Advertisement

Current and Emerging Therapies for Chronic Spontaneous Urticaria: A Narrative Review
- Open access
- Published: 29 June 2023
- Volume 13 , pages 1647–1660, ( 2023 )
Cite this article
You have full access to this open access article

- Gil Yosipovitch 1 ,
- Georgia Biazus Soares 1 &
- Omar Mahmoud 1
5896 Accesses
5 Citations
17 Altmetric
Explore all metrics
Chronic spontaneous urticaria (CSU) is a condition in which wheals, angioedema, and pruritus occur spontaneously and recurrently for at least 6 weeks. The etiology of this disease is partially dependent on production of autoantibodies that activate and recruit inflammatory cells. Although the wheals can resolve within 24 h, symptoms have a significant detrimental impact on the quality of life of these patients. Standard therapy for CSU includes second-generation antihistamines and omalizumab. However, many patients tend to be refractory to these therapies. Available treatments such as cyclosporine, dapsone, dupilumab, and tumor necrosis factor alpha (TNFa) inhibitors have been used with success in some cases. Furthermore, various biologics and other novel drugs have emerged as potential treatments for this condition, and many more are currently under investigation in randomized clinical trials.
Similar content being viewed by others

Pathophysiology, Diagnosis, and Management of Chronic Spontaneous Urticaria: A Literature Review
Treatment of severely recalcitrant chronic spontaneous urticaria: a discussion of relevant issues.

Targeted Therapy for Chronıc Spontaneous Urtıcarıa: Ratıonale and Recent Progress
Avoid common mistakes on your manuscript.
Chronic spontaneous urticaria is a debilitating disease that can negatively impact quality of life |
Few therapies are approved for this condition, and patients can be refractory to these treatments |
In this article, we highlight the various novel drugs that are currently being studied in clinical trials for this disease |
Introduction
Chronic urticaria is characterized by wheals, angioedema, or both that occur continuously or sporadically for at least 6 weeks. The wheals are intensely pruritic, resolve in < 24 h, and tend to recur on a daily basis [ 1 ]. Chronic urticaria typically lasts for 2–5 years before spontaneously resolving [ 2 ]. The point prevalence of the disease ranges from 0.23 to 0.70% in different studies, and it most commonly affects women [ 3 , 4 ]. Pruritus in these patients frequently occurs on a daily basis, with many patients reporting increased itching at night. It most commonly occurs on the back, upper extremities, and lower extremities and is often accompanied by a heat sensation [ 5 ]. Chronic urticaria can cause physical and emotional functional impairments as well as sleep disturbances, fatigue, and irritability due to pruritus, leading to significantly reduce quality of life in these patients [ 6 ]. Patients have also been shown to suffer from psychiatric comorbidities such as anxiety, depression, and somatoform disorders at higher rates than controls [ 7 ].
Chronic urticaria can be further classified as chronic inducible urticaria (CIU), in which symptoms are evoked by temperature changes or pressure, or chronic spontaneous urticaria (CSU), in which lesions occur spontaneously [ 1 ]. The pathogenesis of CSU is thought to involve an autoimmune component that can be characterized by the presence of IgE autoantibodies (type I or autoallergic) or IgG autoantibodies (type IIb) [ 8 ]. Both of these endotypes involve mast cell activation, which is the hallmark of the disease and leads to vasodilation, recruitment of inflammatory cells, and sensory neural activation [ 8 ]. Other mechanisms are also thought to play a role in the etiology of CSU, including alterations in the coagulation cascade, vitamin D deficiency, and infections [ 9 ]. d -dimer levels have been suggested to be a biomarker of CSU severity and may help clinicians in decision making about escalating to more aggressive treatment with biologics or higher medication doses [ 10 , 11 ].
While guidelines that contain a stepwise treatment algorithm with mainstay CSU therapies were recently published, a myriad of drugs have emerged as effective treatments for CSU, and many more are currently under investigation [ 12 ]. In this review, we will discuss available treatments for CSU as well as novel therapies that could provide promising outcomes.
