toronto, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
- 7 Evidence Partners, Ottawa, Canada.
- 8 Clinical Research Institute, American University of Beirut, Beirut, Lebanon; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
- 9 Department of Medical Informatics and Clinical Epidemiology, Oregon Health & Science University, Portland, Oregon, United States of America.
- 10 York Health Economics Consortium (YHEC Ltd), University of York, York, United Kingdom.
- 11 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Department of Medicine, University of Ottawa, Ottawa, Canada.
- 12 Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Open Patient data Exploratory Network (OPEN), Odense University Hospital, Odense, Denmark.
- 13 Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, Blueprint Translational Research Group, Ottawa Hospital Research Institute, Ottawa, Canada; Regenerative Medicine Program, Ottawa Hospital Research Institute, Ottawa, Canada.
- 14 Department of Ophthalmology, School of Medicine, University of Colorado Denver, Denver, Colorado, United States; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America.
- 15 Division of Headache, Department of Neurology, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA; Head of Research, The BMJ, London, United Kingdom.
- 16 Department of Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, Indiana, United States of America.
- 17 Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom.
- 18 Centre for Reviews and Dissemination, University of York, York, United Kingdom.
- 19 EPPI-Centre, UCL Social Research Institute, University College London, London, United Kingdom.
- 20 Li Ka Shing Knowledge Institute of St. Michael's Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Division of the Dalla Lana School of Public Health and the Institute of Health Management, Policy, and Evaluation, University of Toronto, Toronto, Canada; Queen's Collaboration for Health Care Quality Joanna Briggs Institute Centre of Excellence, Queen's University, Kingston, Canada.
- 21 Methods Centre, Bruyère Research Institute, Ottawa, Ontario, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
- 22 Centre for Journalology, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
- PMID: 33780438
- PMCID: PMC8007028
- DOI: 10.1371/journal.pmed.1003583
Matthew Page and co-authors describe PRISMA 2020, an updated reporting guideline for systematic reviews and meta-analyses.

Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Evidence-Based Medicine / standards*
- Publishing / standards*
- Systematic Reviews as Topic* / methods
- Systematic Reviews as Topic* / standards
Grants and funding
- UG1 EY020522/EY/NEI NIH HHS/United States
- DRF-2018-11-ST2-048/DH_/Department of Health/United Kingdom
- UNC Libraries
- HSL Academic Process
- Creating a PRISMA flow diagram
- PRISMA 2020
Creating a PRISMA flow diagram: PRISMA 2020
Created by health science librarians.

What is PRISMA?
Which prisma 2020 flow diagram should i use, step-by-step: prisma 2020 flow diagram, using the covidence prisma diagram, documenting your grey literature search, updating a systematic review with prisma 2020, citing prisma 2020, for more information, prisma 2020 checklist.
- PRISMA 2020 Checklist (.doc)
- PRISMA 2020 Checklist (.pdf)
- PRISMA 2020 Expanded Checklist
PRISMA 2020 Flow Diagram Templates
- PRISMA 2020 V1- New Reviews with Databases and Registers only PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only
- PRISMA 2020 V2 - New Reviews with Databases, Registers, and Other Sources PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources
The format of the PRISMA Step-By-Step was first developed by Glasgow Caledonian University https://www.gcu.ac.uk/library

"PRISMA stands for Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
It is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses.
The aim of the PRISMA Statement is to help authors improve the reporting of systematic reviews and meta-analyses. We have focused on randomized trials, but PRISMA can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. PRISMA may also be useful for critical appraisal of published systematic reviews, although it is not a quality assessment instrument to gauge the quality of a systematic review. The PRISMA Statement consists of a 27-item checklist and a four-phase flow diagram ."
"The PRISMA Explanation and Elaboration document explains and illustrates the principles underlying the PRISMA Statement. It is strongly recommended that it be used in conjunction with the PRISMA Statement.
PRISMA is part of a broader effort, to improve the reporting of different types of health research, and in turn to improve the quality of research used in decision-making in healthcare."
From prisma-statement.org
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol . 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006
Page MJ, Moher D, Bossuyt P, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. doi:10.31222/osf.io/gwdhk.
Rethlefsen M, Kirtley S, Waffenschmidt S, et al. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. doi:10.31219/osf.io/sfc38.
In PRISMA 2020, there are now expanded options depending on where you search and whether you are updating a review. Version 1 of PRISMA 2020 includes databases and clinical trial or preprint registers. Version 2 includes additional sections for elaborating on your grey literature search, such as searches on websites or in citation lists. Both versions are available for new and updated reviews from the Equator Network's PRISMA Flow Diagram page .
Templates for New Reviews

Step 1: Preparation To complete the the PRISMA diagram, save a copy of the diagram to use alongside your searches. It can be downloaded from the PRISMA website .
Step 2: Doing the Database Search Run the search for each database individually, including ALL your search terms, any MeSH or other subject headings, truncation (like hemipleg * ), and/or wildcards (like sul ? ur). Apply all your limits (such as years of search, English language only, and so on). Once all search terms have been combined and you have applied all relevant limits, you should have a final number of records or articles for each database. Enter this information in the top left box of the PRISMA flow chart. You should add the total number of combined results from all databases (including duplicates) after the equal sign where it says Databases (n=) . Many researchers also add notations in the box for the number of results from each database search, for example, Pubmed (n=335), Embase (n= 600), and so on. If you search trial registers, such as ClinicalTrials.gov , CENTRAL , ICTRP , or others, you should enter that number after the equal sign in Registers (n=) .
NOTE: Some citation managers automatically remove duplicates with each file you import. Be sure to capture the number of articles from your database searches before any duplicates are removed.

Step 3: Remove All Duplicates To avoid reviewing duplicate articles, you need to remove any articles that appear more than once in your results. You may want to export the entire list of articles from each database to a citation manager such as EndNote, Sciwheel, Zotero, or Mendeley (including both citation and abstract in your file) and remove the duplicates there. If you are using Covidence for your review, you should also add the duplicate articles identified in Covidence to the citation manager number. Enter the number of records removed as duplicates in the second box on your PRISMA template. If you are using automation tools to help evaluate the relevance of citations in your results, you would also enter that number here.

NOTE: If you are using Covidence to screen your articles , you can copy the numbers from the PRISMA diagram in your Covidence review into the boxes mentioned below. Covidence does not include the number of results from each database, so you will need to keep track of that number yourself.
Step 4: Records Screened- Title/Abstract Screening The next step is to add the number of articles that you will screen. This should be the number of records identified minus the number from the duplicates removed box.

Step 5: Records Excluded- Title/Abstract Screening You will need to screen the titles and abstracts for articles which are relevant to your research question. Any articles that appear to help you provide an answer to your research question should be included. Record the number of articles excluded through title/abstract screening in the box to the right titled "Records excluded." You can optionally add exclusion reasons at this level, but they are not required until full text screening.

Step 6: Reports Sought for Retrieval This is the number of articles you obtain in preparation for full text screening. Subtract the number of excluded records (Step 5) from the total number screened (Step 4) and this will be your number sought for retrieval.

Step 7: Reports Not Retrieved List the number of articles for which you are unable to find the full text. Remember to use Find@UNC and Interlibrary Loan to request articles to see if we can order them from other libraries before automatically excluding them.

Step 8: Reports Assessed for Eligibility- Full Text Screening This should be the number of reports sought for retrieval (Step 6) minus the number of reports not retrieved (Step 7). Review the full text for these articles to assess their eligibility for inclusion in your systematic review.

Step 9: Reports Excluded After reviewing all articles in the full-text screening stage for eligibility, enter the total number of articles you exclude in the box titled "Reports excluded," and then list your reasons for excluding the articles as well as the number of records excluded for each reason. Examples include wrong setting, wrong patient population, wrong intervention, wrong dosage, etc. You should only count an excluded article once in your list even if if meets multiple exclusion criteria.

Step 10: Included Studies The final step is to subtract the number of records excluded during the eligibility review of full-texts (Step 9) from the total number of articles reviewed for eligibility (Step 8). Enter this number in the box labeled "Studies included in review," combining numbers with your grey literature search results in this box if needed. You have now completed your PRISMA flow diagram, unless you have also performed searches in non-database sources.

To view the PRISMA diagram created after using Covidence to screen references for your review, click the PRISMA button on the main menu of your review in Covidence.

If you listed your sources when importing citations, your PRISMA diagram will include the list of databases you used and the number of references from each.

If you imported references from a citation manager, your PRISMA diagram starts with duplicate removal. To have a complete PRISMA diagram, you will need to add the number of results from each database you searched, as well as the number of additional sources you found.
Once you have finished title/abstract and full text screening (and data extraction or quality assessment if applicable), click Download DOCX to download your flow diagram as a Word document, or click View as text to copy and paste the PRISMA data or into an editable template for PRISMA and fill in the numbers.

There are many places articles can get lost in the review process. Remember to make sure your PRISMA numbers add up correctly!

Step 6: Included Studies The final step is to subtract the number of excluded articles or records during the eligibility review of full-texts from the total number of articles reviewed for eligibility. Enter this number in the box labeled "Studies included in review," combining numbers with your database search results in this box if needed. You have now completed your PRISMA flow diagram, which you can now include in the results section of your article or assignment.

If you are updating an existing review, use one of these PRISMA 2020 Updated Review templates, which feature an additional box for the number of studies and reports of studies included in the previous search iterations.
- PRISMA 2020 flow diagram for updated systematic reviews- databases and registers only
- PRISMA 2020 flow diagram for updated systematic reviews- databases, registers and other sources
When referring to PRISMA 2020, The Equator Network recommends using journal article citations (such as those in our For More Information box ) rather than referring to the PRISMA website. If you are not already using a journal article citation, they recommend that you cite one of the original publications of the PRISMA Statement or PRISMA Explanation and Elaboration .
Related HSL Guides
- Systematic Reviews
Additional Readings
- Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement . J Clin Epidemiol. 2021;134:103-112.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews . Bmj. 2021;372:n160.
- Radua J. PRISMA 2020 - An updated checklist for systematic reviews and meta-analyses. Neurosci Biobehav Rev. 2021;124:324-325.
- Sarkis-Onofre R, Catalá-López F, Aromataris E, Lockwood C. How to properly use the PRISMA Statement . Systematic reviews. 2021;10(1):117-117.
- Sohrabi C, Franchi T, Mathew G, et al. PRISMA 2020 statement: What's new and the importance of reporting guidelines. Int J Surg. 2021;88:105918.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews . Int J Surg. 2021;88:105906.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . Bmj. 2021;372:n71.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews . PLoS Med. 2021;18(3):e1003583.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . Syst Rev. 2021;10(1):89.
- Last Updated: Mar 12, 2024 8:53 AM
- URL: https://guides.lib.unc.edu/prisma
Search & Find
- E-Research by Discipline
- More Search & Find
Places & Spaces
- Places to Study
- Book a Study Room
- Printers, Scanners, & Computers
- More Places & Spaces
- Borrowing & Circulation
- Request a Title for Purchase
- Schedule Instruction Session
- More Services
Support & Guides
- Course Reserves
- Research Guides
- Citing & Writing
- More Support & Guides
- Mission Statement
- Diversity Statement
- Staff Directory
- Job Opportunities
- Give to the Libraries
- News & Exhibits
- Reckoning Initiative
- More About Us
- Search This Site
- Privacy Policy
- Accessibility
- Give Us Your Feedback
- 208 Raleigh Street CB #3916
- Chapel Hill, NC 27515-8890
- 919-962-1053
- Open access
- Published: 19 April 2021
How to properly use the PRISMA Statement
- Rafael Sarkis-Onofre 1 ,
- Ferrán Catalá-López 2 , 3 ,
- Edoardo Aromataris 4 &
- Craig Lockwood 4
Systematic Reviews volume 10 , Article number: 117 ( 2021 ) Cite this article
64k Accesses
171 Citations
103 Altmetric
Metrics details
A Research to this article was published on 29 March 2021
It has been more than a decade since the original publication of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [ 1 ], and it has become one of the most cited reporting guidelines in biomedical literature [ 2 , 3 ]. Since its publication, multiple extensions of the PRISMA Statement have been published concomitant with the advancement of knowledge synthesis methods [ 4 , 5 , 6 , 7 ]. The PRISMA2020 statement, an updated version has recently been published [ 8 ], and other extensions are currently in development [ 9 ].
The number of systematic reviews (SRs) has increased substantially over the past 20 years [ 10 , 11 , 12 ]. However, many SRs continue to be poorly conducted and reported [ 10 , 11 ], and it is still common to see articles that use the PRISMA Statement and other reporting guidelines inappropriately, as was highlighted recently [ 13 ].
The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs. This growing set of guidelines have been developed to aid authors with appropriate reporting of different knowledge synthesis methods (such as SRs, scoping reviews, and review protocols) and to ensure that all aspects of this type of research are accurately and transparently reported. In other words, the PRISMA Statement is a road map to help authors best describe what was done, what was found, and in the case of a review protocol, what are they are planning to do.
Despite this clear and well-articulated intention [ 2 , 3 , 4 , 5 ], it is common for Systematic Reviews to receive manuscripts detailing the inappropriate use of the PRISMA Statement and its extensions. Most frequently, improper use appears with authors attempting to use the PRISMA statement as a methodological guideline for the design and conduct reviews, or identifying the PRISMA statement as a tool to assess the methodological quality of reviews, as seen in the following examples:
“This scoping review will be conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Statement.”
“This protocol was designed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
“The methodological quality of the included systematic reviews will be assessed with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement.”
Some organizations (such as Cochrane and JBI) have developed methodological guidelines that can help authors to design or conduct diverse types of knowledge synthesis rigorously [ 14 , 15 ]. While the PRISMA statement is presented to predominantly guide reporting of a systematic review of interventions with meta-analyses, its detailed criteria can readily be applied to the majority of review types [ 13 ]. Differences between the role of the PRISMA Statement to guide reporting versus guidelines detailing methodological conduct is readily illustrated with the following example: the PRISMA Statement recommends that authors report their complete search strategies for all databases, registers, and websites (including any filters and limits used), but it does not include recommendations for designing and conducting literature searches [ 8 ]. If authors are interested in understanding how to create search strategies or which databases to include, they should refer to the methodological guidelines [ 12 , 13 ]. Thus, the following examples can illustrate the appropriate use of the PRISMA Statement in research reporting:
“The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.”
“This scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).”
“The protocol is being reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
Systematic Reviews supports the complete and transparent reporting of research. The Editors require the submission of a populated checklist from the relevant reporting guidelines, including the PRISMA checklist or the most appropriate PRISMA extension. Using the PRISMA statement and its extensions to write protocols or the completed review report, and completing the PRISMA checklists are likely to let reviewers and readers know what authors did and found, but also to optimize the quality of reporting and make the peer review process more efficient.
Transparent and complete reporting is an essential component of “good research”; it allows readers to judge key issues regarding the conduct of research and its trustworthiness and is also critical to establish a study’s replicability.
With the release of a major update to PRISMA in 2021, the appropriate use of the updated PRISMA Statement (and its extensions as those updates progress) will be an essential requirement for review based submissions, and we encourage authors, peer reviewers, and readers of Systematic Reviews to use and disseminate that initiative.
Availability of data and materials
We do not have any additional data or materials to share.
Abbreviations
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
Systematic reviews
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005 .
Article PubMed Google Scholar
Caulley L, Cheng W, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, et al. Citation impact was highly variable for reporting guidelines of health research: a citation analysis. J Clin Epidemiol. 2020;127:96–104. https://doi.org/10.1016/j.jclinepi.2020.07.013 .
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. https://doi.org/10.1186/s13643-017-0663-8 .
Article PubMed PubMed Central Google Scholar
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. https://doi.org/10.1186/s13643-020-01542-z .
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850 .
Article Google Scholar
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi/10.1186/s13643-021-01626-4.
EQUATOR Network: Reporting guidelines under development for systematic reviews. https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/ . Accessed 11 Feb 2021.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. Plos Med. 2016;13(5):e1002028. https://doi.org/10.1371/journal.pmed.1002028 .
Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016;94(3):485–514. https://doi.org/10.1111/1468-0009.12210 .
Niforatos JD, Weaver M, Johansen ME. Assessment of Publication Trends of Systematic Reviews and Randomized Clinical Trials, 1995 to 2017. JAMA Intern Med. 2019;179(11):1593–4. https://doi.org/10.1001/jamainternmed.2019.3013.
Caulley L, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, Cheng W, et al. Reporting guidelines of health research studies are frequently used inappropriately. J Clin Epidemiol. 2020;122:87–94. https://doi.org/10.1016/j.jclinepi.2020.03.006 .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition ed. Chichester: Wiley; 2019.
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. ed. Adelaide: JBI; 2020.
Download references
Acknowledgements
RSO is funded in part by Meridional Foundation. FCL is funded in part by the Institute of Health Carlos III/CIBERSAM.
Author information
Authors and affiliations.
Graduate Program in Dentistry, Meridional Faculty, IMED, Passo Fundo, Brazil
Rafael Sarkis-Onofre
Department of Health Planning and Economics, National School of Public Health, Institute of Health Carlos III, Madrid, Spain
Ferrán Catalá-López
Department of Medicine, University of Valencia/INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain
JBI, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, Australia
Edoardo Aromataris & Craig Lockwood
You can also search for this author in PubMed Google Scholar
Contributions
RSO drafted the initial version. FCL, EA, and CL made substantial additions to the first and subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rafael Sarkis-Onofre .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
CL is Editor-in-Chief of Systematic Reviews, FCL is Protocol Editor of Systematic Reviews, and RSO is Associate Editor of Systematic Reviews.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sarkis-Onofre, R., Catalá-López, F., Aromataris, E. et al. How to properly use the PRISMA Statement. Syst Rev 10 , 117 (2021). https://doi.org/10.1186/s13643-021-01671-z
Download citation
Published : 19 April 2021
DOI : https://doi.org/10.1186/s13643-021-01671-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Med Libr Assoc
- v.110(2); 2022 Apr 1
PRISMA 2020 and PRISMA-S: common questions on tracking records and the flow diagram
Melissa l. rethlefsen.
1 moc.liamg@nesfelhterlm , Executive Director and Professor, Health Sciences Library & Informatics Center, University of New Mexico, Albuquerque, NM
Matthew J. Page
2 [email protected] , Senior Research Fellow and ARC DECRA Fellow, Methods in Evidence Synthesis Unit, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
The PRISMA 2020 and PRISMA-S guidelines help systematic review teams report their reviews clearly, transparently, and with sufficient detail to enable reproducibility. PRISMA 2020, an updated version of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement, is complemented by PRISMA-S, an extension to PRISMA focusing on reporting the search components of systematic reviews. Several significant changes were implemented in PRISMA 2020 and PRISMA-S when compared with the original version of PRISMA in 2009, including the recommendation to report search strategies for all databases, registries, and websites that were searched. PRISMA-S also recommends reporting the number of records identified from each information source. One of the most challenging aspects of the new guidance from both documents has been changes to the flow diagram. In this article, we review some of the common questions about using the PRISMA 2020 flow diagram and tracking records through the systematic review process.
In early 2021, two reporting guidelines were released that provide direct guidance on how to report the literature search components of systematic reviews and related review types: PRISMA 2020, the updated version of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [ 1 , 2 ]; and PRISMA-S, an extension to PRISMA focused solely on reporting the search components of systematic reviews [ 3 ]. PRISMA 2020 and PRISMA-S include several significant changes from the original version of PRISMA published in 2009 [ 4 ], including the recommendation to report search strategies for all databases, registries, and websites that were searched.
One of the most challenging aspects of integrating the new guidance from both documents into practice has been changes to the PRISMA flow diagram, which tracks the flow of information through the systematic review process. In the original version, the flow diagram was broken into four sections: identification, screening, eligibility, and included [ 4 ]. The identification section included boxes for recording the number of records identified through database searching, the number of records identified through other sources, and the number of records after deduplication. Often, using the PRISMA 2009 flow diagram, the “records identified through other sources” box contained only the number of records matching inclusion criteria, not necessarily all the records identified and screened. Building upon the original PRISMA 2009 flow diagram, PRISMA-S recommends constructing the flow diagram to show the number of records retrieved per database in the “records identified through database searching” box. Additionally, PRISMA-S asks authors to record the number of records retrieved for each other information source in the “records identified through other sources” box [ 3 ]. PRISMA-S also suggests reporting the total number of references retrieved from all sources, including updates, in the results section and the total number of references from each database and information source in the supplementary materials.
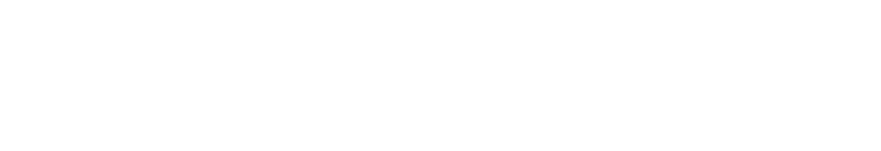
With the new PRISMA 2020 flow diagram template, systematic review teams now have the opportunity to better represent the complexity of the search process [ 1 ]. There are now four templates available, including flow diagram templates designed specifically for updates and systematic reviews that search beyond databases and study registries [ 5 ]. Generally, most systematic review teams will use the “PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources” (see Figure 1 for an example) [ 5 ]. In this flow diagram, records are tracked through two different columns: identification of studies via databases and registers (Column 1) and identification of studies via other methods (Column 2). The flow diagram itself provides guidance on what type of information resource should be reported in which column, specifically noting that records identified from websites, organizations, citation searching, and other methods should be reported in Column 2. The flow diagram template also suggests reporting an overall number for records identified from databases and registers in Column 1.

Example of a “PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources” made using the R ShinyApp [ 1 , 6 , 7 ]
In the PRISMA 2020 flow diagram, Column 2 represents the “Additional records identified through other sources” from the PRISMA 2009 flow diagram but with major improvements to enhance tracking the entire flow of information through the systematic review process. Using the PRISMA 2009 flow diagram, many researchers only put the total number of records that met inclusion criteria in the “Additional records” box, thus excluding the total number of records that were retrieved from each source. The PRISMA 2020 flow diagram makes it explicit that it is expected that the total number of records retrieved from each information source should be tracked, which aligns with PRISMA-S's guidelines.
Since the publication of PRISMA 2020 and PRISMA-S, researchers have posed many questions about the best ways to track records and use the PRISMA 2020 flow diagram appropriately. In the rest of this commentary, we will answer some of the most common ones.
All four versions of the PRISMA 2020 flow diagram are available in Word format on the PRISMA website [ 5 ]. In addition, there is a very useful R ShinyApp that creates downloadable flow diagrams from inputted data [ 6 , 7 ].
Do I need to seek permission from the authors to include a PRISMA 2020 flow diagram in my systematic review manuscript?
No, permission is not required. The PRISMA 2020 papers that include the flow diagram templates were published as open access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt, and build upon this work, even for commercial use, provided the original work is properly cited.
PRISMA-S was released slightly before PRISMA 2020, so the styles do not entirely align, but they are compatible—with a few tweaks. In PRISMA-S, the flow diagram example shows study registries data in the “Additional records identified through other sources” box (e.g., ClinicalTrials.gov ) [ 3 ]. In the PRISMA 2020 flow diagram, Column 1 contains all data related to records and studies identified in study registries, and Column 2 now contains all records identified outside of databases and study registries, such as websites, reference lists, and contacts with manufacturers, among others [ 1 ].
PRISMA-S does recommend that, if space is available, individual databases and other information sources' identified records should be included in the flow diagram. This is not currently possible to do using the R ShinyApp [ 6 ], but any of the Word templates can be modified to add this information [ 5 ]. If it is not possible, the number of records per individual information source should go in the supplementary materials.
It may be helpful to put all citation searching results in Column 2 and reserve Column 1 for reporting subject-based searching, but it is not necessary to do so; users are free to modify the PRISMA 2020 flow diagram templates in a way they consider most optimal for their review. Citation indexes are indeed databases and can be included in Column 1, particularly if the records are assessed as part of the primary screening process [ 2 ]. As with all other searches, researchers conducting citation searches for citing or cited references should report the number of records identified per search in the supplementary materials. It is also important to cite each “base” article examined for citing or cited references in the manuscript text for reproducibility and transparency [ 3 ].
Identifying records and studies from other methods and information sources is one of the trickiest components of a systematic review to report. As acknowledged by the PRISMA 2020 flow diagram, those components of the review often take place outside the “normal” flow of screening that happens during the systematic review process. The ability (or lack thereof) to track the initial number of records identified is often determined by the process used to identify and screen the records—and the system used to manage records identified from other information sources. When records are all centrally tracked, regardless of source, it is easier to produce this data.
Best practice is to count all records identified (by hand or by other means) from each source. This information should be reported individually in supplementary materials, according to PRISMA-S [ 3 ]. It should also be reported in the PRISMA 2020 flow diagram [ 5 ], either by individual information source or by category of information source (i.e., all records identified from websites). If it is not possible or feasible to count all records, report what is feasible.
Google Scholar is both a database and a citation index, and systematic review teams often use Google Scholar for both reasons. For subject-based searching, Google Scholar is considered as a database for the PRISMA 2020 flow diagram and can be reported in Column 1. If Google Scholar is only used as a citation index, data can be reported in either Column 1 or Column 2. The number of records identified should be reported per search in the supplementary materials, per PRISMA-S. If a systematic review team searches Google Scholar as both a traditional bibliographic database and as a citation index, the team may wish to use both Column 1 (subject-based search) and Column 2 (citation searching), but it is also reasonable to combine them in Column 1 in the flow diagram. Each search, however, needs to be reported separately in the supplementary materials.
Google, on the other hand, is not a traditional bibliographic database nor a citation index. It should be considered as an additional information source and reported in Column 2.
A complication of both Google and Google Scholar is that a maximum of 1,000 records is available for any given search, including citation searches [ 3 ]. Therefore, the total number of records identified from these two sources should never be listed in the flow diagram as above 1,000 for any given search. Many times, review teams will pre-identify how many records in Google or Google Scholar they will review per search; this should be the number reported for each search, unless the true number of results identified from a search is smaller.
Yes. Though it is quite convenient to have that detail in the flow diagram, it is not essential to present it there. The number of records identified for each individual database and information source should be reported, however, in the supplementary materials regardless of whether they are included in the flow diagram. If a research team or publication prefers to report these records and sources in both places, the Word templates for the PRISMA 2020 flow diagram are customizable [ 5 ]. The R ShinyApp development team is also actively considering improvements so this feature may be available in future versions [ 6 , 7 ].
PRISMA-S treats all results from the same search, regardless of whether it was the original search or an update, as a single data point [ 3 ]. In the PRISMA 2020 flow diagram, report the total number of items retrieved per database across the lifespan of the systematic review searching process [ 5 ]. If multiple separate searches occurred for a particular database, the total results from each search can be combined in the flow diagram. Authors can consider reporting the number of records retrieved at each search point, original plus update(s), in the supplementary materials.
There are occasions where reports cannot be located, for a multitude of reasons. This may include a journal that cannot be accessed in a local collection or via interlibrary loan, lack of response from authors or contacts, or broken links. Use the appropriate column's “Reports not retrieved” box to indicate how many reports were not able to be retrieved, regardless of reason.
On some occasions, authors might identify a study that has results appearing in two reports (one providing data at three months, another at two years follow-up). In this case, the number of studies included in the review is one, whereas the number of reports of included studies is two. This distinction was introduced in the PRISMA 2020 flow diagram based on our observation that the jump from the number of reports assessed for eligibility to the number of studies included in the review (as was prompted in the original PRISMA flow diagram) sometimes resulted in some reports not being accounted for [ 2 ]. For example, we have seen some flow diagrams where the authors report assessing fifty full-text reports for eligibility, excluding forty reports , and including eight studies (failing to indicate that two of the eight studies were published in two reports).
Loading metrics
Open Access
Guidelines and Guidance
The Guidelines and Guidance section contains advice on conducting and reporting medical research.
See all article types »
The PRISMA 2020 statement: An updated guideline for reporting systematic reviews
* E-mail: [email protected]
Affiliation School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
Affiliation Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
Affiliation Université de Paris, Centre of Epidemiology and Statistics (CRESS), Inserm, Paris, France
Affiliation Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia
Affiliation University of Texas Health Science Center at San Antonio, San Antonio, Texas, United States of America; Annals of Internal Medicine
Affiliation Knowledge Translation Program, Li Ka Shing Knowledge Institute, Toronto, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
Affiliation Evidence Partners, Ottawa, Canada
Affiliation Clinical Research Institute, American University of Beirut, Beirut, Lebanon; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada
Affiliation Department of Medical Informatics and Clinical Epidemiology, Oregon Health & Science University, Portland, Oregon, United States of America
Affiliation York Health Economics Consortium (YHEC Ltd), University of York, York, United Kingdom
Affiliation Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Department of Medicine, University of Ottawa, Ottawa, Canada
Affiliation Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Open Patient data Exploratory Network (OPEN), Odense University Hospital, Odense, Denmark
Affiliation Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, Blueprint Translational Research Group, Ottawa Hospital Research Institute, Ottawa, Canada; Regenerative Medicine Program, Ottawa Hospital Research Institute, Ottawa, Canada
Affiliation Department of Ophthalmology, School of Medicine, University of Colorado Denver, Denver, Colorado, United States; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America
Affiliation Division of Headache, Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA; Head of Research, The BMJ, London, United Kingdom
Affiliation Department of Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, Indiana, United States of America
Affiliation Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom
Affiliation Centre for Reviews and Dissemination, University of York, York, United Kingdom
Affiliation EPPI-Centre, UCL Social Research Institute, University College London, London, United Kingdom
Affiliation Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Division of the Dalla Lana School of Public Health and the Institute of Health Management, Policy, and Evaluation, University of Toronto, Toronto, Canada; Queen’s Collaboration for Health Care Quality Joanna Briggs Institute Centre of Excellence, Queen’s University, Kingston, Canada
Affiliation Methods Centre, Bruyère Research Institute, Ottawa, Ontario, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- [ ... ],
Affiliation Centre for Journalology, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- [ view all ]
- [ view less ]
- Matthew J. Page,
- Joanne E. McKenzie,
- Patrick M. Bossuyt,
- Isabelle Boutron,
- Tammy C. Hoffmann,
- Cynthia D. Mulrow,
- Larissa Shamseer,
- Jennifer M. Tetzlaff,
- Elie A. Akl,

Published: March 29, 2021
- https://doi.org/10.1371/journal.pmed.1003583
- Reader Comments
Citation: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med 18(3): e1003583. https://doi.org/10.1371/journal.pmed.1003583
Copyright: © 2021 Page et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: There was no direct funding for this research. MJP is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200101618) and was previously supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535) during the conduct of this research. JEM is supported by an Australian NHMRC Career Development Fellowship (1143429). TCH is supported by an Australian NHMRC Senior Research Fellowship (1154607). JMT is supported by Evidence Partners Inc. JMG is supported by a Tier 1 Canada Research Chair in Health Knowledge Transfer and Uptake. MML is supported by The Ottawa Hospital Anaesthesia Alternate Funds Association and a Faculty of Medicine Junior Research Chair. TL is supported by funding from the National Eye Institute (UG1EY020522), National Institutes of Health, United States. LAM is supported by a National Institute for Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-048). ACT is supported by a Tier 2 Canada Research Chair in Knowledge Synthesis. DM is supported in part by a University Research Chair, University of Ottawa. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: I have read the journal’s policy and the authors of this manuscript have the following competing interests: EL is head of research for the BMJ; MJP is an editorial board member for PLOS Medicine; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews. None of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health, for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration, which produces systematic reviews, and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocates for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners, creator of DistillerSR software for systematic reviews; Evidence Partners was not involved in the design or outcomes of the statement, and the views expressed solely represent those of the author.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews .
Systematic reviews serve many critical roles. They can provide syntheses of the state of knowledge in a field, from which future research priorities can be identified; they can address questions that otherwise could not be answered by individual studies; they can identify problems in primary research that should be rectified in future studies; and they can generate or evaluate theories about how or why phenomena occur. Systematic reviews therefore generate various types of knowledge for different users of reviews (such as patients, healthcare providers, researchers, and policy makers).[ 1 , 2 ]To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did (such as how studies were identified and selected) and what they found (such as characteristics of contributing studies and results of meta-analyses). Up-to-date reporting guidance facilitates authors achieving this.[ 3 ]
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement published in 2009 (hereafter referred to as PRISMA 2009)[ 4 – 10 ] is a reporting guideline designed to address poor reporting of systematic reviews.[ 11 ] The PRISMA 2009 statement comprised a checklist of 27 items recommended for reporting in systematic reviews and an “explanation and elaboration” paper[ 12 – 16 ] providing additional reporting guidance for each item, along with exemplars of reporting. The recommendations have been widely endorsed and adopted, as evidenced by its co-publication in multiple journals, citation in over 60 000 reports (Scopus, August 2020), endorsement from almost 200 journals and systematic review organisations, and adoption in various disciplines. Evidence from observational studies suggests that use of the PRISMA 2009 statement is associated with more complete reporting of systematic reviews,[ 17 – 20 ] although more could be done to improve adherence to the guideline.[ 21 ]
Many innovations in the conduct of systematic reviews have occurred since publication of the PRISMA 2009 statement. For example, technological advances have enabled the use of natural language processing and machine learning to identify relevant evidence,[ 22 – 24 ] methods have been proposed to synthesise and present findings when meta-analysis is not possible or appropriate,[ 25 – 27 ] and new methods have been developed to assess the risk of bias in results of included studies.[ 28 , 29 ] Evidence on sources of bias in systematic reviews has accrued, culminating in the development of new tools to appraise the conduct of systematic reviews.[ 30 , 31 ] Terminology used to describe particular review processes has also evolved, as in the shift from assessing “quality” to assessing “certainty” in the body of evidence.[ 32 ] In addition, the publishing landscape has transformed, with multiple avenues now available for registering and disseminating systematic review protocols,[ 33 , 34 ] disseminating reports of systematic reviews, and sharing data and materials, such as preprint servers and publicly accessible repositories. To capture these advances in the reporting of systematic reviews necessitated an update to the PRISMA 2009 statement.
Summary points
- To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did, and what they found
- The PRISMA 2020 statement provides updated reporting guidance for systematic reviews that reflects advances in methods to identify, select, appraise, and synthesise studies
- The PRISMA 2020 statement consists of a 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and revised flow diagrams for original and updated reviews
- We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders
Development of PRISMA 2020
A complete description of the methods used to develop PRISMA 2020 is available elsewhere.[ 35 ] We identified PRISMA 2009 items that were often reported incompletely by examining the results of studies investigating the transparency of reporting of published reviews.[ 17 , 21 , 36 , 37 ] We identified possible modifications to the PRISMA 2009 statement by reviewing 60 documents providing reporting guidance for systematic reviews (including reporting guidelines, handbooks, tools, and meta-research studies).[ 38 ] These reviews of the literature were used to inform the content of a survey with suggested possible modifications to the 27 items in PRISMA 2009 and possible additional items. Respondents were asked whether they believed we should keep each PRISMA 2009 item as is, modify it, or remove it, and whether we should add each additional item. Systematic review methodologists and journal editors were invited to complete the online survey (110 of 220 invited responded). We discussed proposed content and wording of the PRISMA 2020 statement, as informed by the review and survey results, at a 21-member, two-day, in-person meeting in September 2018 in Edinburgh, Scotland. Throughout 2019 and 2020, we circulated an initial draft and five revisions of the checklist and explanation and elaboration paper to co-authors for feedback. In April 2020, we invited 22 systematic reviewers who had expressed interest in providing feedback on the PRISMA 2020 checklist to share their views (via an online survey) on the layout and terminology used in a preliminary version of the checklist. Feedback was received from 15 individuals and considered by the first author, and any revisions deemed necessary were incorporated before the final version was approved and endorsed by all co-authors.
The PRISMA 2020 statement
Scope of the guideline.
The PRISMA 2020 statement has been designed primarily for systematic reviews of studies that evaluate the effects of health interventions, irrespective of the design of the included studies. However, the checklist items are applicable to reports of systematic reviews evaluating other interventions (such as social or educational interventions), and many items are applicable to systematic reviews with objectives other than evaluating interventions (such as evaluating aetiology, prevalence, or prognosis). PRISMA 2020 is intended for use in systematic reviews that include synthesis (such as pairwise meta-analysis or other statistical synthesis methods) or do not include synthesis (for example, because only one eligible study is identified). The PRISMA 2020 items are relevant for mixed-methods systematic reviews (which include quantitative and qualitative studies), but reporting guidelines addressing the presentation and synthesis of qualitative data should also be consulted.[ 39 , 40 ] PRISMA 2020 can be used for original systematic reviews, updated systematic reviews, or continually updated (“living”) systematic reviews. However, for updated and living systematic reviews, there may be some additional considerations that need to be addressed. Where there is relevant content from other reporting guidelines, we reference these guidelines within the items in the explanation and elaboration paper [ 41 ] (such as PRISMA-Search [ 42 ] in items 6 and 7, Synthesis without meta-analysis (SWiM) reporting guideline [ 27 ] in item 13d). Box 1 includes a glossary of terms used throughout the PRISMA 2020 statement.
Box 1. Glossary of terms
- Systematic review —A review that uses explicit, systematic methods to collate and synthesise findings of studies that address a clearly formulated question [ 43 ]
- Statistical synthesis —The combination of quantitative results of two or more studies. This encompasses meta-analysis of effect estimates (described below) and other methods, such as combining P values, calculating the range and distribution of observed effects, and vote counting based on the direction of effect (see McKenzie and Brennan [ 25 ] for a description of each method)
- Meta-analysis of effect estimates —A statistical technique used to synthesise results when study effect estimates and their variances are available, yielding a quantitative summary of results [ 25 ]
- Outcome —An event or measurement collected for participants in a study (such as quality of life, mortality)
- Result —The combination of a point estimate (such as a mean difference, risk ratio, or proportion) and a measure of its precision (such as a confidence/credible interval) for a particular outcome
- Report —A document (paper or electronic) supplying information about a particular study. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information
- Record —The title or abstract (or both) of a report indexed in a database or website (such as a title or abstract for an article indexed in Medline). Records that refer to the same report (such as the same journal article) are “duplicates”; however, records that refer to reports that are merely similar (such as a similar abstract submitted to two different conferences) should be considered unique.
- Study —An investigation, such as a clinical trial, that includes a defined group of participants and one or more interventions and outcomes. A “study” might have multiple reports. For example, reports could include the protocol, statistical analysis plan, baseline characteristics, results for the primary outcome, results for harms, results for secondary outcomes, and results for additional mediator and moderator analyses
PRISMA 2020 is not intended to guide systematic review conduct, for which comprehensive resources are available.[ 43 – 46 ] However, familiarity with PRISMA 2020 is useful when planning and conducting systematic reviews to ensure that all recommended information is captured. PRISMA 2020 should not be used to assess the conduct or methodological quality of systematic reviews; other tools exist for this purpose.[ 30 , 31 ] Furthermore, PRISMA 2020 is not intended to inform the reporting of systematic review protocols, for which a separate statement is available (PRISMA for Protocols (PRISMA-P) 2015 statement [ 47 , 48 ]). Finally, extensions to the PRISMA 2009 statement have been developed to guide reporting of network meta-analyses,[ 49 ] meta-analyses of individual participant data,[ 50 ] systematic reviews of harms, [ 51 ] systematic reviews of diagnostic test accuracy studies, [ 52 ] and scoping reviews [ 53 ]; for these types of reviews we recommend authors report their review in accordance with the recommendations in PRISMA 2020 along with the guidance specific to the extension.
How to use PRISMA 2020
The PRISMA 2020 statement (including the checklists, explanation and elaboration, and flow diagram) replaces the PRISMA 2009 statement, which should no longer be used. Box 2 summarises noteworthy changes from the PRISMA 2009 statement. The PRISMA 2020 checklist includes seven sections with 27 items, some of which include sub-items ( Table 1 ). A checklist for journal and conference abstracts for systematic reviews is included in PRISMA 2020. This abstract checklist is an update of the 2013 PRISMA for Abstracts statement,[ 54 ] reflecting new and modified content in PRISMA 2020 ( Table 2 ). A template PRISMA flow diagram is provided, which can be modified depending on whether the systematic review is original or updated ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
The new design is adapted from flow diagrams proposed by Boers,[ 55 ] Mayo-Wilson et al.[ 56 ] and Stovold et al.[ 57 ] The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
https://doi.org/10.1371/journal.pmed.1003583.g001
https://doi.org/10.1371/journal.pmed.1003583.t001
https://doi.org/10.1371/journal.pmed.1003583.t002
Box 2. Noteworthy changes to the PRISMA 2009 statement
- Inclusion of the abstract reporting checklist within PRISMA 2020 (see item #2 and Table 2 ).
- Movement of the ‘Protocol and registration’ item from the start of the Methods section of the checklist to a new Other section, with addition of a sub-item recommending authors describe amendments to information provided at registration or in the protocol (see item #24a-24c).
- Modification of the ‘Search’ item to recommend authors present full search strategies for all databases, registers and websites searched, not just at least one database (see item #7).
- Modification of the ‘Study selection’ item in the Methods section to emphasise the reporting of how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process (see item #8).
- Addition of a sub-item to the ‘Data items’ item recommending authors report how outcomes were defined, which results were sought, and methods for selecting a subset of results from included studies (see item #10a).
- Splitting of the ‘Synthesis of results’ item in the Methods section into six sub-items recommending authors describe: the processes used to decide which studies were eligible for each synthesis; any methods required to prepare the data for synthesis; any methods used to tabulate or visually display results of individual studies and syntheses; any methods used to synthesise results; any methods used to explore possible causes of heterogeneity among study results (such as subgroup analysis, meta-regression); and any sensitivity analyses used to assess robustness of the synthesised results (see item #13a-13f).
- Addition of a sub-item to the ‘Study selection’ item in the Results section recommending authors cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded (see item #16b).
- Splitting of the ‘Synthesis of results’ item in the Results section into four sub-items recommending authors: briefly summarise the characteristics and risk of bias among studies contributing to the synthesis; present results of all statistical syntheses conducted; present results of any investigations of possible causes of heterogeneity among study results; and present results of any sensitivity analyses (see item #20a-20d).
- Addition of new items recommending authors report methods for and results of an assessment of certainty (or confidence) in the body of evidence for an outcome (see items #15 and #22).
- Addition of a new item recommending authors declare any competing interests (see item #26).
- Addition of a new item recommending authors indicate whether data, analytic code and other materials used in the review are publicly available and if so, where they can be found (see item #27).
We recommend authors refer to PRISMA 2020 early in the writing process, because prospective consideration of the items may help to ensure that all the items are addressed. To help keep track of which items have been reported, the PRISMA statement website ( http://www.prisma-statement.org/ ) includes fillable templates of the checklists to download and complete (also available in S1 PRISMA 2020 checklist ). We have also created a web application that allows users to complete the checklist via a user-friendly interface [ 58 ] (available at https://prisma.shinyapps.io/checklist/ and adapted from the Transparency Checklist app [ 59 ]). The completed checklist can be exported to Word or PDF. Editable templates of the flow diagram can also be downloaded from the PRISMA statement website.
We have prepared an updated explanation and elaboration paper, in which we explain why reporting of each item is recommended and present bullet points that detail the reporting recommendations (which we refer to as elements).[ 41 ] The bullet-point structure is new to PRISMA 2020 and has been adopted to facilitate implementation of the guidance.[ 60 , 61 ] An expanded checklist, which comprises an abridged version of the elements presented in the explanation and elaboration paper, with references and some examples removed, is available in S2 PRISMA 2020 expanded checklist . Consulting the explanation and elaboration paper is recommended if further clarity or information is required.
Journals and publishers might impose word and section limits, and limits on the number of tables and figures allowed in the main report. In such cases, if the relevant information for some items already appears in a publicly accessible review protocol, referring to the protocol may suffice. Alternatively, placing detailed descriptions of the methods used or additional results (such as for less critical outcomes) in supplementary files is recommended. Ideally, supplementary files should be deposited to a general-purpose or institutional open-access repository that provides free and permanent access to the material (such as Open Science Framework, Dryad, figshare). A reference or link to the additional information should be included in the main report. Finally, although PRISMA 2020 provides a template for where information might be located, the suggested location should not be seen as prescriptive; the guiding principle is to ensure the information is reported.
Use of PRISMA 2020 has the potential to benefit many stakeholders. Complete reporting allows readers to assess the appropriateness of the methods, and therefore the trustworthiness of the findings. Presenting and summarising characteristics of studies contributing to a synthesis allows healthcare providers and policy makers to evaluate the applicability of the findings to their setting. Describing the certainty in the body of evidence for an outcome and the implications of findings should help policy makers, managers, and other decision makers formulate appropriate recommendations for practice or policy. Complete reporting of all PRISMA 2020 items also facilitates replication and review updates, as well as inclusion of systematic reviews in overviews (of systematic reviews) and guidelines, so teams can leverage work that is already done and decrease research waste.[ 36 , 62 , 63 ]
We updated the PRISMA 2009 statement by adapting the EQUATOR Network’s guidance for developing health research reporting guidelines.[ 64 ] We evaluated the reporting completeness of published systematic reviews,[ 17 , 21 , 36 , 37 ] reviewed the items included in other documents providing guidance for systematic reviews,[ 38 ] surveyed systematic review methodologists and journal editors for their views on how to revise the original PRISMA statement,[ 35 ] discussed the findings at an in-person meeting, and prepared this document through an iterative process. Our recommendations are informed by the reviews and survey conducted before the in-person meeting, theoretical considerations about which items facilitate replication and help users assess the risk of bias and applicability of systematic reviews, and co-authors’ experience with authoring and using systematic reviews.
Various strategies to increase the use of reporting guidelines and improve reporting have been proposed. They include educators introducing reporting guidelines into graduate curricula to promote good reporting habits of early career scientists [ 65 ]; journal editors and regulators endorsing use of reporting guidelines [ 18 ]; peer reviewers evaluating adherence to reporting guidelines [ 61 , 66 ]; journals requiring authors to indicate where in their manuscript they have adhered to each reporting item[ 67 ]; and authors using online writing tools that prompt complete reporting at the writing stage.[ 60 ] Multi-pronged interventions, where more than one of these strategies are combined, may be more effective (such as completion of checklists coupled with editorial checks).[ 68 ] However, of 31 interventions proposed to increase adherence to reporting guidelines, the effects of only 11 have been evaluated, mostly in observational studies at high risk of bias due to confounding.[ 69 ] It is therefore unclear which strategies should be used. Future research might explore barriers and facilitators to the use of PRISMA 2020 by authors, editors, and peer reviewers, designing interventions that address the identified barriers, and evaluating those interventions using randomised trials. To inform possible revisions to the guideline, it would also be valuable to conduct think-aloud studies [ 70 ] to understand how systematic reviewers interpret the items, and reliability studies to identify items where there is varied interpretation of the items.
We encourage readers to submit evidence that informs any of the recommendations in PRISMA 2020 (via the PRISMA statement website: http://www.prisma-statement.org/ ). To enhance accessibility of PRISMA 2020, several translations of the guideline are under way (see available translations at the PRISMA statement website). We encourage journal editors and publishers to raise awareness of PRISMA 2020 (for example, by referring to it in journal “Instructions to authors”), endorsing its use, advising editors and peer reviewers to evaluate submitted systematic reviews against the PRISMA 2020 checklists, and making changes to journal policies to accommodate the new reporting recommendations. We recommend existing PRISMA extensions [ 47 , 49 – 53 , 71 , 72 ] be updated to reflect PRISMA 2020 and advise developers of new PRISMA extensions to use PRISMA 2020 as the foundation document.
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders. Ultimately, we hope that uptake of the guideline will lead to more transparent, complete, and accurate reporting of systematic reviews, thus facilitating evidence based decision making.
Supporting information
S1 prisma 2020 checklist..
https://doi.org/10.1371/journal.pmed.1003583.s001
S2 PRISMA 2020 expanded checklist.
https://doi.org/10.1371/journal.pmed.1003583.s002
Acknowledgments
We dedicate this paper to the late Douglas G Altman and Alessandro Liberati, whose contributions were fundamental to the development and implementation of the original PRISMA statement.
We thank the following contributors who completed the survey to inform discussions at the development meeting: Xavier Armoiry, Edoardo Aromataris, Ana Patricia Ayala, Ethan M Balk, Virginia Barbour, Elaine Beller, Jesse A Berlin, Lisa Bero, Zhao-Xiang Bian, Jean Joel Bigna, Ferrán Catalá-López, Anna Chaimani, Mike Clarke, Tammy Clifford, Ioana A Cristea, Miranda Cumpston, Sofia Dias, Corinna Dressler, Ivan D Florez, Joel J Gagnier, Chantelle Garritty, Long Ge, Davina Ghersi, Sean Grant, Gordon Guyatt, Neal R Haddaway, Julian PT Higgins, Sally Hopewell, Brian Hutton, Jamie J Kirkham, Jos Kleijnen, Julia Koricheva, Joey SW Kwong, Toby J Lasserson, Julia H Littell, Yoon K Loke, Malcolm R Macleod, Chris G Maher, Ana Marušic, Dimitris Mavridis, Jessie McGowan, Matthew DF McInnes, Philippa Middleton, Karel G Moons, Zachary Munn, Jane Noyes, Barbara Nußbaumer-Streit, Donald L Patrick, Tatiana Pereira-Cenci, Ba’ Pham, Bob Phillips, Dawid Pieper, Michelle Pollock, Daniel S Quintana, Drummond Rennie, Melissa L Rethlefsen, Hannah R Rothstein, Maroeska M Rovers, Rebecca Ryan, Georgia Salanti, Ian J Saldanha, Margaret Sampson, Nancy Santesso, Rafael Sarkis-Onofre, Jelena Savović, Christopher H Schmid, Kenneth F Schulz, Guido Schwarzer, Beverley J Shea, Paul G Shekelle, Farhad Shokraneh, Mark Simmonds, Nicole Skoetz, Sharon E Straus, Anneliese Synnot, Emily E Tanner-Smith, Brett D Thombs, Hilary Thomson, Alexander Tsertsvadze, Peter Tugwell, Tari Turner, Lesley Uttley, Jeffrey C Valentine, Matt Vassar, Areti Angeliki Veroniki, Meera Viswanathan, Cole Wayant, Paul Whaley, and Kehu Yang. We thank the following contributors who provided feedback on a preliminary version of the PRISMA 2020 checklist: Jo Abbott, Fionn Büttner, Patricia Correia-Santos, Victoria Freeman, Emily A Hennessy, Rakibul Islam, Amalia (Emily) Karahalios, Kasper Krommes, Andreas Lundh, Dafne Port Nascimento, Davina Robson, Catherine Schenck-Yglesias, Mary M Scott, Sarah Tanveer and Pavel Zhelnov. We thank Abigail H Goben, Melissa L Rethlefsen, Tanja Rombey, Anna Scott, and Farhad Shokraneh for their helpful comments on the preprints of the PRISMA 2020 papers. We thank Edoardo Aromataris, Stephanie Chang, Toby Lasserson and David Schriger for their helpful peer review comments on the PRISMA 2020 papers.
Patient and public involvement: Patients and the public were not involved in this methodological research. We plan to disseminate the research widely, including to community participants in evidence synthesis organisations.
Author Contributions
JEM and DM are joint senior authors. MJP, JEM, PMB, IB, TCH, CDM, LS, and DM conceived this paper and designed the literature review and survey conducted to inform the guideline content. MJP conducted the literature review, administered the survey and analysed the data for both. MJP prepared all materials for the development meeting. MJP and JEM presented proposals at the development meeting. All authors except for TCH, JMT, EAA, SEB, and LAM attended the development meeting. MJP and JEM took and consolidated notes from the development meeting. MJP and JEM led the drafting and editing of the article. JEM, PMB, IB, TCH, LS, JMT, EAA, SEB, RC, JG, AH, TL, EMW, SM, LAM, LAS, JT, ACT, PW, and DM drafted particular sections of the article. All authors were involved in revising the article critically for important intellectual content. All authors approved the final version of the article. MJP is the guarantor of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
- View Article
- PubMed/NCBI
- Google Scholar
- 25. McKenzie JE, Brennan SE. Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions . Cochrane, 2019 https://doi.org/10.1002/9781119536604.ch12
- 43. Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions : Version 6.0. Cochrane, 2019. Available from https://training.cochrane.org/handbook .
- 45. Cooper H, Hedges LV, Valentine JV, eds. The Handbook of Research Synthesis and Meta-Analysis . Russell Sage Foundation, 2019.
- 46. IOM (Institute of Medicine). Finding What Works in Health Care : Standards for Systematic Reviews . The National Academies Press, 2011.