A literature review was performed on PubMed to retrieve relevant English-language articles on the mentioned chronic spontaneous urticaria treatments between 2010 and 2023. The national clinical trial database ClinicalTrials.gov was used to obtain information regarding any ongoing clinical trials. References and citations from retrieved articles were also assessed to obtain other relevant literature. The considered relevant papers were accessed and included in the review. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Current and Available Treatments
Second-generation h1-antihistamines (sgahs).
Second-generation H1-antihistamines (sgAHs) such as loratadine, desloratadine, cetirizine, and levocetirizine are the first-line treatment for CSU. These are preferred over first-generation antihistamines for they selectively block peripheral receptors, therefore causing fewer side effects, including sedation and anticholinergic effects [ 9 ]. In general, no significant differences are seen between the different sgAHs in controlling CSU symptoms [ 13 ]. Standard doses of antihistamines may not provide relief for many CSU patients, however, with a recent study reporting a response rate of only 38.6% [ 14 ]. Guidelines recommend that the dose should be increased up to fourfold in patients who do not adequately respond to standard doses, with bilastine, fexofenadine, levocetirizine, and cetirizine having good-quality evidence for up-dosing [ 12 , 15 ]. Dose escalation by two- or four-fold has been shown to lead to complete remission in 63.2% of CSU cases [ 14 ]. Furthermore, a cohort study of 175 pediatric chronic urticaria patients showed that 92% of cases were well controlled with sgAHs, and these medications were found to have a favorable safety profile in randomized controlled trials (RTCs) evaluating children [ 16 , 17 , 18 ]. The addition of a second sgAH or another medication such as a H2-antihistamine or leukotriene receptor antagonist (LTRA) can also be considered in certain cases [ 19 ]. The addition of LTRA montelukast to first-line therapy with sgAHs, for example, was found to be particularly effective in patients with angioedema-predominant CSU, leading to disease resolution in about two-thirds of cases [ 20 ].
Omalizumab is a recombinant humanized monoclonal antibody against IgE that was approved by the Food and Drug Administration (FDA) for the treatment of antihistamine-refractory CSU. It works by binding to free IgE and inhibiting interaction with the FcεRI receptor on basophils and mast cells, thus preventing their activation (Fig. 1 ). It also increases free IgE levels, leading to downregulation of FcεRI receptors [ 9 ]. Many RTCs have been conducted to evaluate the efficacy of omalizumab in CSU patients. One meta-analysis including seven trials reported that patients treated with omalizumab had significantly reduced itch scores and wheal scores compared to placebo, with the strongest reduction in weekly itch scores seen in those taking 300 mg every 4 weeks [ 21 ]. Improvements in quality of life are also seen with this therapy, with one systematic review reporting an improvement of 13.12 points on a scale of 23–115 using the validated chronic urticaria quality of life questionnaire [ 22 ].
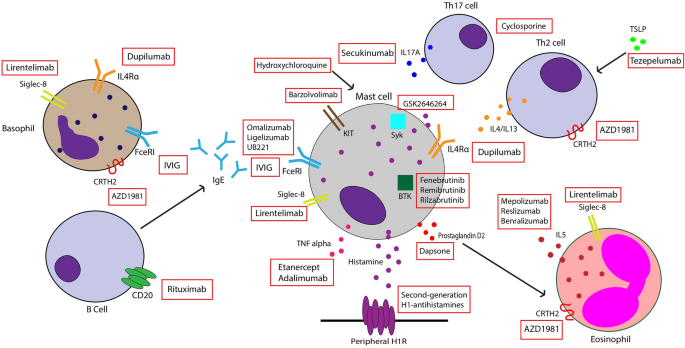
Mechanism of action of some existing and novel therapies for chronic spontaneous urticaria
The response to treatment may vary depending on a patient’s disease endotype. Patients with type I CSU who have IgE autoantibodies against allergens such as thyroperoxidase (TPO) usually respond quickly and effectively to omalizumab. A RTC assessing patients who had antihistamine-refractory CSU and IgE anti-TPO reported that 70.4% of patients demonstrated complete protection from wheal development compared to 4.5% in the placebo group, and complete absence of pruritus was seen in 59.3% of patients compared to 9.1% of controls [ 23 ]. Those with type IIb CSU are less likely to respond to omalizumab and have delayed onset of response to the drug [ 8 ]. Many studies have shown that patients who partially respond to the standard dose of 300 mg every 4 weeks can benefit from dose increases [ 24 ]. Omalizumab is generally well tolerated, and the most common adverse effects include injection site reactions, viral infections, upper respiratory infections, sinusitis, and headaches [ 25 ]. Patient monitoring is often recommended after the first three doses of the drug because anaphylaxis—although rare—is more likely to occur within this time frame [ 26 ]. In the last 2 years, the FDA has also approved home self-administration of omalizumab [ 27 ].
Cyclosporine
Cyclosporine is an immunosuppressant that has been used as an off-label add-on therapy in patients who are refractory to monotherapy with antihistamines or omalizumab [ 9 ]. A meta-analysis evaluating efficacy of cyclosporine in CSU revealed a significant change from baseline in mean urticaria activity score (which measures the intensity of wheals and pruritus) for those taking cyclosporine compared to placebo [ 28 ]. However, Savic et al. found that omalizumab was superior to cyclosporine in disease resolution and quality of life improvements [ 29 ]. Patients with biomarkers indicative of type IIb CSU were found to have quicker and more favorable response to cyclosporine [ 30 ]. There are a variety of adverse effects associated with this agent, including nausea, vomiting, headaches, hypertension, and nephrotoxicity, and many of these are dose-dependent. Although most side effects resolve with dose reduction, they have been shown to lead to treatment discontinuation in up to 18.1% of CSU patients [ 28 ].
Other immunosuppressants
Other immunosuppressants such as methotrexate (MTX), azathioprine, and mycophenolate mofetil (MMF) have been used off-label to treat CSU. MTX did not demonstrate efficacy as an add-on treatment to antihistamines in randomized clinical trials [ 31 , 32 , 33 ]. Azathioprine has been shown to reduce chronic urticaria symptoms and was reported to be efficacious in patients with refractory CSU [ 34 , 35 ]. Furthermore, small studies have shown that MMF improved urticarial symptoms in 88% of patients with CSU and that it significantly decreased the number of wheals and itch severity in patients refractory to antihistamines and corticosteroids [ 36 , 37 ].
Dapsone is a sulfone antibiotic that has been used to treat various dermatologic conditions, and its anti-inflammatory properties could be helpful in treating CSU. In a small open study of 11 refractory CSU patients, 9 patients experienced a complete response after 3 months of treatment with dapsone and cetirizine [ 38 ]. The effect of dapsone has been reported to last as long as 1 year, with a case report showing no recurrence in CSU symptoms despite discontinuation of all treatments [ 39 ]. Treatment with dapsone for 6 weeks showed a significant reduction in both itch and visual analog scale (VAS) ratings of urticaria severity compared to placebo in a cohort of antihistamine-refractory patients. However, only 3 out of 22 patients showed complete resolution of pruritus and hives with this therapy [ 40 ]. A retrospective chart review by Liang et al. found that 78% of patients with CSU improved with dapsone therapy, and 44% of these patients achieved complete response after a mean time of 5.2 months. Adverse effects were mild and most commonly included reticulocytosis and anemia [ 41 ]. Furthermore, a recent systematic review and meta-analysis found that treatment with dapsone resulted in a large increase in clinical improvement, although it caused little to no difference in remission rates [ 42 ]. The authors of these studies concluded that dapsone is an effective second-line treatment modality in CSU patients.
Hydroxychloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug that is useful in managing some autoimmune and inflammatory conditions. Although its mechanism of action in CSU is not well understood, several studies have shown it can reduce symptoms in these patients. A study evaluating antihistamine-refractory CSU patients found that 20.8% of patients who received hydroxychloroquine daily in addition to H1-antihhistamines experienced disease remission at 12 weeks compared to no patients achieving remission in the placebo group (antihistamines only) [ 43 ]. Furthermore, there were significant improvements in severity scores and quality of life in those treated with HCQ compared to placebo. However, no significant difference was found in urticarial severity scores or urticarial responses between HCQ and a leukotriene receptor antagonist in a follow-up study [ 43 ]. Other studies have also found that HCQ improves the quality of life in CSU [ 44 ]. A recent retrospective chart review including a cohort of 264 refractory CSU patients found that although omalizumab add-on therapy was associated with a higher rate of complete response, 66% of patients on HCQ achieved complete, sustained disease response at 1 year. No retinal abnormalities were found on serial visual field testing in those treated with HCQ. Therefore, the study found that HCQ is an acceptable alternative to omalizumab, especially due to its lower cost [ 45 ].
Intravenous immunoglobulin (IVIG)
IVIG is a polyvalent antibody product derived from plasma of healthy donors. Its various effects on the immune system include inactivating autoreactive T cells, downregulating B-cell antibody production, and blocking FcεR-mediated activity (Fig. 1 ) [ 46 ]. IVIG has been used in the treatment of autoimmune disorders and was proposed for treatment of refractory CSU by O’Donnell et al., who reported significant improvement in urticaria activity scores after IVIG treatment compared to baseline [ 47 ]. In another case series, administration of IVIG led to remission after 12 months in four out of six patients. Many of these patients experienced a decrease in urticaria activity score after 1–3 cycles of treatment. Adverse effects included headache, chills, fever, and increased blood pressure [ 46 ]. Another study found that 73% of patients had complete remission of symptoms with use of IVIG every 4 weeks, although the number of infusions needed to achieve disease control varied widely between patients [ 48 ]. However, no randomized clinical trials have evaluated the efficacy of this therapy in CSU patients, and more robust data are needed to support its use.
Autologous serum therapy
Autologous serum therapy (AST) involves injecting a patient’s own serum subcutaneously or intramuscularly (IM). Studies have showed mixed results in the efficacy of IM AST in patients with a positive autologous serum skin test (ASST). In a study with 66 CSU patients, AST therapy led to significant improvement in disease activity after 8 weeks [ 49 ]. However, another prospective study found that, after 12 weeks, only 12% of patients experienced partial or complete remission with this therapy [ 50 ]. Subcutaneous AST has been reported to significantly decrease urticarial activity scores (UAS) and to improve quality of life in CSU patients [ 51 , 52 ].
Inhibition of Th2 cytokines such as interleukin (IL)-4, IL-5, and IL-13 leads to decreased IgE production and limited activation of mast cells and basophils, all of which play a role in CSU pathogenesis. Dupilumab is a monoclonal antibody against IL-4/13Rα that has been used off-label for the treatment of CSU. It has been shown to be effective within 3 months in patients with refractory CSU, with a case series reporting that 67% of patients started on dupilumab remained in remission after treatment discontinuation [ 53 ]. Results from a phase III RTC show that patients on a standard dose of antihistamines who received add-on dupilumab reported significant changes in itch severity scores and urticaria activity scores compared to those treated with antihistamine monotherapy [ 54 ]. Other RTCs to further evaluate dupilumab efficacy and safety in CSU patients are currently ongoing, and further studies are needed to assess dupilumab therapy in omalizumab-refractory patients [ 8 , 9 ].
Rituximab is a monoclonal antibody against CD20 on B cells and has been proposed as an alternative therapy in CSU patients because of its potential ability to inhibit IgG autoantibodies against FcεRI or IgE [ 55 ]. There have been several case reports in which rituximab has been successfully used to treat refractory CSU [ 56 , 57 , 58 ].One phase I/II open-label trial aimed to assess rituximab use in these patients but was terminated because of safety concerns [ 9 ]. Currently, more data are needed to support the use of this drug in CSU patients.