Literature Reviews: systematic searching at various levels
- for assignments
- for dissertations / theses
- Search strategy and searching
- Boolean Operators
- Search strategy template
- Screening & critiquing
- Citation Searching
- Google Scholar (with Lean Library)
- Resources for literature reviews
- Adding a referencing style to EndNote
- Exporting from different databases
PRISMA Flow Diagram
- Grey Literature
- What is the PRISMA Flow Diagram?
- How should I use it?
- When should I use it?
- PRISMA Links
The PRISMA Flow Diagram is a tool that can be used to record different stages of the literature search process--across multiple resources--and clearly show how a researcher went from, 'These are the databases I searched for my terms', to, 'These are the papers I'm going to talk about'.
PRISMA is not inflexible; it can be modified to suit the research needs of different people and, indeed, if you did a Google images search for the flow diagram you would see many different versions of the diagram being used. It's a good idea to have a look at a couple of those examples, and also to have a look at a couple of the articles on the PRISMA website to see how it has--and can--be used.
The PRISMA 2020 Statement was published in 2021. It consists of a checklist and a flow diagram , and is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document.
In order to encourage dissemination of the PRISMA 2020 Statement, it has been published in several journals.
- How to use the PRISMA Flow Diagram for literature reviews A PDF [3.81MB] of the PowerPoint used to create the video. Each slide that has notes has a callout icon on the top right of the page which can be toggled on or off to make the notes visible.
There is also a PowerPoint version of the document but the file size is too large to upload here.
If you would like a copy, please email the Academic Librarians' mailbox from your university account to ask for it to be sent to you.
This is an example of how you could fill in the PRISMA flow diagram when conducting a new review. It is not a hard and fast rule but it should give you an idea of how you can use it.
For more detailed information, please have a look at this article:
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting,P. & Moher, D. (2021) 'The PRISMA 2020 statement: an updated guideline for reporting systematic reviews', BMJ 372:(71). doi: 10.1136/bmj.n71 .
- Example of PRISMA 2020 diagram This is an example of *one* of the PRISMA 2020 flow diagrams you can use when reporting on your research process. There is more than one form that you can use so for other forms and advice please look at the PRISMA website for full details.
Start using the flow diagram as you start searching the databases you've decided upon.
Make sure that you record the number of results that you found per database (before removing any duplicates) as per the filled in example. You can also do a Google images search for the PRISMA flow diagram to see the different ways in which people have used them to express their search processes.
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. PRISMA focuses on the reporting of reviews evaluating randomized trials, but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions.
- Prisma Flow Diagram This link will take you to downloadable Word and PDF copies of the flow diagram. These are modifiable and act as a starting point for you to record the process you engaged in from first search to the papers you ultimately discuss in your work. more... less... Do an image search on the internet for the flow diagram and you will be able to see all the different ways that people have modified the diagram to suit their personal research needs.
You can access the various checklists via the Equator website and the articles explaining PRISMA and its various extensions are available via PubMed.
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & Moher, D. (2021) ' The PRISMA 2020 statement: an updated guideline for reporting systematic reviews,' BMJ . Mar 29; 372:n71. doi: 10.1136/bmj.n71 .
Page, M.J., Moher, D., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & McKenzie, J.E. (2021) 'PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews', BMJ, Mar 29; 372:n160. doi: 10.1136/bmj.n160 .
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., & Moher, D. (2021) ' The PRISMA 2020 statement: An updated guideline for reporting systematic reviews,' Journal of Clinical Epidemiology, June; 134:178-189. doi: 10.1016/j.jclinepi.2021.03.001 .
- << Previous: Exporting from different databases
- Next: Grey Literature >>
- Last Updated: Mar 12, 2024 3:44 PM
- URL: https://libguides.derby.ac.uk/literature-reviews
- Open access
- Published: 31 July 2023
In search of an international multidimensional action plan for second victim support: a narrative review
- Deborah Seys 1 , 2 ,
- Massimiliano Panella 3 ,
- Sophia Russotto 3 ,
- Reinhard Strametz 4 ,
- José Joaquín Mira 5 , 6 ,
- Astrid Van Wilder 2 ,
- Lode Godderis 2 , 7 &
- Kris Vanhaecht 2 , 8
BMC Health Services Research volume 23 , Article number: 816 ( 2023 ) Cite this article
1196 Accesses
4 Citations
1 Altmetric
Metrics details
Insights around second victims (SV) and patient safety has been growing over time. An overview of the available evidence is lacking. This review aims to describe (i) the impact a patient safety incident can have and (ii) how healthcare professionals can be supported in the aftermath of a patient safety incident.
A literature search in Medline, EMBASE and CINAHL was performed between 1 and 2010 and 26 November 2020 with studies on SV as inclusion criteria. To be included in this review the studies must include healthcare professionals involved in the aftermath of a patient safety incident.
In total 104 studies were included. SVs can suffer from both psychosocial (negative and positive), professional and physical reactions. Support can be provided at five levels. The first level is prevention (on individual and organizational level) referring to measures taken before a patient safety incident happens. The other four levels focus on providing support in the aftermath of a patient safety incident, such as self-care of individuals and/or team, support by peers and triage, structured support by an expert in the field (professional support) and structured clinical support.
The impact of a patient safety incident on healthcare professionals is broad and diverse. Support programs should be organized at five levels, starting with preventive actions followed by self-care, support by peers, structured professional support and clinical support. This multilevel approach can now be translated in different countries, networks and organizations based on their own culture, support history, structure and legal context. Next to this, they should also include the stage of recovery in which the healthcare professional is located in.
Peer Review reports
Introduction
Since the publication of “To Err is Human” by the Institute of Medicine (IOM) back in 1999, attention to patient safety has been surging [ 1 ]. Several actions have been undertaken to reduce the number of preventable errors and patient safety incidents (PSIs) in the hospital. As mentioned in the Global Patient Safety Action Plan 2021–2030 (Strategy 4.4) by the WHO, attention for PSIs continues to be important and it needs to be “ensured that patients, families and health care staff are given ongoing psychological and other support in the aftermath of a serious PSI” [ 2 ]. Next to the ‘first victim’, i.e. the patient and their kin affected by the PSI, the health care staff is considered to be ‘the second victim’ (SV), a term introduced by Wu in 2000 [ 3 ]. Since Wu’s seminal publication in BMJ, research on the impact of a PSI on the SV and on how to support them in the most optimal way has been numerous with collective consensus that the SV should not be treated punitively [ 4 , 5 , 6 , 7 , 8 ].
The term ‘second victims’ has been in use for over two decades and has been on the receiving end of some criticism [ 9 , 10 , 11 , 12 ]. Recently, the definition has been further improved upon by the COST Action on European Researchers’ Network Working on Second Victims (ERNST) [ 13 ] and a SV is now defined as “Any health care worker, directly or indirectly involved in an unanticipated adverse patient event, unintentional healthcare error, or patient injury, and who becomes victimized in the sense that they are also negatively impacted” [ 12 ]. In the aftermath of a PSI, healthcare professionals are highly affected by different psychological and psychosomatic symptoms, e.g. troubling memories, anxiety, anger, remorse, distress and fear of future errors [ 4 , 5 , 14 ].
When a healthcare professional is involved, the symptoms in the aftermath of a PSI can differ dependent on the stage of recovery healthcare professionals find themselves in. Scott et al. described the natural history of recovery in the aftermath of a PSI to first start with a chaos and accident response (1), followed by intrusive reflections (2), restoring personal integrity (3), enduring the inquisition (4), obtaining emotional first aid (5) and finally moving on (6) [ 15 ]. The different recovery stages described are comparable with the different stages of grief in the aftermath of a trauma as described by Kübler-Ross, which are denial, anger, bargaining, depression and acceptance [ 16 ]. This implies that being involved in a PSI is associated with a spiral of emotions [ 17 ]. Finally, a PSI can have a long impact on the healthcare professional which can be negative when symptoms are getting worse or the duration lasts longer than expected [ 18 ].
Since 2006, several support resources have been described in the literature, most established in the United States, trying to help healthcare professionals in dealing with the impact of a PSI [ 19 ]. In 2013, a review with possible support mechanisms was published concluding that support can be provided on the individual and on organizational level. These SV programmes should include support to be provided immediately after PSI occurrence as well as on the middle long and long term [ 4 ]. Scott et al. described the “Scott three-tiered emotional support system”, which includes department support and leadership mentoring (Tier 1), support by peer experts (Tier 2) and professional resources (Tier 3) [ 20 ]. However, the recent attention given towards assessments of a ‘just culture’ indicate that a lot of work is still required to put this into practice [ 21 ]. A recent meta-analysis confirmed that second victims use different types of coping strategies, e.g. being task-oriented or avoidance-oriented, to deal with the emotional impact of a PSI [ 8 , 22 ]. Further actions to increase awareness about the second victim after a PSI and implement support protocols are necessary to improve the complex and multi-layered impact of a PSI [ 23 ].
As the evidence-base around SVs and patient safety has evolved, and several of the available literature reviews were published over ten years ago, the international community lacks an update of the available insights and findings. How healthcare professionals can reduce the impact of a PSI has not been explored within current evidence-base. Therefore, this review wants to describe the different kinds of impact a PSI can cause, not only on a personal and physical level, but also on a professional level. Additionally, it wants to recount which support and prevention strategies are required. The following two research questions were defined: (i) What kind of impact of PSIs on healthcare professionals can be identified? and (ii) How can healthcare professionals be supported in the aftermath of a PSI?
Information sources and search strategy
A literature search was performed in Medline, EMBASE and CINAHL from 1 October 2010 onwards, following on two reviews published by Seys et al. [ 4 , 5 ], and collected literature until 26 November 2020. The following search strategy was employed: (“second victim,” OR “medical error” OR “adverse event”) AND (“psychology” OR “emotions” OR “feelings” OR “burnout” OR “depression” OR “empathy” OR “attitude of health personnel,”) OR “medical error”[MeSH] AND “Burnout, Professional”[MeSH] OR “Depressive Disorder”[MeSH] OR “Empathy”[MeSH] for Medline. For EMBASE: (“second victim,” OR “medical error” OR “adverse event”) AND (“psychology” OR “emotions” OR “feelings” OR “burnout” OR “depression” OR “empathy” OR “attitude of health personnel,”) OR “medical error”/exp AND “professional burnout’/exp OR “depression’/exp OR ‘empathy’/exp. For CINAHL: (“second victim,” OR “medical error” OR “adverse event”) AND (“psychology” OR “emotions” OR “feelings” OR “burnout” OR “depression” OR “empathy” OR “attitude of health personnel,”). This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standard [ 24 ]. The studies should include healthcare professionals involved in a patient safety incident (PSI), where a PSI is defined as “an event or circumstance that could have resulted, or did result, in unnecessary harm to a patient.” [ 25 ].
Eligibility criteria
Studies were included if any of the following criteria were met. (i) They described a definition or described the term second victims. (ii) They mentioned the prevalence of health care professionals involved in a PSI, which could have occurred over the time span of their career or during a well-defined period. (iii) They studied the impact of a PSI on the involved health care professional without restrictions regarding the level of impact or harm. (iv) Finally, studies were included if they assessed what support was provided and/or needed in the aftermath of a PSI.
We excluded studies not published in English, reviews, conference reports, newspaper stories, and anecdotal evidence. Publications on analyses occurring during the COVID-19 pandemic were excluded due to the fact that these publications are no longer able to purely distinguish the effect of a PSI on the second victim, but have COVID-19 as a confounding factor.
Article screening
All citations were imported into the citations manager, Endnote X9, and duplicates were initially removed by this citation manager. Duplicates found during the title analyses were removed manually. In the next phase, the titles and abstracts were first screened by two reviewers (DS, KV) to eliminate unrelated studies. Any discrepancies were resolved by two other investigators (MP, SR). For all remaining relevant articles, the full text was retrieved, and two reviewers examined them independently according to the eligibility criteria.
Qualitative analysis: data extraction and data synthesis
Data were extracted from the full text by a single investigator (DS) using a data extraction sheet. The sheet included study summary information (authors, country, year of study), study design, patient population reports (sample size, type of respondents) and outcome measurements (impact of PSIs, support in the aftermath of a PSI). The outcome measurements related to the impact of a PSI were clustered by the researchers into psychosocial reactions, positive professional reactions, negative professional reactions or physical reactions which the authors defined before the study.Each of the outcomes related to support in the aftermath of a PSI were extracted from each article. Based on these results the support in the aftermath of a PSI could be clustered into five separate levels, whereby clustering occurred on the degree of support received by SVs. The data extraction was thoroughly discussed with the members of the review team (DS, KV, SR, MP).
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Figure 1 illustrates the outcome of the search process. A total of 120,635 titles were identified after the initial search. After the removal of duplicates and title, abstract and full text analysis, 104 studies remained.

PRISMA flow diagram
Description of the included studies
Out of the 104 included publications, eight involved opinion papers (Hauk et al. 2018 [ 26 ], MacLeod 2014 [ 27 ], Petersen 2019 [ 28 ], Saver 2013 [ 29 ], Schrøder et al. 2019 [ 30 ], Scott et al. 2016 [ 15 ], Scott 2015 [ 31 ], Smetzer 2012 [ 32 ]), six were perspectives (Ahc 2019 [ 10 ], Tebala 2020 [ 33 ], Tamburri et al. 2017 [ 34 ], Gómez-Durán et al. 2019 [ 35 ], Shapiro et al. 2016 [ 36 ], Slykerman et al. 2019 [ 37 ]), six were editorials (Anderson et al. [ 38 ], Clarkson et al. 2019 [ 11 ], Edrees et al. 2015 [ 39 ], Everly Jr et al. 2020 [ 40 ], Thompson et al. 2015 [ 41 ], Sataloff 2020 [ 42 ]) and one was a case study (Rappaport et al. 2019 [ 43 ]). The other 83 publications were research articles (Supplementary File 1 ). Most studies were performed in the USA (n = 30), followed by Iran (n = 5), Canada (n = 5) and China (n = 4). More than half of the studies were observational studies and one out of four were qualitative studies. More than 9 out of 10 studies were in-hospital studies of which 25% were multi-centre studies.
What kinds of impact of a PSI on healthcare professionals could be identified?
Symptoms can involve psychosocial reactions, professional reactions or a physical response. The PSI could have had an impact on the mental health of the healthcare professional as a SV, leading to a wave of emotions, or a psychosocial reaction. These psychosocial reactions can for example be. feelings of guilt, sleep disturbances, anxiety or fear. Additionally, both negative and positive impacts on their way of working can emerge, Negative professional reactions can e.g. be wanting to change wards, decrease professional self-efficacy or loss of confidence. Examples of positive professional reactions include raised attention, being more careful or being more critical/self-critical. Last, physical health, mentioned by physical response have been described including e.g. eating disorder, headache or muscle tension. The details of these reactions can be found in Supplementary Files 2 – 5 . Figure 2 provides an overview of the most commonly reported psychosocial, negative and positive reactions. Three studies mentioned that some healthcare professionals were not at all impacted by a PSI [ 18 , 44 , 45 ]. In contrast, other studies have posed that a PSI will always affect the involved healthcare professionals [ 46 ].

Most reported psychosocial, negative and positive professional reactions based on number of studies
How can healthcare professionals be supported in the aftermath of a PSI?
For the optimal support in the aftermath of a PSI, actions should be undertaken at five -levels (Fig. 3 , Supplementary File 6 ). The first level includes prevention on both the individual healthcare professional level and the organization level. Several actions can be taken in advance which can lead to a lower impact in the aftermath of a PSI. Examples include investments in good relationships with colleagues [ 47 ]; being a supportive and/or an active listener [ 26 , 39 , 43 , 46 , 48 , 49 , 50 ]; having an environment with no punitive response, where blame is avoided, an environment that is family oriented, or a just and no-macho culture [ 30 , 39 , 45 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ]; and being educated about the concept of the second victim and related feelings [ 30 , 48 , 50 , 54 , 59 , 60 , 61 ]. The second level includes self-care of the healthcare individual and team and starts when a PSI had occurred [ 17 , 31 , 36 , 42 , 43 , 46 , 47 , 48 , 49 , 50 , 52 , 55 , 57 , 58 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 ]. Some examples of support in this level are: wanting to talk in great detail about PSI/discuss the PSI [ 17 , 46 , 50 , 54 ], trying to understand what happened and how to avoid the PSI in the future [ 31 , 36 , 43 , 46 , 50 , 52 , 73 , 75 ], looking for support or feedback by supervisor/senior person [ 31 , 43 , 47 , 48 , 49 , 50 , 55 , 57 , 63 , 67 , 68 , 69 , 70 , 82 , 83 ]. If this level is insufficient, support by peers and triage [ 15 , 17 , 30 , 31 , 39 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 62 , 63 , 64 , 65 , 66 , 67 , 69 , 70 , 71 , 72 , 73 , 74 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 ] (Level 3) can be provided, which is, if necessary, followed by structured professional support [ 39 , 42 , 43 , 45 , 46 , 47 , 49 , 50 , 52 , 54 , 56 , 57 , 58 , 61 , 64 , 67 , 69 , 73 , 76 , 78 , 79 , 80 , 81 , 87 , 89 , 90 , 92 , 93 ] (Level 4) and clinical support (Level 5). In level 3 (Support by peers and triage), healthcare professionals want e.g. timely support or support soon in the aftermath of a PSI [ 15 , 31 , 39 , 54 , 64 , 87 ], support which is available by hotline, telephone, email, intranet [ 81 , 85 , 86 ] and they want to be informed about the next steps in the hospital’s process for follow-up a PSI [ 31 , 46 , 52 , 60 , 76 , 78 , 79 , 90 , 91 , 92 ]. The structured professional support (Level 4), is support given by an expert in the field and can include e.g. mental health support [ 39 , 42 , 45 , 52 , 58 , 64 , 69 , 73 , 76 , 78 , 79 , 93 ] or access to specialized support (Formal organizational support) [ 39 , 56 , 57 , 92 ], but can also be debriefings [ 39 , 43 , 47 , 50 , 52 , 61 , 76 , 78 , 79 , 80 , 87 ] or mortality and morbidity meetings/clinical incident reviews [ 45 , 50 , 81 , 89 ]. In general it treats the second victim as a person with a condition that requires e.g. short-term psychotherapy or psychosocial support, while clinical support (Level 5) treats the second victim as having a disease treatable with medication [ 32 , 42 , 44 , 48 , 52 , 67 , 73 , 76 , 93 , 94 ] and long-term psychotherapy [ 32 , 42 , 44 , 48 , 52 , 67 , 73 , 76 , 80 , 81 , 93 , 94 ].

Overview of the five-level of support
Next to this, national and local initiatives exist that include several levels of support [ 37 , 49 , 50 , 54 , 56 , 57 , 64 , 69 , 73 , 81 , 86 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 ] (Supplementary File 7 ).
Methodological observations
Although professional and psychosocial reactions are described in many publications, only three validated questionnaires that evaluated the impact of a PSI could be identified [ 68 , 78 , 79 , 91 , 120 , 121 , 122 ]. First, the Second Victim Experience and Support Tool (SVEST) is increasingly being implemented as a questionnaire in second victim research [ 68 , 69 , 123 ]. The SVEST was originally validated in the United States and contains 29 items within seven psychosocial domains (psychological stress, physical distress, colleague support, supervisor support, institutional support, non-work-related support, professional self-efficacy) and two employment-related domains or outcomes (turnover intentions and absenteeism) [ 68 , 121 , 122 , 123 ]. The questionnaire is available in English [ 69 ] and evaluates psychological distress, physical distress, professional self-efficacy, colleague support, supervisor support, institutional support, non-work-related support, turnover intentions, and absenteeism [ 68 , 121 , 122 , 123 ]. Another questionnaire often applied in second victim research, is the Impact of Event Scale (IES). This evaluates the subjective distress caused by traumatic events such as PSIs [ 91 , 120 ]. Last, the Second Victims im Deutschsprachigen Raum (Second Victims in German-speaking Countries) (SeViD) questionnaire was developed in Germany and Austria and covers prevalence, symptoms and support strategies of the second victim phenomenon [ 78 , 79 ].
This is the first review focusing on preventive actions which can be taking in advance of a PSI, both by healthcare professionals and by organizations. Additionally our results showed that a difference should be made in different levels of professional support, whereby support can be either structured professionally for the SV on the short and middle term, or could be clinical, with medication or clinical-related aid on the long term. This lead to a shift of the “Scott three-tiered emotional support system towards a five-level support. These results are based on reviewing a total of 104 articles across different healthcare professionals, healthcare settings and countries. Based on the differences in published psychosocial and professional reactions, we argue that no general detailed support program is available for second victims, but that the actions should be based on the needs of the involved individual healthcare professional and should happen at different levels.
In general, the impact of a PSI on healthcare professionals is diverse, ranging from no reactions at all to psychosocial, negative and positive professional reactions, or physical reactions, which on their own could become an obstacle to patient safety. Previous studies showed that the greater the harm to the patient, the more intense the SV phenomenon is, culminating to the most severe symptoms in PSIs [ 22 ]. Healthcare organizations should also take into account that trainees and students can also become SVs in the aftermath of a PSI [ 62 , 66 , 81 , 124 , 125 ].
Only a few instruments to measure the impact of a PSI are published and the most common instrument is the Second Victim Experience and Support Tool (SVEST)., [ 68 , 121 , 122 , 123 ] In the last two years, this instrument has been validated in several languages, e.g. Danish [ 126 ], Spanish [ 127 ], German [ 128 ]; Korean [ 63 ], Argentinian [ 68 ], Chinese [ 71 ], Italian [ 129 ], Malaysian [ 130 ] and Persian [ 121 ]. A revised version of the instrument (SVEST-R) was developed and validated in December 2020, right after the inclusion date of this review. It was first published in English [ 123 ], followed by publication of a German version [ 79 ]. The revised instrument comprises 35 items and has adapted the original domain on ‘non-work-related support’ to ‘resilience’. Additionally, seven items were added regarding desirability of second victim support options. Some of the items in the SVEST and SVEST-R questionnaire are scored inversely to reduce bias [ 131 ]. The SVEST and SVEST-R questionnaires include support and impact items by using these questionnaires in practice. This implies that the results of the questionnaire should be presented at all levels of the organization, not only on how to improve support in general to management and board, but also to supervisors and colleagues. As healthcare professionals go through different stages in their recovery [ 15 ], the SVEST or SVEST-R questionnaire should be taken at different timepoints to check the healthcare professional’s needs. This leads to the question if it is possible to translate one instrument into different languages. In other words: are these questionnaires sufficiently sensitive to capture the differences in culture, language and reactions of the healthcare professionals in the aftermath of a PSI over time? Healthcare professionals go through different stages in the aftermath of a PSI [ 15 , 16 , 17 ] and each stage can differ in time but can also need a different kind of support. For example, 23,6% of the involved healthcare professionals are still hypervigilant, 8,7% continue to have flashbacks, 8,2% have feelings of shame and 8,1% doubt their knowledge and skills six months after a PSI [ 18 ]. If countries, network or organizations want to set up a peer-support protocol, they should not start from scratch, but they should rely on the multilevel approach presented in this paper (see Fig. 3 ). However, these programs could be adapted based on their own cultural context and specific needs of the SV during the different stages of their recovery.
It is still unclear how many healthcare professionals are in need of a specific level of support according to our five-level support system (Fig. 3 ) in the aftermath of a PSI. Our five-level support system is an enlargement of the “Scott three-tiered emotional support system”, The “Scott three-tiered emotional support system” showed that 60% of the SVs found that the support given by colleagues and peers is very helpful and that there was no need for specially trained peer-support [ 20 ]. This review mentioned different prevention strategies which can be used by the individual healthcare professional or by the organization. A safety culture promoting support for emotional needs and increasing awareness of the SV phenomenon are seen as important preventive actions [ 132 ]. An online program such as the ‘Mitigating Impact in Second Victims (MISE) online program’ can help to improve the knowledge about PSIs, possible support models and actions in the aftermath of a PSI [ 133 ]. Professional treatment for SVs doesn’t immediately include medication or clinically-related treatment, it can also include structured professional support. However, if structured professional support is not sufficient, the next step could be treating the involved healthcare professional clinically or with medication. Interventions including visuospatial cognitive ‘Tetris’-like tasks could be implemented to reduce intrusive memories, which are common after PSIs [ 134 ]. Other methods such as cognitive processing therapy (CPT), prolonged exposure therapy (PE) and eye movement, desensitization, and restructuring (EMDR) are the current golden standards for the treatment of trauma-associated symptoms of post-traumatic stress disorder [ 135 ].
Next to learning from other SV support literature, the post-COVID era provides opportunities to enhance the SV evidence-base. A study comparing the reaction in the aftermath of a PSI by doctors and nurses with the reactions during COVID-19 showed that the impact on doctors and nurses is larger during COVID-19 than in the aftermath of a PSI [ 136 ]. Most organizations have set up a support program for their healthcare professionals during the COVID-19 pandemic, which implies that these program can be extended to SVs. Not only the level of PSIs can have an impact on the reactions, being involved in formal complaints or lawsuits can put an additional layer on the personal and professional reactions of the involved healthcare professionals [ 137 , 138 ].
Some studies have shown positive reactions in the aftermath of a PSI, such as raised attention or becoming more critical. During the COVID-19 pandemic, it was shown that the pandemic can be a trigger for developing posttraumatic growth, as it is disruptive enough to affect the individual’s values and perspectives [ 139 ]. Being involved in a PSI can similarly be disruptive enough, meaning there is potential for posttraumatic growth for SVs, while for others the disruption can be a possible reason why they want to leave profession.
Strengths and limitations
The strength of this review is that it draws together and reports all the possible reactions and support in the aftermath of a PSI. We chose to extract the reactions ad verbatim from the primary articles as to not lose subtle nuances that might depend on the language or culture of the respondents. Due to this type of research, recall bias in reactions is possible due to the fact that the symptoms were collected after the PSI had happened. These reactions were collected by (validated) questionnaires or based on qualitative research. For this reason the results of the symptoms should be interpreted by general trends and with caution. Other limitations of this review are caused by the heterogeneity of the included studies, in terms of type and severity of adverse events studied, involved healthcare professionals and types of study design. A critical appraisal of the individual studies was not performed as the aim of our study was to have a broad overview of both the reactions and support in the aftermath of a PSI.Next to this, the studies have been carried out in countries with different legal frameworks, which may affect the scope and type of intervention. Besides this, some of the studies have a small sample size or were performed at one organization which limits the generalizability of the results. Aspects of the safety culture of the individual organization should be considered when interpreting and implementing these results as differences between countries may also play a role.
Recommendations
Further research is needed to optimize the validated instruments which measure the impact and support mechanisms for healthcare professionals in the aftermath of a PSI. This five-level support system can be used for longitudinal research but can also evaluate the impact and support needed in the aftermath of different types of PSI and different levels of support, ranging from prevention to professional and cultural support. Age, gender, professional group, working experience and personality dimensions should also be included to understand the SV phenomenon better and give the most optimal support to them. Therefore, knowledge sharing networks within and across countries should be organized. Besides measuring cultural differences, possible reactions and their duration, interventional research is needed, including randomized controlled trials to solidify different interventions at different stages of recovery and evaluate the effectiveness of the interventions.
Conclusions
The impact of a PSI on healthcare professionals is broad, ranging from psychosocial and physical reactions to negative and positive professional reactions. This review shows that support programs should be organized at five levels. It should start with preventive actions followed by self-care of the healthcare professionals, support by peers, structured professional support and clinical support. Based on this, the involved healthcare professionals can be supported in the most optimal way in the aftermath of a PSI. Finally, organizations and healthcare professionals should take preventive actions to reduce the impact of a PSI and should provide support based on the individual needs of each healthcare professional in the aftermath of a PSI and should evolve in time based on the different stages of recovery. As each SV is a unique individual, we should be careful in implementing standard protocols or generalized approaches and provide support fitting for the SV’s personal reaction and recovering stage.
Data Availability
All data presented in this study are available in results section. Deborah Seys can be contacted if there are questions about it.
Kohn LT, Corrigan JM, Donaldson MS. To err Is Human: Building A Safer Health System. Washington DC: National Academic Press; 1999.
Google Scholar
World Health Organization. World Health Organization Global Patient Safety Action Plan 2021–2030. Towards zero patients harm in Healthcare. In.; 2020.
Wu AW. Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–7.
Article CAS PubMed PubMed Central Google Scholar
Seys D, Scott S, Wu A, Van Gerven E, Vleugels A, Euwema M, Panella M, Conway J, Sermeus W, Vanhaecht K. Supporting involved health care professionals (second victims) following an adverse health event: a literature review. Int J Nurs Stud. 2013;50(5):678–87.
Article PubMed Google Scholar
Seys D, Wu AW, Gerven EV, Vleugels A, Euwema M, Panella M, Scott SD, Conway J, Sermeus W, Vanhaecht K. Health Care Professionals as second victims after adverse events: a systematic review. Eval Health Prof. 2013;36(2):135–62.
Quadrado ERS, Tronchin DMR, Maia FOM. Strategies to support health professionals in the condition of second victim: scoping review. Rev Esc Enferm USP. 2021;55:e03669.
Harrison R, Johnson J, McMullan RD, Pervaz-Iqbal M, Chitkara U, Mears S, Shapiro J, Lawton R. Toward constructive change after making a medical error: recovery from situations of Error Theory as a Psychosocial Model for Clinician Recovery. J Patient Saf 2022, 18(6).
Busch IM, Moretti F, Purgato M, Barbui C, Wu AW, Rimondini M. Dealing with adverse events: a Meta-analysis on second victims’ coping strategies. J Patient Saf 2020, 16(2).
Tumelty ME. The second victim: a contested term? J Patient Saf 2018.
Ahc M. Second victim’ may not be the Best Approach to adverse events. Healthc Risk Manage. 2019;41(9):NPAG–NPAG.
Clarkson MD, Haskell H, Hemmelgarn C, Skolnik PJ. Abandon the term “second victim”. BMJ (Online) 2019, 364.
Vanhaecht K, Seys D, Russotto S, Strametz R, Mira J, Sigurgeirsdóttir S, Wu AW, Põlluste K, Popovici DG, Sfetcu R, et al. An evidence and Consensus-Based definition of second victim: a Strategic Topic in Healthcare Quality, Patient Safety, Person-Centeredness and Human Resource Management. Int J Environ Res Public Health. 2022;19(24):16869.
Article PubMed PubMed Central Google Scholar
The European Researchers’ Network Working on Second Victims. [ https://cost-ernst.eu/about/ ]. Accessed 15 Jul 2023
Busch IM, Moretti F, Purgato M, Barbui C, Wu AW, Rimondini M. Psychological and psychosomatic symptoms of second victims of adverse events: a systematic review and Meta-analysis. J Patient Saf. 2020;16(2):e61–e74.
Scott SD, McCoig MM. Care at the point of impact: insights into the second-victim experience. J Healthc risk management: J Am Soc Healthc Risk Manage. 2016;35(4):6–13.
Article Google Scholar
Kübler-Ross E, Byock I. On death and dying: what the Dying have to teach doctors, nurses, clergy and their own families. Manhattan: Scribner; 2014.
Luu S, Patel P, St-Martin L, Leung ASO, Regehr G, Murnaghan ML, Gallinger S. Moulton C-a: waking up the next morning: surgeons’ emotional reactions to adverse events. Med Educ. 2012;46(12):1179–88.
Vanhaecht K, Seys D, Schouten L, Bruyneel L, Coeckelberghs E, Panella M, Zeeman G. Duration of second victim symptoms in the aftermath of a patient safety incident and association with the level of patient harm: a cross-sectional study in the Netherlands. BMJ Open. 2019;9(7):e029923.
Busch IM, Moretti F, Campagna I, Benoni R, Tardivo S, Wu AW, Rimondini M. Promoting the Psychological Well-Being of Healthcare Providers facing the Burden of adverse events: a systematic review of second victim support resources. Int J Environ Res Public Health. 2021;18(10):5080.
Scott SD, Hirschinger LE, Cox KR, McCoig M, Hahn-Cover K, Epperly KM, Phillips EC, Hall LW. Caring for our own: deploying a systemwide second victim Rapid Response Team. Joint Comm J Qual Patient Saf. 2010;36(5):233–40.
White RM, Delacroix R. Second victim phenomenon: is ‘just culture’ a reality? An integrative review. Appl Nurs Res. 2020;56:151319.
Kappes M, Romero-García M, Delgado-Hito P. Coping strategies in health care providers as second victims: a systematic review. Int Nurs Rev. 2021;68(4):471–81.
Liukka M, Steven A, Moreno MFV, Sara-Aho AM, Khakurel J, Pearson P, Turunen H, Tella S. Action after adverse events in Healthcare: an integrative literature review. Int J Environ Res Public Health. 2020;17(13):4717.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
World Health Organization.: The Conceptual Framework for the International Classification for Patient Safety. Version 1.1. Final Technical Report. 2009.
Hauk L. Support strategies for health care professionals who are second victims. AORN J. 2018;107(6):P7–P9.
MacLeod L. Second victim” casualties and how physician leaders can help. Phys Exec. 2014;40(1):8–12.
Petersen IG. The term “second victim” is appropriate for frontline workers. BMJ. 2019;365:l2157.
Saver C. Second victim’ rapid-response team helps fellow clinicians recover from trauma. OR Manager. 2013;29(5):10–2.
Schrøder K, Hvidt NC, Jørgensen JS, Lamont RF, Jørgensen JS, Lamont RF, Hvidt NC. Second victims need emotional support after adverse events: even in a just safety culture. BJOG: An International Journal of Obstetrics & Gynaecology. 2019;126(4):440–2.
Scott SD. The second victim experience: mitigating the harm. Am Nurse Today. 2015;10(9):8–11.
Smetzer J. Don’t abandon the “second victims” of medical errors. Nursing. 2012;42(2):54–8.
Tebala GD. Is there a standard reaction of surgeons to surgical complications? Study on an interesting historical case. Med Hypotheses. 2020;144:110006.
Tamburri LM. Creating healthy work environments for second victims of adverse events. AACN Adv Crit Care. 2017;28(4):366–74.
Gómez-Durán EL, Tolchinsky G, Martin-Fumadó C, Arimany-Manso J. Neglecting the “second victim” will not help harmed patients or improve patient safety. BMJ. 2019;365:l2167.
Shapiro J, Galowitz P. Peer support for Clinicians: a Programmatic Approach. Acad medicine: J Association Am Med Colleges. 2016;91(9):1200–4.
Slykerman G, Wiemers MJ, Wyssusek KH. Peer support in anaesthesia: development and implementation of a peer-support programme within the Royal Brisbane and Women’s Hospital Department of Anaesthesia and Perioperative Medicine. Anaesth Intensive Care. 2019;47(6):497–502.
Anderson J, Gianola FJ. After the error, then what? The emotional impact of errors on clinicians. JAAPA: J Am Acad Physician Assistants (Haymarket Media Inc). 2011;24(12):71–2.
Edrees H, Federico F. Supporting clinicians after medical error. BMJ (Online) 2015, 350.
Everly GS Jr. Psychological first aid to support healthcare professionals. J Patient Saf Risk Manage. 2020;25(4):159–62.
Thompson CV, Suggett N, Fellows J. Must “second victims” always be in the wrong? BMJ (Online) 2015, 350.
Sataloff RT. Adverse Surgical events: Effects on the Surgeon. Ear Nose and Throat Journal. 2020;99(4):225–6.
Rappaport DI, Selbst SM, Mull CC. Medical errors and malpractice lawsuits: impact on Providers- Part 2 of 6. Pediatr Emerg Care. 2019;35(6):440–2.
McLennan SR, Engel-Glatter S, Meyer AH, Schwappach DLB, Scheidegger DH, Elger BS. The impact of medical errors on swiss anaesthesiologists: a cross-sectional survey. Acta Anaesthesiol Scand. 2015;59(8):990–8.
Article CAS PubMed Google Scholar
Biggs S, Waggett HB, Shabbir J. Impact of surgical complications on the operating surgeon. Colorectal Dis 2020.
Ullström S, Sachs MA, Hansson J, Øvretveit J, Brommels M. Suffering in silence: a qualitative study of second victims of adverse events. BMJ Qual Saf. 2014;23(4):325–31.
Schrøder K, Edrees HH, Christensen RD, Jørgensen JS, Lamont RF, Hvidt NC. Second victims in the labor ward: are danish midwives and obstetricians getting the support they need? Int J Qual Health Care. 2019;31(8):583–9.
PubMed Google Scholar
Rinaldi C, Leigheb F, Vanhaecht K, Donnarumma C, Panella M. Becoming a “second victim” in health care: pathway of recovery after adverse event. Rev Calid Asist. 2016;31(Suppl 2):11–9.
Dukhanin V, Edrees HH, Connors CA, Kang E, Norvell M, Wu AW. Case: a second victim support program in Pediatrics: Successes and Challenges to implementation. J Pediatr Nurs. 2018;41:54–9.
Kobe C, Blouin S, Moltzan C, Koul R. The second victim phenomenon: perspective of canadian Radiation therapists. J Med Imaging Radiation Sci. 2019;50(1):87–97.
Pinto A, Faiz O, Bicknell C, Vincent C. Acute traumatic stress among surgeons after major surgical complications. Am J Surg. 2014;208(4):642–7.
Joesten L, Cipparrone N, Okuno-Jones S, DuBose ER. Assessing the Perceived Level of Institutional Support for the second victim after a Patient Safety Event. J Patient Saf. 2015;11(2):73–8.
Treiber LA, Jones JH. Making an infusion error: the second victims of infusion therapy-related medication errors. J Infus Nurs. 2018;41(3):156–63.
Edrees H, Connors C, Paine L, Norvell M, Taylor H, Wu AW. Implementing the RISE second victim support programme at the Johns Hopkins Hospital: a case study. BMJ Open 2016, 6(9).
Harrison R, Sharma A, Walton M, Esguerra E, Onobrakpor S, Nghia BT, Chinh ND. Responding to adverse patient safety events in Viet Nam. BMC Health Serv Res. 2019;19(1):677.
Merandi J, Winning AM, Liao N, Rogers E, Lewe D, Gerhardt CA. Implementation of a second victim program in the neonatal intensive care unit: an interim analysis of employee satisfaction. J Patient Saf Risk Manage. 2018;23(6):231–8.
Mok WQ, Chin GF, Yap SF, Wang W. A cross-sectional survey on nurses’ second victim experience and quality of support resources in Singapore. J Nurs Manag. 2020;28(2):286–93.
Han K, Bohnen JD, Peponis T, Martinez M, Nandan A, Yeh DD, Lee J, Demoya M, Velmahos G, Kaafarani HMA. The Surgeon as the second victim? Results of the Boston intraoperative adverse events Surgeons’ attitude (BISA) Study. J Am Coll Surg. 2017;224(6):1048–56.
Edrees H, Brock DM, Wu AW, McCotter PI, Hofeldt R, Shannon SE, Gallagher TH, White AA. The experiences of risk managers in providing emotional support for health care workers after adverse events. J Healthc Risk Manage. 2016;35(4):14–21.
Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf/Jt Comm Resour. 2012;38(5):235–40.
Chung AS, Smart J, Zdradzinski M, Roth S, Gende A, Conroy K, Battaglioli N. Educator Toolkits on second victim syndrome, mindfulness and meditation, and positive psychology: the 2017 Resident Wellness Consensus Summit. Western J Emerg Medicine: Integrating Emerg Care Popul Health. 2018;19(2):327–31.
Huang H, Chen J, Xiao M, Cao S, Zhao Q. Experiences and responses of nursing students as second victims of patient safety incidents in a clinical setting: a mixed-methods study. J Nurs Manage (John Wiley Sons Inc). 2020;28(6):1317–25.
Kim EM, Kim SA, Lee JR, Burlison JD, Oh EG. Psychometric Properties of Korean Version of the second victim experience and Support Tool (K-SVEST). J Patient Saf. 2020;16(3):179–86.
Stone M. Second victim support: nurses’ perspectives of Organizational Support after an adverse event. J Nurs Adm. 2020;50(10):521–5.
Tan R, Luo K, Hu D, Zhao Y, Han Y, Xu K. Inpatient suicide second victim experience and Support Tool: psychometric properties of a scale for nurses who experienced inpatient suicide at chinese general hospitals. Nurs Health Sci 2020.
Pijl Zieber M, Williams B. The experience of nursing students who make mistakes in clinical. Int J Nurs Educ Scholarsh. 2015;12(1):1–9.
Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101–7.
Brunelli MV, Estrada S, Celano C. Cross-cultural adaptation and psychometric evaluation of a second victim experience and Support Tool (SVEST). J Patient Saf 2018.
Burlison JD, Scott SD, Browne EK, Thompson SG, Hoffman JM. The second victim experience and Support Tool: validation of an Organizational Resource for assessing second victim Effects and the quality of Support Resources. J Patient Saf. 2017;13(2):93–102.
Zhang X, Li Q, Guo Y, Lee SY. From organisational support to second victim-related distress: role of patient safety culture. J Nurs Manage (John Wiley Sons Inc). 2019;27(8):1818–25.
Article CAS Google Scholar
Zhang X, Chen J, Lee SY. Psychometric testing of the chinese version of second victim experience and Support Tool. J Patient Saf 2020.
Winning AM, Merandi JM, Lewe D, Stepney LMC, Liao NN, Fortney CA, Gerhardt CA. The emotional impact of errors or adverse events on healthcare providers in the NICU: the protective role of coworker support. J Adv Nurs. 2018;74(1):172–80.
Kable A, Kelly B, Adams J. Effects of adverse events in health care on acute care nurses in an australian context: a qualitative study. Nurs Health Sci. 2018;20(2):238–46.
Chen J, Yang Q, Zhao Q, Zheng S, Xiao M. Psychometric validation of the chinese version of the second victim experience and Support Tool (C-SVEST). J Nurs Manage (John Wiley Sons Inc). 2019;27(7):1416–22.
Koehn AR, Ebright PR, Draucker CB. Nurses’ experiences with errors in nursing. Nurs Outlook. 2016;64(6):566–74.
Edrees HH, Morlock L, Wu AW. Do hospitals support second victims? Collective insights from patient safety leaders in Maryland. Joint Comm J Qual Patient Saf. 2017;43(9):471–83.
Harrison R, Lawton R, Stewart K. Doctors’ experiences of adverse events in secondary care: the professional and personal impact. Clin Med J Royal Coll Physicians Lond. 2014;14(6):585–90.
Strametz R, Koch P, Vogelgesang A, Burbridge A, Rösner H, Abloescher M, Huf W, Ettl B, Raspe M. Prevalence of second victims, risk factors and support strategies among young german physicians in internal medicine (SeViD-I survey). J Occup Med Toxicol. 2021;16(1):11–1.
Strametz R, Siebold B, Heistermann P, Haller S, Bushuven S. Validation of the german version of the second victim experience and Support Tool-Revised. J Patient Saf 2021.
Graham P, Zerbi G, Norcross W, Montross-Thomas L, Lobbestael L, Davidson J. Testing of a Caregiver Support Team. Explore. 2019;15(1):19–26.
El Hechi MW, Bohnen JD, Westfal M, Han K, Cauley C, Wright C, Schulz J, Mort E, Ferris T, Lillemoe KD, et al. Design and impact of a novel surgery-specific second victim peer support program. J Am Coll Surg. 2020;230(6):926–33.
Abusalem SK, Coty M-B. Home health nurses coping with practice care errors. J Res Nurs. 2013;18(4):336–48.
Deringer E, Caligor E. Supervision and responses of psychiatry residents to adverse patient events. Acad Psychiatry. 2014;38(6):761–7.
Finney RE, Torbenson VE, Riggan KA, Weaver AL, Long ME, Allyse MA, Rivera-Chiauzzi E. Second victim experiences of Nurses in Obstetrics and Gynecology: a SVEST Survey. J Nurs Adm Manag 2020.
Merandi J, Liao N, Lewe D, Morvay S, Stewart B, Catt C, Scott SD. Deployment of a second victim peer support program: a replication study. Pediatr Qual Saf. 2017;2(4):e031.
White AA, Brock DM, McCotter PI, Hofeldt R, Edrees HH, Wu AW, Shannon S, Gallagher TH. Risk managers’ descriptions of programs to support second victims after adverse events. J Healthc risk management: J Am Soc Healthc Risk Manage. 2015;34(4):30–40.
Edrees HH, Wu AW. Does one size fit all? Assessing the need for organizational second victim support programs. J Patient Saf 2017.
Mohsenpour M, Hosseini M, Abbaszadeh A, Shahboulaghi FM, Khankeh H. Iranian nurses’ experience of “being a wrongdoer”: a phenomenological study. Nurs Ethics. 2018;25(5):653–64.
Vinson AE, Mitchell JD. Assessing levels of support for residents following adverse outcomes: a national survey of anesthesia residency programs in the United States. Med Teach. 2014;36(10):858–66.
Van Gerven E, Deweer D, Scott SD, Panella M, Euwema M, Sermeus W, Vanhaecht K. Personal, situational and organizational aspects that influence the impact of patient safety incidents: a qualitative study. Revista de calidad asistencial: organo de la Sociedad Espanola de Calidad Asistencial. 2016;31:34–46.
Van Gerven E, Bruyneel L, Panella M, Euwema M, Sermeus W, Vanhaecht K. Psychological impact and recovery after involvement in a patient safety incident: a repeated measures analysis. BMJ Open 2016, 6(8).
Mira JJ, Lorenzo S, Carrillo I, Ferrús L, Pérez-Pérez P, Iglesias F, Silvestre C, Olivera G, Zavala E, Nuño-Solinís R, et al. Interventions in health organisations to reduce the impact of adverse events in second and third victims. BMC Health Serv Res. 2015;15:341.
Gupta K, Lisker S, Rivadeneira NA, Mangurian C, Linos E, Sarkar U. Decisions and repercussions of second victim experiences for mothers in medicine (SAVE DR MoM). BMJ Qual Saf. 2019;28(7):564–73.
Mira JJ, Carrillo I, Lorenzo S, Ferrús L, Silvestre C, Pérez-Pérez P, Olivera G, Iglesias F, Zavala E, Maderuelo-Fernández JA, et al. The aftermath of adverse events in spanish primary care and hospital health professionals. BMC Health Serv Res. 2015;15(1):151–1.
Hopkins Medicine. [ http://m.hopkinsmedicine.org/news/publications/dome/november_2010/the_second_victims ]. Accessed 16 Jun 2022.
Brigham and Women’s Hospital[ http://www.brighamandwomens.org/medical_professionals/career/cpps/PeerSupport.aspx ]. Accessed 15 Jun 2023.
Mike Greenstein. [ http://mikegreenstein.com/media/docs/Physicians-Insurance-Annual-Report-2010.pdf ]. Accessed 10 Jun 2020.
Physicians Insurance. [ https://www.phyins.com/magazine/provider-support-program ]. Accessed 15 Jun 2021.
Conway J, Federico F, Stewart K, Campbell MJ. Respectful Management of Serious Clinical Adverse Events (Second Edition). IHI Innovation Series white paper. Cambridge, Massachusetts: Institute for Healthcare Improvement; 2011. (Available on www.IHI.org )
UCLA Health. [ https://www.uclahealth.org/quality/Workfiles/quality/NQF-Safety.pdf ]. Accessed 10 Jun 2022.
The Schwartz Center. [ https://www.theschwartzcenter.org/about/who-we-are/ ]. Accessed 10 Jun 2022.
Medically Induced Trauma Support Services (MITSS). [ http://www.mitss.org/ ]. Accessed 10 Jun 2022.
Institute for Safe Medication Practices Canada. ISMP Canada Safety Bulletin. 2017;17(9):1-6. [ https://www.ismp-canada.org/download/safetyBulletins/2017/ISMPCSB2017-10-SecondVictim.pdf ]. Accessed 15 Jun 2023.
For YOU team. [ https://www.muhealth.org/sites/default/files/forYOUstaff_brochure.pdf ]. 16 Jun 2022.
Foundations for Healthy Communities. [ https://healthynh.org/images/Implementationofasecondvictimprograminapediatrichospital_2.pdf ]. Accessed 10 Jun 2022.
Kaiser Permanente. [ http://kpnet.kp.org/qrrm/risk/CareforCaregivers/care_for_caregivers.htm ]. Accessed 19 Jun 2022.
Denham CR. TRUST: the 5 rights of the second victim. J Patient Saf. 2007;3(2):107–19.
Lane MA, Newman BM, Taylor MZ, OʼNeill M, Ghetti C, Woltman RM, Waterman AD. Supporting Clinicians after adverse events: development of a clinician peer support program. J Patient Saf. 2018;14(3):e56–e60.
Richtlijn Omgaan met Ernstige Klinische Incidenten. [ https://secondvictim.be/richtlijn-in-dutch-only/ ]. Accessed 16 Jun 2023.
University of Illnois Chicago. [ https://www.uic.edu/apps/departments-az/search?dispatch=find&orgid=94765 ]. Access 19 Feb 2021.
McCarthy SE, O’Boyle CA, O’Shaughnessy A, Walsh G. Online patient safety education programme for junior doctors: is it worthwhile? Ir J Med Sci. 2016;185(1):51–8.
American Association of Nurse Anesthesiology. [ https://www.aana.com/docs/default-source/aana-journal-web-documents-1/design-second-victim-0416-pp107-113.pdf?sfvrsn=f1d448b1_6 ]. Accessed 19 Feb 2021.
Patient Safety Switzerland. [ https://www.patientensicherheit.ch/ ]. Accessed 19 Feb 2021.
Gardner Webb University. [ https://digitalcommons.gardner-webb.edu/cgi/viewcontentcgi?article=1025&context=nursing_etd ]. Accessed 19 Feb 2021.
Australian and New Zealand College of Anaesthetists. [ https://www.anzca.edu.au/about-us/doctors-health-and-wellbeing ]. Accessed 15 Jun 2023.
The Association of Anaesthetists of Great Britain and Ireland. Catastrophes in Anaesthetic Practice – dealing with the aftermath. September 2005
Mitchell I, Schuster A, Smith K, Pronovost P, Wu A: Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after ‘ To Err is Human’. BMJ Quality & Safety 2016, 25(2):92–99
Segundas y Terceras victimas. [ http://www.segundasvictimas.es/acceso.php ]. Accessed 15 Jun 2023.
International Critical Incident Stress Foundation, Inc. [ https://www.icisf.org/ ]. Accessed 15 Jun 2023.
Stukalin I, Lethebe BC, Temple W. The physician’s Achilles heel-surviving an adverse event. Curr Oncol. 2019;26(6):e742–7.
Ajoudani F, Habibzadeh H, Baghaei R. Second victim experience and Support Tool: persian translation and psychometric characteristics evaluation. Int Nurs Rev 2020.
Burlison JD, Quillivan RR, Scott SD, Johnson S, Hoffman JM. The Effects of the second victim phenomenon on work-related outcomes: connecting self-reported caregiver distress to turnover intentions and absenteeism. J Patient Saf 2016.
Winning AM, Merandi J, Rausch JR, Liao N, Hoffman JM, Burlison JD, Gerhardt CA. Validation of the second victim experience and Support Tool-Revised in the neonatal Intensive Care Unit. J Patient Saf 2020.
Van Slambrouck L, Verschueren R, Seys D, Bruyneel L, Panella M, Vanhaecht K. Second victims among baccalaureate nursing students in the aftermath of a patient safety incident: an exploratory cross-sectional study. J Prof Nurs. 2021;37(4):765–70.
Cebeci F, Karazeybek E, Sucu G, Kahveci R. Nursing students’ medication errors and their opinions on the reasons of errors: a cross-sectional survey. J Pak Med Assoc. 2015;65(5):457–62.
Knudsen T, Abrahamsen C, Jørgensen JS, Schrøder K. Validation of the danish version of the second victim experience and Support Tool. Scand J Public Health. 2022;50(4):497–506.
Santana-Domínguez I, González-de la Torre H, Verdú-Soriano J, Nolasco A, Martín-Martínez A. Validation and Psychometric Properties of the Spanish Version of the second victim experience and support Tool Questionnaire. J Patient Saf 2022.
Trifunovic-Koenig M, Strametz R, Gerber B, Mantri S, Bushuven S. Validation of the german version of the Moral Injury Symptom and Support Scale for Health Professionals (G-MISS-HP) and its correlation to the second victim phenomenon. Int J Environ Res Public Health 2022, 19(8).
Scarpis E, Castriotta L, Ruscio E, Bianchet B, Doimo A, Moretti V, Cocconi R, Farneti F, Quattrin R. The second victim experience and Support Tool: a cross-cultural adaptation and psychometric evaluation in Italy (IT-SVEST). J Patient Saf. 2022;18(2):88–93.
Mohd Kamaruzaman AZ, Ibrahim MI, Mokhtar AM, Mohd Zain M, Satiman SN, Yaacob NM. Translation and validation of the malay revised second victim experience and Support Tool (M-SVEST-R) among Healthcare Workers in Kelantan, Malaysia. Int J Environ Res Public Health 2022, 19(4).
Suárez-Álvarez J, Pedrosa I, Lozano LM, García-Cueto E, Cuesta M, Muñiz J. Using reversed items in likert scales: a questionable practice. Psicothema. 2018;30(2):149–58.
Mira JJ, Lorenzo S, Carrillo I, Ferrús L, Silvestre C, Astier P, Iglesias-Alonso F, Maderuelo JA, Pérez-Pérez P, Torijano ML, et al. Lessons learned for reducing the negative impact of adverse events on patients, health professionals and healthcare organizations. Int J Qual Health Care. 2017;29(4):450–60.
Mira JJ, Carrillo I, Guilabert M, Lorenzo S, Pérez-Pérez P, Silvestre C, Ferrús L. The second victim Phenomenon after a clinical error: the design and evaluation of a website to reduce caregivers’ emotional responses after a clinical error. J Med Internet Res. 2017;19(6):e203.
Kanstrup M, Singh L, Göransson KE, Widoff J, Taylor RS, Gamble B, Iyadurai L, Moulds ML, Holmes EA. Reducing intrusive memories after trauma via a brief cognitive task intervention in the hospital emergency department: an exploratory pilot randomised controlled trial. Transl Psychiatry. 2021;11(1):30.
Schrader C, Ross A. A review of PTSD and current treatment strategies. Mo Med. 2021;118(6):546–51.
PubMed PubMed Central Google Scholar
Seys D, De Decker E, Waelkens H, Claes S, Panella M, Danckaerts M, Vanhaecht K. A comparative study measuring the difference of Healthcare workers reactions among those involved in a patent Safety Incident and Healthcare Professionals while Working during COVID-19. J Patient Saf 2022.
Zeeman G, Schouten L, Seys D, Coeckelberghs E, Weijenborg P, Bruyneel L, Vanhaecht K. Prolonged mental health sequelae among doctors and nurses involved in patient safety incidents with formal complaints and lawsuits. Eur J Public Health. 2020;30(4):777–9.
Sigurgeirsdóttir S. Criminalisation of human error in health care: how and why legal accountability can crowd out professional accountability and undermine patient safety. Icelandic Rev Politics Adm 2020, 16(2).
Olson K, Shanafelt T, Southwick S. Pandemic-Driven Posttraumatic Growth for Organizations and individuals. JAMA. 2020;324(18):1829–30.
Download references
Acknowledgements
Not applicable.
This publication is based upon work from COST ACTION 19113, supported by COST (European Cooperation in Science and Technology) www.cost.eu .
Author information
Authors and affiliations.
Department Public Health and Primary Care, KU Leuven, Kapucijnenvoer 35, Leuven, Belgium
Deborah Seys
Department Public Health and Primary Care, KU Leuven, Leuven, Belgium
Deborah Seys, Astrid Van Wilder, Lode Godderis & Kris Vanhaecht
Department of Translational Medicine, University of Eastern Piedmont, Novara, Italy
Massimiliano Panella & Sophia Russotto
RheinMain University of Applied Science, Wiesbaden, Germany
Reinhard Strametz
The Foundation for the Promotion of Health and Biomedical Research of Valencia Region, Alicante, Spain
José Joaquín Mira
Health Psychology Department, Miguel Hernandez University, Elche, Spain
External Service for Prevention and Protection at Work, IDEWE, Heverlee, Belgium
Lode Godderis
Department of Quality, University Hospitals Leuven, 3000, Leuven, Belgium
Kris Vanhaecht
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, D.S., M.P., S.R., R.S, J.M., L.G. and K.V., methodology, K.V., D.S., S.R. and M.P.; validation, K.V., D.S., S.R. and M.P.; formal analysis, D.S., M.P., S.R., R.S, J.M., A.V.W. and K.V. writing —original draft preparation, K.V., D.S., S.R. and M.P.; writing—review and editing, D.S., M.P., S.R., R.S, J.M., A.V.W., LG and K.V., supervision, K.V. and M.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Deborah Seys .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
Ethical approval was not required for this narrative review, since all data came from information freely available in the published articles.
Consent for publication
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Seys, D., Panella, M., Russotto, S. et al. In search of an international multidimensional action plan for second victim support: a narrative review. BMC Health Serv Res 23 , 816 (2023). https://doi.org/10.1186/s12913-023-09637-8
Download citation
Received : 29 March 2023
Accepted : 03 June 2023
Published : 31 July 2023
DOI : https://doi.org/10.1186/s12913-023-09637-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Healthcare professionals
- Patients safety incident
- Second victim

BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,885,667 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.
Author information, affiliations.
- McKenzie JE 1
- Brennan SE 1
- McDonald S 1
- Bossuyt PM 2
- Boutron I 3
- Hoffmann TC 4
- Mulrow CD 5
ORCIDs linked to this article
- Hoffmann TC | 0000-0001-5210-8548
- Li T | 0000-0001-5371-4558
- Mayo-Wilson E | 0000-0001-6126-2459
- Grimshaw JM | 0000-0001-8015-8243
- McGuinness LA | 0000-0001-8730-9761
Plos Medicine , 29 Mar 2021 , 18(3): e1003583 https://doi.org/10.1371/journal.pmed.1003583 PMID: 33780438 PMCID: PMC8007028
Abstract
Free full text .

The PRISMA 2020 statement: An updated guideline for reporting systematic reviews
Matthew j. page.
1 School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
Joanne E. McKenzie
Patrick m. bossuyt.
2 Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
Isabelle Boutron
3 Université de Paris, Centre of Epidemiology and Statistics (CRESS), Inserm, Paris, France
Tammy C. Hoffmann
4 Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia
Cynthia D. Mulrow
5 University of Texas Health Science Center at San Antonio, San Antonio, Texas, United States of America; Annals of Internal Medicine
Larissa Shamseer
6 Knowledge Translation Program, Li Ka Shing Knowledge Institute, Toronto, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
Jennifer M. Tetzlaff
7 Evidence Partners, Ottawa, Canada
Elie A. Akl
8 Clinical Research Institute, American University of Beirut, Beirut, Lebanon; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada
Sue E. Brennan
9 Department of Medical Informatics and Clinical Epidemiology, Oregon Health & Science University, Portland, Oregon, United States of America
Julie Glanville
10 York Health Economics Consortium (YHEC Ltd), University of York, York, United Kingdom
Jeremy M. Grimshaw
11 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Department of Medicine, University of Ottawa, Ottawa, Canada
Asbjørn Hróbjartsson
12 Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Open Patient data Exploratory Network (OPEN), Odense University Hospital, Odense, Denmark
Manoj M. Lalu
13 Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, Blueprint Translational Research Group, Ottawa Hospital Research Institute, Ottawa, Canada; Regenerative Medicine Program, Ottawa Hospital Research Institute, Ottawa, Canada
Tianjing Li
14 Department of Ophthalmology, School of Medicine, University of Colorado Denver, Denver, Colorado, United States; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America
Elizabeth W. Loder
15 Division of Headache, Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA; Head of Research, The BMJ , London, United Kingdom
Evan Mayo-Wilson
16 Department of Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, Indiana, United States of America
Steve McDonald
Luke a. mcguinness.
17 Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom
Lesley A. Stewart
18 Centre for Reviews and Dissemination, University of York, York, United Kingdom
James Thomas
19 EPPI-Centre, UCL Social Research Institute, University College London, London, United Kingdom
Andrea C. Tricco
20 Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Division of the Dalla Lana School of Public Health and the Institute of Health Management, Policy, and Evaluation, University of Toronto, Toronto, Canada; Queen’s Collaboration for Health Care Quality Joanna Briggs Institute Centre of Excellence, Queen’s University, Kingston, Canada
Vivian A. Welch
21 Methods Centre, Bruyère Research Institute, Ottawa, Ontario, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
Penny Whiting
David moher.
22 Centre for Journalology, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- Associated Data
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews .
Systematic reviews serve many critical roles. They can provide syntheses of the state of knowledge in a field, from which future research priorities can be identified; they can address questions that otherwise could not be answered by individual studies; they can identify problems in primary research that should be rectified in future studies; and they can generate or evaluate theories about how or why phenomena occur. Systematic reviews therefore generate various types of knowledge for different users of reviews (such as patients, healthcare providers, researchers, and policy makers).[ 1 , 2 ]To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did (such as how studies were identified and selected) and what they found (such as characteristics of contributing studies and results of meta-analyses). Up-to-date reporting guidance facilitates authors achieving this.[ 3 ]
Many innovations in the conduct of systematic reviews have occurred since publication of the PRISMA 2009 statement. For example, technological advances have enabled the use of natural language processing and machine learning to identify relevant evidence,[ 22 – 24 ] methods have been proposed to synthesise and present findings when meta-analysis is not possible or appropriate,[ 25 – 27 ] and new methods have been developed to assess the risk of bias in results of included studies.[ 28 , 29 ] Evidence on sources of bias in systematic reviews has accrued, culminating in the development of new tools to appraise the conduct of systematic reviews.[ 30 , 31 ] Terminology used to describe particular review processes has also evolved, as in the shift from assessing “quality” to assessing “certainty” in the body of evidence.[ 32 ] In addition, the publishing landscape has transformed, with multiple avenues now available for registering and disseminating systematic review protocols,[ 33 , 34 ] disseminating reports of systematic reviews, and sharing data and materials, such as preprint servers and publicly accessible repositories. To capture these advances in the reporting of systematic reviews necessitated an update to the PRISMA 2009 statement.
Summary points
To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did, and what they found
The PRISMA 2020 statement provides updated reporting guidance for systematic reviews that reflects advances in methods to identify, select, appraise, and synthesise studies
The PRISMA 2020 statement consists of a 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and revised flow diagrams for original and updated reviews
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders
- Development of PRISMA 2020
A complete description of the methods used to develop PRISMA 2020 is available elsewhere.[ 35 ] We identified PRISMA 2009 items that were often reported incompletely by examining the results of studies investigating the transparency of reporting of published reviews.[ 17 , 21 , 36 , 37 ] We identified possible modifications to the PRISMA 2009 statement by reviewing 60 documents providing reporting guidance for systematic reviews (including reporting guidelines, handbooks, tools, and meta-research studies).[ 38 ] These reviews of the literature were used to inform the content of a survey with suggested possible modifications to the 27 items in PRISMA 2009 and possible additional items. Respondents were asked whether they believed we should keep each PRISMA 2009 item as is, modify it, or remove it, and whether we should add each additional item. Systematic review methodologists and journal editors were invited to complete the online survey (110 of 220 invited responded). We discussed proposed content and wording of the PRISMA 2020 statement, as informed by the review and survey results, at a 21-member, two-day, in-person meeting in September 2018 in Edinburgh, Scotland. Throughout 2019 and 2020, we circulated an initial draft and five revisions of the checklist and explanation and elaboration paper to co-authors for feedback. In April 2020, we invited 22 systematic reviewers who had expressed interest in providing feedback on the PRISMA 2020 checklist to share their views (via an online survey) on the layout and terminology used in a preliminary version of the checklist. Feedback was received from 15 individuals and considered by the first author, and any revisions deemed necessary were incorporated before the final version was approved and endorsed by all co-authors.
- The PRISMA 2020 statement
Scope of the guideline
The PRISMA 2020 statement has been designed primarily for systematic reviews of studies that evaluate the effects of health interventions, irrespective of the design of the included studies. However, the checklist items are applicable to reports of systematic reviews evaluating other interventions (such as social or educational interventions), and many items are applicable to systematic reviews with objectives other than evaluating interventions (such as evaluating aetiology, prevalence, or prognosis). PRISMA 2020 is intended for use in systematic reviews that include synthesis (such as pairwise meta-analysis or other statistical synthesis methods) or do not include synthesis (for example, because only one eligible study is identified). The PRISMA 2020 items are relevant for mixed-methods systematic reviews (which include quantitative and qualitative studies), but reporting guidelines addressing the presentation and synthesis of qualitative data should also be consulted.[ 39 , 40 ] PRISMA 2020 can be used for original systematic reviews, updated systematic reviews, or continually updated (“living”) systematic reviews. However, for updated and living systematic reviews, there may be some additional considerations that need to be addressed. Where there is relevant content from other reporting guidelines, we reference these guidelines within the items in the explanation and elaboration paper [ 41 ] (such as PRISMA-Search [ 42 ] in items 6 and 7, Synthesis without meta-analysis (SWiM) reporting guideline [ 27 ] in item 13d). Box 1 includes a glossary of terms used throughout the PRISMA 2020 statement.
Box 1. Glossary of terms
Systematic review —A review that uses explicit, systematic methods to collate and synthesise findings of studies that address a clearly formulated question [ 43 ]
Statistical synthesis —The combination of quantitative results of two or more studies. This encompasses meta-analysis of effect estimates (described below) and other methods, such as combining P values, calculating the range and distribution of observed effects, and vote counting based on the direction of effect (see McKenzie and Brennan [ 25 ] for a description of each method)
Meta-analysis of effect estimates —A statistical technique used to synthesise results when study effect estimates and their variances are available, yielding a quantitative summary of results [ 25 ]
Outcome —An event or measurement collected for participants in a study (such as quality of life, mortality)
Result —The combination of a point estimate (such as a mean difference, risk ratio, or proportion) and a measure of its precision (such as a confidence/credible interval) for a particular outcome
Report —A document (paper or electronic) supplying information about a particular study. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information
Record —The title or abstract (or both) of a report indexed in a database or website (such as a title or abstract for an article indexed in Medline). Records that refer to the same report (such as the same journal article) are “duplicates”; however, records that refer to reports that are merely similar (such as a similar abstract submitted to two different conferences) should be considered unique.
Study —An investigation, such as a clinical trial, that includes a defined group of participants and one or more interventions and outcomes. A “study” might have multiple reports. For example, reports could include the protocol, statistical analysis plan, baseline characteristics, results for the primary outcome, results for harms, results for secondary outcomes, and results for additional mediator and moderator analyses
PRISMA 2020 is not intended to guide systematic review conduct, for which comprehensive resources are available.[ 43 – 46 ] However, familiarity with PRISMA 2020 is useful when planning and conducting systematic reviews to ensure that all recommended information is captured. PRISMA 2020 should not be used to assess the conduct or methodological quality of systematic reviews; other tools exist for this purpose.[ 30 , 31 ] Furthermore, PRISMA 2020 is not intended to inform the reporting of systematic review protocols, for which a separate statement is available (PRISMA for Protocols (PRISMA-P) 2015 statement [ 47 , 48 ]). Finally, extensions to the PRISMA 2009 statement have been developed to guide reporting of network meta-analyses,[ 49 ] meta-analyses of individual participant data,[ 50 ] systematic reviews of harms, [ 51 ] systematic reviews of diagnostic test accuracy studies, [ 52 ] and scoping reviews [ 53 ]; for these types of reviews we recommend authors report their review in accordance with the recommendations in PRISMA 2020 along with the guidance specific to the extension.
How to use PRISMA 2020
The PRISMA 2020 statement (including the checklists, explanation and elaboration, and flow diagram) replaces the PRISMA 2009 statement, which should no longer be used. Box 2 summarises noteworthy changes from the PRISMA 2009 statement. The PRISMA 2020 checklist includes seven sections with 27 items, some of which include sub-items ( Table 1 ). A checklist for journal and conference abstracts for systematic reviews is included in PRISMA 2020. This abstract checklist is an update of the 2013 PRISMA for Abstracts statement,[ 54 ] reflecting new and modified content in PRISMA 2020 ( Table 2 ). A template PRISMA flow diagram is provided, which can be modified depending on whether the systematic review is original or updated ( Fig 1 ).

The new design is adapted from flow diagrams proposed by Boers,[ 55 ] Mayo-Wilson et al.[ 56 ] and Stovold et al.[ 57 ] The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
*This abstract checklist retains the same items as those included in the PRISMA for Abstracts statement published in 2013, [ 54 ] but has been revised to make the wording consistent with the PRISMA 2020 statement and includes a new item recommending authors specify the methods used to present and synthesise results (item #6).
Box 2. Noteworthy changes to the PRISMA 2009 statement
Inclusion of the abstract reporting checklist within PRISMA 2020 (see item #2 and Table 2 ).
Movement of the ‘Protocol and registration’ item from the start of the Methods section of the checklist to a new Other section, with addition of a sub-item recommending authors describe amendments to information provided at registration or in the protocol (see item #24a-24c).
Modification of the ‘Search’ item to recommend authors present full search strategies for all databases, registers and websites searched, not just at least one database (see item #7).
Modification of the ‘Study selection’ item in the Methods section to emphasise the reporting of how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process (see item #8).
Addition of a sub-item to the ‘Data items’ item recommending authors report how outcomes were defined, which results were sought, and methods for selecting a subset of results from included studies (see item #10a).
Splitting of the ‘Synthesis of results’ item in the Methods section into six sub-items recommending authors describe: the processes used to decide which studies were eligible for each synthesis; any methods required to prepare the data for synthesis; any methods used to tabulate or visually display results of individual studies and syntheses; any methods used to synthesise results; any methods used to explore possible causes of heterogeneity among study results (such as subgroup analysis, meta-regression); and any sensitivity analyses used to assess robustness of the synthesised results (see item #13a-13f).
Addition of a sub-item to the ‘Study selection’ item in the Results section recommending authors cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded (see item #16b).
Splitting of the ‘Synthesis of results’ item in the Results section into four sub-items recommending authors: briefly summarise the characteristics and risk of bias among studies contributing to the synthesis; present results of all statistical syntheses conducted; present results of any investigations of possible causes of heterogeneity among study results; and present results of any sensitivity analyses (see item #20a-20d).
Addition of new items recommending authors report methods for and results of an assessment of certainty (or confidence) in the body of evidence for an outcome (see items #15 and #22).
Addition of a new item recommending authors declare any competing interests (see item #26).
Addition of a new item recommending authors indicate whether data, analytic code and other materials used in the review are publicly available and if so, where they can be found (see item #27).
We recommend authors refer to PRISMA 2020 early in the writing process, because prospective consideration of the items may help to ensure that all the items are addressed. To help keep track of which items have been reported, the PRISMA statement website ( http://www.prisma-statement.org/ ) includes fillable templates of the checklists to download and complete (also available in S1 PRISMA 2020 checklist ). We have also created a web application that allows users to complete the checklist via a user-friendly interface [ 58 ] (available at https://prisma.shinyapps.io/checklist/ and adapted from the Transparency Checklist app [ 59 ]). The completed checklist can be exported to Word or PDF. Editable templates of the flow diagram can also be downloaded from the PRISMA statement website.
We have prepared an updated explanation and elaboration paper, in which we explain why reporting of each item is recommended and present bullet points that detail the reporting recommendations (which we refer to as elements).[ 41 ] The bullet-point structure is new to PRISMA 2020 and has been adopted to facilitate implementation of the guidance.[ 60 , 61 ] An expanded checklist, which comprises an abridged version of the elements presented in the explanation and elaboration paper, with references and some examples removed, is available in S2 PRISMA 2020 expanded checklist . Consulting the explanation and elaboration paper is recommended if further clarity or information is required.
Journals and publishers might impose word and section limits, and limits on the number of tables and figures allowed in the main report. In such cases, if the relevant information for some items already appears in a publicly accessible review protocol, referring to the protocol may suffice. Alternatively, placing detailed descriptions of the methods used or additional results (such as for less critical outcomes) in supplementary files is recommended. Ideally, supplementary files should be deposited to a general-purpose or institutional open-access repository that provides free and permanent access to the material (such as Open Science Framework, Dryad, figshare). A reference or link to the additional information should be included in the main report. Finally, although PRISMA 2020 provides a template for where information might be located, the suggested location should not be seen as prescriptive; the guiding principle is to ensure the information is reported.
Use of PRISMA 2020 has the potential to benefit many stakeholders. Complete reporting allows readers to assess the appropriateness of the methods, and therefore the trustworthiness of the findings. Presenting and summarising characteristics of studies contributing to a synthesis allows healthcare providers and policy makers to evaluate the applicability of the findings to their setting. Describing the certainty in the body of evidence for an outcome and the implications of findings should help policy makers, managers, and other decision makers formulate appropriate recommendations for practice or policy. Complete reporting of all PRISMA 2020 items also facilitates replication and review updates, as well as inclusion of systematic reviews in overviews (of systematic reviews) and guidelines, so teams can leverage work that is already done and decrease research waste.[ 36 , 62 , 63 ]
We updated the PRISMA 2009 statement by adapting the EQUATOR Network’s guidance for developing health research reporting guidelines.[ 64 ] We evaluated the reporting completeness of published systematic reviews,[ 17 , 21 , 36 , 37 ] reviewed the items included in other documents providing guidance for systematic reviews,[ 38 ] surveyed systematic review methodologists and journal editors for their views on how to revise the original PRISMA statement,[ 35 ] discussed the findings at an in-person meeting, and prepared this document through an iterative process. Our recommendations are informed by the reviews and survey conducted before the in-person meeting, theoretical considerations about which items facilitate replication and help users assess the risk of bias and applicability of systematic reviews, and co-authors’ experience with authoring and using systematic reviews.
Various strategies to increase the use of reporting guidelines and improve reporting have been proposed. They include educators introducing reporting guidelines into graduate curricula to promote good reporting habits of early career scientists [ 65 ]; journal editors and regulators endorsing use of reporting guidelines [ 18 ]; peer reviewers evaluating adherence to reporting guidelines [ 61 , 66 ]; journals requiring authors to indicate where in their manuscript they have adhered to each reporting item[ 67 ]; and authors using online writing tools that prompt complete reporting at the writing stage.[ 60 ] Multi-pronged interventions, where more than one of these strategies are combined, may be more effective (such as completion of checklists coupled with editorial checks).[ 68 ] However, of 31 interventions proposed to increase adherence to reporting guidelines, the effects of only 11 have been evaluated, mostly in observational studies at high risk of bias due to confounding.[ 69 ] It is therefore unclear which strategies should be used. Future research might explore barriers and facilitators to the use of PRISMA 2020 by authors, editors, and peer reviewers, designing interventions that address the identified barriers, and evaluating those interventions using randomised trials. To inform possible revisions to the guideline, it would also be valuable to conduct think-aloud studies [ 70 ] to understand how systematic reviewers interpret the items, and reliability studies to identify items where there is varied interpretation of the items.
We encourage readers to submit evidence that informs any of the recommendations in PRISMA 2020 (via the PRISMA statement website: http://www.prisma-statement.org/ ). To enhance accessibility of PRISMA 2020, several translations of the guideline are under way (see available translations at the PRISMA statement website). We encourage journal editors and publishers to raise awareness of PRISMA 2020 (for example, by referring to it in journal “Instructions to authors”), endorsing its use, advising editors and peer reviewers to evaluate submitted systematic reviews against the PRISMA 2020 checklists, and making changes to journal policies to accommodate the new reporting recommendations. We recommend existing PRISMA extensions [ 47 , 49 – 53 , 71 , 72 ] be updated to reflect PRISMA 2020 and advise developers of new PRISMA extensions to use PRISMA 2020 as the foundation document.
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders. Ultimately, we hope that uptake of the guideline will lead to more transparent, complete, and accurate reporting of systematic reviews, thus facilitating evidence based decision making.
- Supporting information
S1 PRISMA 2020 checklist
S2 prisma 2020 expanded checklist.
- Acknowledgments
We dedicate this paper to the late Douglas G Altman and Alessandro Liberati, whose contributions were fundamental to the development and implementation of the original PRISMA statement.
We thank the following contributors who completed the survey to inform discussions at the development meeting: Xavier Armoiry, Edoardo Aromataris, Ana Patricia Ayala, Ethan M Balk, Virginia Barbour, Elaine Beller, Jesse A Berlin, Lisa Bero, Zhao-Xiang Bian, Jean Joel Bigna, Ferrán Catalá-López, Anna Chaimani, Mike Clarke, Tammy Clifford, Ioana A Cristea, Miranda Cumpston, Sofia Dias, Corinna Dressler, Ivan D Florez, Joel J Gagnier, Chantelle Garritty, Long Ge, Davina Ghersi, Sean Grant, Gordon Guyatt, Neal R Haddaway, Julian PT Higgins, Sally Hopewell, Brian Hutton, Jamie J Kirkham, Jos Kleijnen, Julia Koricheva, Joey SW Kwong, Toby J Lasserson, Julia H Littell, Yoon K Loke, Malcolm R Macleod, Chris G Maher, Ana Marušic, Dimitris Mavridis, Jessie McGowan, Matthew DF McInnes, Philippa Middleton, Karel G Moons, Zachary Munn, Jane Noyes, Barbara Nußbaumer-Streit, Donald L Patrick, Tatiana Pereira-Cenci, Ba’ Pham, Bob Phillips, Dawid Pieper, Michelle Pollock, Daniel S Quintana, Drummond Rennie, Melissa L Rethlefsen, Hannah R Rothstein, Maroeska M Rovers, Rebecca Ryan, Georgia Salanti, Ian J Saldanha, Margaret Sampson, Nancy Santesso, Rafael Sarkis-Onofre, Jelena Savović, Christopher H Schmid, Kenneth F Schulz, Guido Schwarzer, Beverley J Shea, Paul G Shekelle, Farhad Shokraneh, Mark Simmonds, Nicole Skoetz, Sharon E Straus, Anneliese Synnot, Emily E Tanner-Smith, Brett D Thombs, Hilary Thomson, Alexander Tsertsvadze, Peter Tugwell, Tari Turner, Lesley Uttley, Jeffrey C Valentine, Matt Vassar, Areti Angeliki Veroniki, Meera Viswanathan, Cole Wayant, Paul Whaley, and Kehu Yang. We thank the following contributors who provided feedback on a preliminary version of the PRISMA 2020 checklist: Jo Abbott, Fionn Büttner, Patricia Correia-Santos, Victoria Freeman, Emily A Hennessy, Rakibul Islam, Amalia (Emily) Karahalios, Kasper Krommes, Andreas Lundh, Dafne Port Nascimento, Davina Robson, Catherine Schenck-Yglesias, Mary M Scott, Sarah Tanveer and Pavel Zhelnov. We thank Abigail H Goben, Melissa L Rethlefsen, Tanja Rombey, Anna Scott, and Farhad Shokraneh for their helpful comments on the preprints of the PRISMA 2020 papers. We thank Edoardo Aromataris, Stephanie Chang, Toby Lasserson and David Schriger for their helpful peer review comments on the PRISMA 2020 papers.
Patient and public involvement: Patients and the public were not involved in this methodological research. We plan to disseminate the research widely, including to community participants in evidence synthesis organisations.
Author Contributions
JEM and DM are joint senior authors. MJP, JEM, PMB, IB, TCH, CDM, LS, and DM conceived this paper and designed the literature review and survey conducted to inform the guideline content. MJP conducted the literature review, administered the survey and analysed the data for both. MJP prepared all materials for the development meeting. MJP and JEM presented proposals at the development meeting. All authors except for TCH, JMT, EAA, SEB, and LAM attended the development meeting. MJP and JEM took and consolidated notes from the development meeting. MJP and JEM led the drafting and editing of the article. JEM, PMB, IB, TCH, LS, JMT, EAA, SEB, RC, JG, AH, TL, EMW, SM, LAM, LAS, JT, ACT, PW, and DM drafted particular sections of the article. All authors were involved in revising the article critically for important intellectual content. All authors approved the final version of the article. MJP is the guarantor of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
- Funding Statement
There was no direct funding for this research. MJP is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200101618) and was previously supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535) during the conduct of this research. JEM is supported by an Australian NHMRC Career Development Fellowship (1143429). TCH is supported by an Australian NHMRC Senior Research Fellowship (1154607). JMT is supported by Evidence Partners Inc. JMG is supported by a Tier 1 Canada Research Chair in Health Knowledge Transfer and Uptake. MML is supported by The Ottawa Hospital Anaesthesia Alternate Funds Association and a Faculty of Medicine Junior Research Chair. TL is supported by funding from the National Eye Institute (UG1EY020522), National Institutes of Health, United States. LAM is supported by a National Institute for Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-048). ACT is supported by a Tier 2 Canada Research Chair in Knowledge Synthesis. DM is supported in part by a University Research Chair, University of Ottawa. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pmed.1003583
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Article citations
The biopsychosocial factors associated with development of chronic musculoskeletal pain. an umbrella review and meta-analysis of observational systematic reviews..
Dunn M , Rushton AB , Mistry J , Soundy A , Heneghan NR
PLoS One , 19(4):e0294830, 01 Apr 2024
Cited by: 0 articles | PMID: 38557647 | PMCID: PMC10984407
Progress and challenges in achieving tuberculosis elimination in India by 2025: A systematic review and meta-analysis.
Padhi A , Agarwal A , Bhise M , Chaudhary A , Joshi K , Katoch CDS
PLoS One , 19(3):e0301060, 27 Mar 2024
Cited by: 0 articles | PMID: 38536792 | PMCID: PMC10971764
Effect of maternal diet on the frequency of micronuclei in pregnant women and newborns: A protocol for systematic review and meta-analysis.
Araújo AC , Medeiros MCS , Nascimento PKDSBD , Cobucci RN , Bortolin RH , Rezende AA
PLoS One , 19(3):e0300714, 25 Mar 2024
Cited by: 0 articles | PMID: 38527051 | PMCID: PMC10962814
Web-Based Initiatives to Prevent Sexual Offense Perpetration: A Systematic Review.
Hillert J , Haubrock LS , Dekker A , Briken P
Curr Psychiatry Rep , 26(4):121-133, 21 Mar 2024
Cited by: 0 articles | PMID: 38509408 | PMCID: PMC10978615
Waist Circumference and Body Mass Index as Predictors of Disability Progression in Multiple Sclerosis: A Systematic Review and Meta-Analysis.
Giannopapas V , Stefanou MI , Smyrni V , Kitsos DK , Kosmidou M , Stasi S , Chasiotis AK , Stavrogianni K , Papagiannopoulou G , Tzartos JS , Paraskevas GP , Tsivgoulis G , Giannopoulos S
J Clin Med , 13(6):1739, 18 Mar 2024
Cited by: 0 articles | PMID: 38541964 | PMCID: PMC10970884
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
- http://www.ebi.ac.uk/biostudies/studies/S-EPMC8007028?xr=true
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Shamseer L , Tetzlaff JM , Akl EA , Brennan SE , Chou R , Glanville J , Grimshaw JM , Hróbjartsson A , Lalu MM , Li T , Loder EW , Mayo-Wilson E , McDonald S , [...] Moher D
J Clin Epidemiol , 134:178-189, 29 Mar 2021
Cited by: 531 articles | PMID: 33789819
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JP , Clarke M , Devereaux PJ , Kleijnen J , Moher D
PLoS Med , 6(7):e1000100, 21 Jul 2009
Cited by: 6101 articles | PMID: 19621070 | PMCID: PMC2707010
The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations.
Hutton B , Salanti G , Caldwell DM , Chaimani A , Schmid CH , Cameron C , Ioannidis JP , Straus S , Thorlund K , Jansen JP , Mulrow C , Catalá-López F , Gøtzsche PC , Dickersin K , Boutron I , Altman DG , Moher D
Ann Intern Med , 162(11):777-784, 01 Jun 2015
Cited by: 2603 articles | PMID: 26030634
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration.
BMJ , 339:b2700, 21 Jul 2009
Cited by: 7602 articles | PMID: 19622552 | PMCID: PMC2714672
J Clin Epidemiol , 62(10):e1-34, 23 Jul 2009
Cited by: 4490 articles | PMID: 19631507
Ann Intern Med , 151(4):W65-94, 20 Jul 2009
Cited by: 2511 articles | PMID: 19622512
Funding
Funders who supported this work.
NEI NIH HHS (1)
Grant ID: UG1 EY020522
101 publication s
National Institute for Health Research (NIHR) (1)
Determining the causal effects of lipid levels on risk of dementia: a triangulation of the available evidence and individual participant data meta-analysis.
Mr Luke McGuinness, University of Bristol
Grant ID: DRF-2018-11-ST2-048
23 publication s
Europe PMC is part of the ELIXIR infrastructure
Brand hate: a systematic literature review and future perspectives
- Published: 22 January 2024
Cite this article
- Fakhra Malik Mushtaq ORCID: orcid.org/0000-0003-2250-5221 1 ,
- Ezlika M. Ghazali ORCID: orcid.org/0000-0001-7824-4433 1 &
- Zalfa Laili Hamzah ORCID: orcid.org/0000-0002-9994-310X 1
532 Accesses
Explore all metrics
The duplex theory posits that consumer animosity towards brands emerges when expectations remain unmet, culminating in dissatisfaction. Despite increasing interest in brand hate within the realms of consumerism, academic discourse on this multifaceted emotion remains limited. Consequently, this paper endeavours to execute a systematic review, synthesising extant literature on brand hate. An initial screening encompassed 182 articles, following an exhaustive evaluation of six databases spanning 2009–2022. Of these, only 60 peer-reviewed articles satisfied the stringent criteria for in-depth analysis. This analysis offers a comprehensive overview of brand hate's conceptualisation and its developmental trajectory, focusing on publication years, journals, countries, antecedents, and consequences. Furthermore, this review delineates prospective research trajectories, potentially enriching the corpus of brand hate literature. For effective consumer recovery strategies, organisations and brands must grasp the underlying catalysts of such antipathy. To our knowledge, this study represents a thorough literature-based investigation in the domain of brand hate, charting new directions for future inquiry.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The Antecedents and Consequences of Brand Hate: A Review of Current Literature

I Hate This Brand! A Classification of Brand Haters Based on their Motivations and Reactions: An Abstract
Brand love: conceptual and empirical investigation of a holistic causal model
Renée Rahman, Tobias Langner & Dirk Temme
Data availability
During this study, No datasets were generated or analysed. Therefore, the sharing or availability of data is not applicable.
Aaker J (1997) Dimensions of brand personality. J Mark Res 34(3):347. https://doi.org/10.2307/3151897
Article Google Scholar
Abbasi AZ, Fayyaz MS, Ting DH, Munir M, Bashir S, Chun Z (2022) The moderating role of complaint handling on brand hate in the cancel culture. Asia Pac J Bus Adm 15(1):46–71. https://doi.org/10.1108/apjba-06-2021-0246
Adams JS (1963) Towards an understanding of inequity. J Abnorm Soc Psychol 67(5):422–436. https://doi.org/10.1037/h0040968
Ahmed S, Hashim S (2018) The moderating effect of brand recovery on brand hate and desire for reconciliation: a PLS-MGA approach. Int J Bus Soc 19(3):833–850
Google Scholar
Alba JW, Lutz RJ (2013) Broadening (and narrowing) the scope of brand relationships. J Consum Psychol Soc Consum Psychol 23(2):265–268
Albert N, Merunka D (2013) The role of brand love in consumer-brand relationships. J Consum Mark 30(3):258–266
Ali S, Attiq S, Talib N (2020) Antecedents of brand hate: mediating role of customer dissatisfaction and moderating role of narcissism. Pak J Commer Soc Sci 14(3):603–628
Alvarez C, David ME, George M (2023) Types of consumer-brand relationships: a systematic review and future research agenda. J Bus Res 160:113753. https://doi.org/10.1016/j.jbusres.2023.113753
Atwal G, Bryson D, Kaiser M (2020) The chopsticks debacle: how brand hate flattened Dolce & Gabbana in China. J Bus Strategy 43(1):37–43. https://doi.org/10.1108/jbs-07-2020-0160
Aziz R, Rahman Z (2022) Brand hate: a literature review and future research agenda. Eur J Mark 56(7):2014–2051. https://doi.org/10.1108/ejm-03-2021-0189
Banerjee S (2016) Influence of consumer personality, brand personality, and corporate personality on brand preference: an empirical investigation of interaction effect. Asia Pac J Mark Logistics 28(2). https://doi.org/10.1108/apjml-05-2015-0073
Banerjee S, Goel P (2020) Party brand hate in political market: antecedents and consequences. Asian J Polit Sci 28(2):97–121
Batra R, Ahuvia A, Bagozzi RP (2012) Brand love. J Mark 76(2):1–16
Bavik A, Bavik YL (2015) Effect of employee incivility on customer retaliation through psychological contract breach: the moderating role of moral identity. Int J Hosp Manag 50:66–76
Bayarassou O, Becheur I, Valette-Florence P (2020) “Fight or flight”: coping responses to brand hate. J Prod Brand Manag 30(3):492–511. https://doi.org/10.1108/jpbm-08-2019-2519
Benton B, Peterka-Benton D (2019) Hating in plain sight: the hatejacking of brands by extremist groups. Public Relat Inq 9(1):7–26. https://doi.org/10.1177/2046147x19863838
Ben-Ze'ev A (2001) The explanation of emotions. In Explanation. Springer, Dordrecht, pp 167–184
Bitner MJ, Booms BH, Tetreault MS (1990) The service encounter: diagnosing favorable and unfavorable incidents. J Mark 54(1):71–84
Bloemer JMM, Kasper HDP (1995) The complex relationship between consumer satisfaction and brand loyalty. J Econ Psychol 16(2):311–329
Briner RB, Denyer D (2012) Systematic review and evidence synthesis as a practice and scholarship tool. In Oxford University Press eBooks, pp 112–129. https://doi.org/10.1093/oxfordhb/9780199763986.013.0007
Bryson D, Atwal G (2019) Brand hate: the case of Starbucks in France. Br Food J 121(1):172–182
Bryson D, Atwal G, Hultén P (2013) Towards the conceptualisation of the antecedents of extreme negative affect towards luxury brands. Qual Mark Res 16(4):393–405
Bryson D, Atwal G, Hultén P, Heine K (2021) Antecedents of luxury brand hate: A quantitative study. Strateg Chang 30(1):35–43. https://doi.org/10.1002/jsc.2387
Curina I, Francioni B, Cioppi M, Savelli E (2019) Traits and peculiarities of different brand hate behaviours. J Strateg Mark 29(3):227–246. https://doi.org/10.1080/0965254x.2019.1676293
Curina I, Francioni B, Hegner SM, Cioppi M (2020) Brand hate and non-repurchase intention: a service context perspective in a cross-channel setting. J Retail Consum Serv 54:102031. https://doi.org/10.1016/j.jretconser.2019.102031
Dalli D, Romani S, Gistri G (2006) Brand dislike: representing the negative side of consumer preferences. Adv Consum Res 32:234–250
Das K (2009) Relationship marketing research (1994–2006) an academic literature review and classification. Mark Intell Plan 27:326–363
Farhat Z, Chaney D (2020) Introducing destination brand hate: an exploratory study. Curr Issue Tour 24(17):2472–2488. https://doi.org/10.1080/13683500.2020.1844160
Fatma M, Rahman Z (2015) Consumer perspective on csr literature review and future research agenda. Manag Res Rev 38(2):195–216
Fetscherin M (2019) The five types of brand hate: how they affect consumer behavior. J Bus Res 101:116–127
Fetscherin M, Sampedro A (2019) Brand forgiveness. J Prod Brand Manag 28(5):633–652
Fetscherin M, Usunier J (2012) Corporate branding: an interdisciplinary literature review. Eur J Mark 46(5):733–753. https://doi.org/10.1108/03090561211212494
Fetscherin M, Guzman F, Veloutsou C, Cayolla RRRR (2019) Latest research on brand relationships: introduction to the special issue. J Prod Brand Manag 28(2):133–139
Foscht T, Maloles C, Swoboda B, Morschett D, Sinha I (2008) The impact of culture on brand perceptions: a six-nation study. J Prod Brand Manag 17(3):131–142
Fournier S (1998) Consumers and their brands: developing relationship theory in consumer research. J Consum Res 24(4):343–353
Fournier S, Alvarez C (2013) Relating badly to brands. J Consum Psychol Soc Consum Psychol 23(2):253–264
Grégoire Y, Fisher RJ (2006) The effects of relationship quality on customer retaliation. Mark Lett 17(1):31–46
Grégoire Y, Tripp TM, Legoux R (2009) When customer love turns into lasting hate: the effects of relationship strength and time on customer revenge and avoidance. J Mark 73(6):18–32
Grisaffe DB, Nguyen H (2011) Antecedents of emotional attachment to brands. J Bus Res 64(10):1052–1059. https://doi.org/10.1016/j.jbusres.2010.11.002
Hao F (2020) The landscape of customer engagement in hospitality and tourism: a systematic review. Int J Contemp Hosp Manag 32(5):1837–1860
Hashim S, Kasana S (2019) Antecedents of brand hate in the fast food industry. Span J Mark ESIC 23(2):227–248
Hegner SM, Fetscherin M, van Delzen M (2017) Determinants and outcomes of brand hate. J Prod Brand Manag 26(1):13–25
Hogg MK, Banister EN, Stephenson CA (2009) Mapping symbolic (anti-) consumption. J Bus Res 62(2):148–159
Hogreve J, Iseke A, Derfuß K, Eller TF (2017) The service–profit chain: a meta-analytic test of a comprehensive theoretical framework. J Mark 81(3):41–61. https://doi.org/10.1509/jm.15.0395
Husnain M, Wang Z, Poulová P, Syed F, Akbar A, Akhtar MW, Akbar M, Usman M (2021) Exploring brand hate and the association between similar competitor offer and brand equity: a moderated-mediation model. Front Psychol. https://doi.org/10.3389/fpsyg.2020.533216
Husnain M, Syed F, Hussain K, Zhang Q, Usman M, Javed M (2022) Explaining the mechanism of brand hate: a mixed-method investigation using moderated mediation in emerging markets. Kybernetes. https://doi.org/10.1108/k-12-2021-1246
Islam JU, Hollebeek LD, Rahman Z, Khan I, Rasool A (2019a) Customer engagement in the service context: an empirical investigation of the construct, its antecedents and consequences. J Retail Consum Serv 50:277–285
Islam T, Attiq S, Hameed Z, Khokhar MN, Sheikh Z (2019b) The impact of self-congruity (symbolic and functional) on the brand hate: a study based on self-congruity theory. Br Food J 121(1):71–88
Islam T, Li J, Ali A, Xiaobei L, Sheikh Z, Ullah Zafar A (2020) Mapping online app hate: determinants and consequences. Telemat Inform 51:101401
Itani OS (2020) “Us” to co-create value and hate “them”: examining the interplay of consumer-brand identification, peer identification, value co-creation among consumers, competitor brand hate and individualism. Eur J Mark 55(4):1023–1066. https://doi.org/10.1108/ejm-06-2019-0469
Jabeen F, Kaur P, Talwar S, Malodia S, Dhir A (2022) I love you, but you let me down! How hate and retaliation damage customer-brand relationship. Technol Forecast Soc Chang 174:121183. https://doi.org/10.1016/j.techfore.2021.121183
Jain K, Sharma I (2019) Negative outcomes of positive brand relationships. J Consum Mark 36(7):986–1002
Japutra A, Roy SK, Pham TA (2021) Relating brand anxiety, brand hatred and obsess: moderating role of age and brand affection. J Retail Consum Serv 60:102465. https://doi.org/10.1016/j.jretconser.2021.102465
Jeesha K (2018) Book review: S. Umit Kucuk, brand hate: navigating consumer negativity in the digital world. IIM Kozhikode Society & Management Review
Johnson AR, Matear M, Thomson M (2011) A coal in the heart: self-relevance as a post-exit predictor of consumer anti-brand actions. J Consum Res 38(1):108–125
Joshi R, Yadav R (2020) Captivating brand hate using contemporary metrics: a structural equation modelling approach. Vision 25(4):439–447. https://doi.org/10.1177/0972262919892173
Kähr A, Nyffenegger B, Krohmer H, Hoyer WD (2016) When hostile consumers wreak havoc on your brand: the phenomenon of consumer brand sabotage. J Mark 80(3):25–41
Kashif M, Devrani TK, Rehman A, Samad S (2021) Love is not blind: investigating a love-hate transition among luxury fashion brand consumers. J Fashion Mark Manag 25(4):625–643. https://doi.org/10.1108/jfmm-04-2020-0058
Keller KL (2012) Understanding the richness of brand relationships: research dialogue on brands as intentional agents. J Consum Psychol Soc Consum Psychol 22(2):186–190
Keller KL (2014) Strategic brand management, 4th edn. Pearson, London
Keller KL, Keller KL (2014) Special issue : Consumer brand relationships S. J Brand Manag
Khan MA, Lee MSW (2014) Prepurchase determinants of brand avoidance: the moderating role of country-of-origin familiarity. J Glob Mark 27(5):329–343
Khatoon S, Rehman V (2021) Negative emotions in consumer brand relationship: a review and future research agenda. Int J Consum Stud 45(4):719–749. https://doi.org/10.1111/ijcs.12665
Krishnamurthy S, Kucuk SU (2009) Anti-branding on the internet. J Bus Res 62(11):1119–1126
Krszjzaniek E (2020) Book review: brand hate: navigating consumer negativity in the digital world. J Macromarketing 41(2):432–435. https://doi.org/10.1177/0276146720978251
Kucuk SU (2008) Negative double jeopardy: the role of anti-brand sites on the internet. J Brand Manag 15(3):209–222
Kucuk SU (2010) Negative double jeopardy revisited: a longitudinal analysis. J Brand Manag 18(2):150–158
Kucuk SU (2016) Brand hate: navigating consumer negativity in the digital world. Palgrave, London
Book Google Scholar
Kucuk SU (2018) Brand hate: navigating consumer negativity in the digital world. Springer International Publishing, Berlin
Kucuk SU (2019) Consumer brand hate: steam rolling whatever I see. Psychol Mark 36(5):431–443
Kucuk SU (2020) Reverse (brand) anthropomorphism: the case of brand hitlerization. J Consum Mark 37(6):651–659. https://doi.org/10.1108/jcm-11-2019-3487
Kucuk SU (2021) Developing a theory of brand hate: Where are we now? Strateg Chang 30(1):29–33
Kumar A, Paul J, Unnithan AB (2020) ‘Masstige’ marketing: a review, synthesis and research agenda. J Bus Res 113:384–398. https://doi.org/10.1016/j.jbusres.2019.09.030
Lee MSW, Motion J, Conroy D (2009) Anti-consumption and brand avoidance. J Bus Res 62(2):169–180
Lee MSW (2007) Brands we love to hate: an exploration of brand avoidance. Thèse En Philosophie, The University of Auckland 1994: 1–282
Lim WM, Yap SF, Makkar M (2021) Home sharing in marketing and tourism at a tipping point: what do we know, how do we know, and where should we be heading? J Bus Res 122:534–566. https://doi.org/10.1016/j.jbusres.2020.08.051
Lin L (2010) The relationship of consumer personality trait, brand personality and brand loyalty: an empirical study of toys and video games buyers. J Prod Brand Manag 19(1):4–17. https://doi.org/10.1108/10610421011018347
Maghzi A (2018) Exploring the influence of hospitality on guest satisfaction in luxury hotel services. e-Review Tour Res 15(1)
McCullough ME (2000) Forgiveness as human strength: theory, measurement, and links to well-being. J Soc Clin Psychol 19(1):43–55
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ Br Med J 340:869
Muhammad L, Gul-E-Rana R (2020) Mediating role of customer forgiveness between perceived justice and satisfaction. J Retail Consum Serv 52(1):101886
Opotow S, McClelland SI (2007) The intensification of hating: a theory. Soc Justice Res 20(1):68–97
Osuna Ramírez SASA, Veloutsou C, Morgan-Thomas A (2019) I hate what you love: brand polarisation and negativity towards brands as an opportunity for brand management. J Prod Brand Manag 28(5):614–632
Pantano E (2021) When a luxury brand bursts: modelling the social media viral effects of negative stereotypes adoption leading to brand hate. J Bus Res 123:117–125. https://doi.org/10.1016/j.jbusres.2020.09.049
Park CW, Eisingerich AB, Park JW (2013) Attachment–aversion (AA) model of customer–brand relationships. J Consum Psychol 23(2):229–248. https://doi.org/10.1016/j.jcps.2013.01.002
Paul J, Benito GRG (2017) A review of research on outward foreign direct investment from emerging countries, including China: what do we know, how do we know and where should we be heading? Asia Pac Bus Rev 24(1):90–115. https://doi.org/10.1080/13602381.2017.1357316
Paul J, Rosado-Serrano A (2019) Gradual internationalization vs born-global/International new venture models. Int Mark Rev 36(6):830–858. https://doi.org/10.1108/imr-10-2018-0280
Pinto O, Brandão A (2020) Antecedents and consequences of brand hate: empirical evidence from the telecommunication industry. Eur J Manag Bus Econ 30(1):18–35
Platania S, Morando M, Santisi G (2017) The phenomenon of brand hate: analysis of predictors and outcomes. Qual Access Success 18:342–347
Platania S, Morando M, Santisi G (2020) Psychometric properties, measurement invariance, and construct validity of the Italian version of the brand Hate Short Scale (BHS). Sustainability 12(5):2103. https://doi.org/10.3390/su12052103
Prayag G, Ryan C (2011) Antecedents of tourists’ loyalty to Mauritius: the role and influence of destination image, place attachment, personal involvement, and satisfaction. J Travel Res 51:342–356
Rasouli N, Rasoolimanesh SM, Rahmani AK, Momayez A, Torabi MA (2022) Effects of customer forgiveness on brand betrayal and brand hate in restaurant service failures: Does apology letter matter? J Hosp Mark Manag. https://doi.org/10.1080/19368623.2022.2043800
Rempel JK, Burris CT (2005) Let me count the ways: an integrative theory of love and hate. Pers Relatsh 12(2):297–313
Rodrigues C, Rodrigues P (2019) Brand love matters to Millennials: the relevance of mystery, sensuality and intimacy to neo-luxury brands. J Prod Brand Manag 28(7):830–848
Rodrigues C, Brandão A, Rodrigues P (2020) I can’t stop hating you: an anti-brand-community perspective on apple brand hate. J Prod Brand Manag 30:1115–1133
Romani S, Grappi S, Dalli D (2012) Emotions that drive consumers away from brands: measuring negative emotions toward brands and their behavioral effects. Int J Res Mark 29(1):55–67
Romani S, Grappi S, Zarantonello L, Bagozzi RP (2015) The revenge of the consumer how brand moral violations lead to consumer anti-brand activism. J Brand Manag 22(8):658–672
Roy SK, Sharma A, Bose S, Singh G (2022) Consumer—brand relationship: a brand hate perspective. J Bus Res 144:1293–1304. https://doi.org/10.1016/j.jbusres.2022.02.065
Rui-Ying C, Qu H (2017) Customers’ perceived justice, emotions, direct and indirect reactions to service recovery: moderating effects of recovery efforts. J Hospitality Mark Manag 27(3):323–345. https://doi.org/10.1080/19368623.2018.1385434
Sandikci Ö, Ekici A (2009) Politically motivated brand rejection. J Bus Res 62(2):208–217
Sarkar A, Sarkar JG, Sreejesh S, Anusree MR, Rishi B (2019) You are so embarrassing, still, I hate you less! Investigating consumers’ brand embarrassment and brand hate. J Brand Manag 27(1):93–107. https://doi.org/10.1057/s41262-019-00164-8
Shahab MH, Ghazali EM, Mohtar M (2021) The role of elaboration likelihood model in consumer behaviour research and its extension to new technologies: a review and future research agenda. Int J Consum Stud 45(4):664–689. https://doi.org/10.1111/ijcs.12658
Sharma I, Jain K, Gupta R (2021) The power to voice my hate! Exploring the effect of brand hate and perceived social media power on negative eWOM. J Asia Bus Stud 16(4):652–675. https://doi.org/10.1108/jabs-10-2020-0423
Sharma I, Jain K, Behl A (2022) Motives of the self and brand hate. J Consum Mark 39(7):708–725. https://doi.org/10.1108/jcm-04-2021-4635
Sternberg RJ (1986) A triangular theory of love. Psychol Rev 93(2):119–135. https://doi.org/10.1037/0033-295x.93.2.119
Sternberg RJ (2003) A duplex theory of hate: development and application to terrorism, massacres, and genocide. Rev Gen Psychol 7(3):299–328. https://doi.org/10.1037/1089-2680.7.3.299
Thomson M, MacInnis DJ, Park CW (2005) The ties that bind: measuring the strength of consumers’ emotional attachments to brands. J Consum Psychol 15(1):77–91
Thomson M, Whelan J, Johnson AR (2012) Why brands should fear fearful consumers: how attachment style predicts retaliation. J Consum Psychol Soc Consum Psychol 22(2):289–298
Tifferet S, Herstein R (2012) Gender differences in brand commitment, impulse buying, and hedonic consumption. J Prod Brand Manag 21(3):176–182
Tranfield D, Denyer D, Smart P (2003) Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br J Manag 14:207–222
Tupes EC, Christal RE (1992) Recurrent personality factors based on trait ratings. J Pers 60(2):225–251. https://doi.org/10.1111/j.1467-6494.1992.tb00973.x
Van Eck NJ, Waltman L (2009) Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84(2):523–538. https://doi.org/10.1007/s11192-009-0146-3
Waqas M, Hamzah ZL, Salleh NAM (2021) Customer experience: a systematic literature review and consumer culture theory-based conceptualisation. Manag Rev Q 71(1):135–176
Weingarten K (2006) On hating to hate. Fam Process 45(3):277–288
Weiten W, Dunn DS, Hammer EY (2014) Psychology applied to modern life: adjustment in the 21st century. Cengage Learning
Wisker ZL (2020) The effect of fake news in marketing halal food: a moderating role of religiosity. J Islamic Mark 12(3):558–575. https://doi.org/10.1108/jima-09-2020-0276
Wolter JS, Brach S, Cronin JJ, Bonn M (2016) Symbolic drivers of consumer-brand identification and disidentification. J Bus Res 69(2):785–793
Xie Y, Peng S (2009) How to repair customer trust after negative publicity: the roles of competence, integrity, benevolence, and forgiveness. Psychol Mark 26(7):572–589
Yadav A, Chakrabarti S (2022) Brand hate: a systematic literature review and future research agenda. Int J Consum Stud 46(5):1992–2019. https://doi.org/10.1111/ijcs.12772
Yang J, Mundel J (2021) Effects of brand feedback to negative eWOM on brand love/hate: an expectancy violation approach. J Prod Brand Manag 31(2):279–292. https://doi.org/10.1108/jpbm-05-2020-2900
Zarantonello L (2020) Book review: brand hate: navigating consumer negativity in the digital world by S. Umit Kucuk. Psychol Mark 37(11):1635. https://doi.org/10.1002/mar.21402
Zarantonello L, Romani S, Grappi S, Bagozzi RP (2016) Brand hate. J Prod Brand Manag 25(1):11–25
Zarantonello L, Romani S, Grappi S, Fetscherin M (2018) Trajectories of brand hate. J Brand Manag 25(6):549–560
Zhang C, Laroche M (2020) Brand hate: a multidimensional construct. J Prod Brand Manag 30:392–414
Zourrig H, Chebat JC, Toffoli R (2009) Exploring cultural differences in customer forgiveness behavior. J Serv Manag 20(4):404–419
Download references
Acknowledgements
We express our gratitude to the editor-in-chief, Professor Dr. Joern Block, as well as the anonymous reviewers for their valuable feedback on this manuscript.
No financial support was received by the author for this research.
Author information
Authors and affiliations.
Faculty of Business and Economics, University of Malaya, 50603, Kuala Lumpur, Malaysia
Fakhra Malik Mushtaq, Ezlika M. Ghazali & Zalfa Laili Hamzah
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zalfa Laili Hamzah .
Ethics declarations
Conflict of interest.
There are no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Mushtaq, F.M., Ghazali, E.M. & Hamzah, Z.L. Brand hate: a systematic literature review and future perspectives. Manag Rev Q (2024). https://doi.org/10.1007/s11301-023-00402-z
Download citation
Received : 25 May 2023
Accepted : 17 December 2023
Published : 22 January 2024
DOI : https://doi.org/10.1007/s11301-023-00402-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brand hatred
- Systematic literature review
- Negative consumer emotions
- Consumer-brand relationships
JEL Classification
- M310 Marketing
- M370 Advertising
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement ...
The PRISMA 2020 statement comprises a 27-item checklist addressing the introduction, methods, results and discussion sections of a systematic review report. PRISMA 2020 Checklist (PDF) PRISMA 2020 Checklist (Word)
PRISMA 2020 is published as a suite of three papers: a statement paper ... Terms such as "review," "literature review," "evidence synthesis," or "knowledge synthesis" are not recommended because they do not distinguish systematic and non-systematic approaches. We also discourage using the terms "systematic review" and ...
PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. PRISMA primarily focuses on the reporting of reviews evaluating the effects of interventions, but can also be used as a basis for reporting systematic reviews with objectives other than evaluating interventions (e.g. evaluating aetiology ...
Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the ...
Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the ...
Matthew Page and co-authors describe PRISMA 2020, an updated reporting guideline for systematic reviews and meta-analyses. PLoS Med . 2021 Mar 29;18(3):e1003583. doi: 10.1371/journal.pmed.1003583.
Different templates are available depending on the type of review (new or updated) and sources used to identify studies. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources
In PRISMA 2020, there are now expanded options depending on where you search and whether you are updating a review. Version 1 of PRISMA 2020 includes databases and clinical trial or preprint registers. Version 2 includes additional sections for elaborating on your grey literature search, such as searches on websites or in citation lists.
"The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement." ... J Clin Epidemiol. 2020;127:96-104. ... Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic ...
The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. ... MJP conducted the literature review, administered the survey and ...
PRISMA 2020 - development and improvements. The proposed modifications to the PRISMA 2020 statement were initially derived from a total of 60 documents providing reporting guidance for systematic reviews [6]. These articles were used to inform the contents of a survey with suggested alterations, and was distributed to 220 systematic review ...
In early 2021, two reporting guidelines were released that provide direct guidance on how to report the literature search components of systematic reviews and related review types: PRISMA 2020, the updated version of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [1, 2]; and PRISMA-S, an extension to PRISMA focused solely on reporting the search ...
to PRISMA 2020 and has been adopted to facilitate implementation of the guidance.25 26 Authors familiar with PRISMA 2020 may opt to use the standalone ... "review," "literature review," "evidence synthesis," or "knowledge synthesis" are not recommended because they do not distinguish systematic and non-
The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded ...
The PRISMA 2020 Statement was published in 2021. It consists of a checklist and a flow diagram , and is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document. In order to encourage dissemination of the PRISMA 2020 Statement, it has been published in several journals.
The PRISMA 2020 Statement was published in 2021. It consists of a checklist and a flow diagram, and is intended to be accompanied by the PRISMA 2020 Explanation and Elaboration document. In order to encourage dissemination of the PRISMA 2020 Statement, it has been published in several journals. BMJ (OPEN ACCESS) Page MJ, McKenzie JE, Bossuyt PM ...
The PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is a robust framework designed to guide researchers in conducting systematic reviews.It goes beyond the scope of regular literature reviews, which are essentially summaries of existing research on a particular topic. In contrast, a systematic review is a comprehensive and methodical exploration of the ...
The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and ...
This review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) scoping review extension (PRISMA-ScR). Following systematic searches of academic databases and grey literature, 17 studies meeting eligibility criteria were included.
The PRISMA 2020 statement. Scope of the guideline. The PRISMA 2020 statement has been designed primarily for systematic reviews of studies that evaluate the efects of health interventions, irrespective of the design of the included studies. However, the checklist items are applicable to reports of systematic reviews evaluating other ...
A literature search in Medline, EMBASE and CINAHL was performed between 1 and 2010 and 26 November 2020 with studies on SV as inclusion criteria. To be included in this review the studies must include healthcare professionals involved in the aftermath of a patient safety incident. In total 104 studies were included.
The present PRISMA-guided article systematically reviews the current state of research on the autonomous sensory meridian response (ASMR). A systematic literature search was conducted in Pubmed, SCOPUS, and Web of Science (last search: March 2022) selecting all studies that conducted quantitative scientific research on the ASMR phenomenon. Fifty-four studies focusing on ASMR were retrieved ...
PRISMA 2020 should not be used to assess the conduct or methodological quality of systematic reviews; other tools exist for this purpose.[30, 31] Furthermore, PRISMA 2020 is not intended to inform the reporting of systematic review protocols, for which a separate statement is available (PRISMA for Protocols (PRISMA-P) 2015 statement [47,48]).
The purpose of this study is to develop a proposal for a future research agenda on university rankings, based on a systematic review of the existing literature, ith emphasis on the following criteria: objectives, countries, types, variables, methodologies, and future lines of research. The analysis of university rankings revealed a need to review evaluation methodologies and reflect on their ...
Objective: The present systematic review assessed the efficacy of peri-procedurally administered trimetazidine in the prevention of contrast-induced nephropathy (CIN) in patients undergoing coronary interventions with contrast agents. Methods: We performed a systematic literature review of articles published in PubMed and Google Scholar by 7 December 2023 and included articles from the last 15 ...
In this section you can find out more about the PRISMA Statement, obtain downloads of PRISMA documents, find out more about PRISMA development, and information about funding. Find out more about the following here: PRISMA 2020 Statement. PRISMA 2020 Explanation and Elaboration (E&E) PRISMA 2020 checklist. PRISMA 2020 flow diagram.
The duplex theory posits that consumer animosity towards brands emerges when expectations remain unmet, culminating in dissatisfaction. Despite increasing interest in brand hate within the realms of consumerism, academic discourse on this multifaceted emotion remains limited. Consequently, this paper endeavours to execute a systematic review, synthesising extant literature on brand hate. An ...
Background: Persistent symptoms in coeliac disease (CD) can be due to not only poor gluten-free diet (GFD) adherence and complications of CD, but also functional gastrointestinal disorders such as irritable bowel syndrome (IBS). Although the role of a low fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet is well-established in IBS, little data are available on its role in ...
Object moved to here.