TNFa Inhibitors
Mast cell activation leads to the release of various inflammatory mediators, including TNFa. TNFa production is increased in CSU patients, and these patients also have increased TNFa expression in both lesional and nonlesional skin compared to healthy controls [ 59 , 60 ]. Furthermore, serum levels of TNFa are positively correlated to disease severity assessed by the UAS [ 61 ]. TNFa inhibitors such as etanercept and adalimumab commonly used for psoriasis have been tried off-label in CSU patients. One retrospective study reported 60% of patients had complete or almost complete symptom resolution after treatment with TNFa inhibitors, and many had sustained responses with a mean of 13 months [ 62 ]. TNFa inhibitors have also been used to successfully treat immunosuppressive-refractory CSU patients, and even led to disease remission in three out of six cases [ 63 ]. However, RTCs with a larger study population are needed to provide a better assessment of the efficacy and safety of these agents in CSU.
Secukinumab
IL-17 is implicated in cutaneous diseases such as psoriasis, and it may also play a role in the pathogenesis of CSU. Levels of this cytokine were found to be elevated in the serum of CSU patients and correlated with disease activity [ 64 , 65 ]. IL-17 is also highly expressed in both lesional and nonlesional skin of CSU patients compared to controls, with increased IL-17A expression observed in skin mast cells [ 66 ]. This indicates that IL-17 may contribute to the increased mast cell degranulation seen in this disease. Secukinumab, an IL-17 inhibitor, was used to treat eight patients who were refractory to standard CSU treatments. The intensity of wheals and pruritus—measured by the urticaria activity score—was significantly reduced by 55% from baseline after 30 days, and by 82% after 90 days, and patients remained in remission throughout the duration of therapy [ 66 ]. More studies evaluating this drug in CSU patients are warranted.
Novel Therapies and Therapies Under Development
Anti-ige monoclonal antibodies: ligelizumab, ub221.
Ligelizumab is a novel humanized monoclonal anti-IgE antibody that works by binding to IgE with a much higher affinity than omalizumab. It has been shown that this drug is more potent than omalizumab at suppressing free IgE levels and at reducing expression of FcεRI and IgE on basophils [ 67 ]. A phase IIb study evaluated three doses of ligelizumab (24 mg, 72 mg, and 240 mg) and found that this drug achieved complete symptom control in up to 44% of CSU patients by week 12 compared to 26% of patients taking omalizumab [ 68 ]. Furthermore, the percentage of patients achieving a weekly itch severity score of 0 was higher in all ligelizumab groups compared to omalizumab at week 12 [ 68 ]. An extension, open-label study was subsequently performed and demonstrated sustained efficacy of the drug, with 84.2% of patients having cumulatively achieved minimal disease activity by week 52 [ 69 ]. A multicenter phase III RTC evaluating the use of this drug in adolescents and adults with CSU and comparing ligelizumab with omalizumab has recently been completed. Preliminary data confirm the efficacy of ligelizumab in CSU patients compared to placebo, although it did not find this drug to be superior to omalizumab when assessing changes from baseline in urticaria activity scores at week 12 (NCT03580369, https://www.hcplive.com/view/phase-3-ligelizumab-not-superior-omalizumab ). A phase III clinical trial evaluating the efficacy and safety of ligelizumab in CSU was recently terminated, but this was not due to safety concerns (NCT04210843). Ligelizumab was found to have a favorable safety profile, with the most common adverse events being injection site reactions, nasopharyngitis, headache, upper respiratory tract infection, and urticaria [ 69 ].
UB221 is a monoclonal antibody that binds to CD23-bound IgE, thus downregulating CD23-mediated IgE production. It also binds to IgE with higher affinity than omalizumab, with a rapid reduction in serum IgE observed after one IV dose of UB221 in mice models [ 70 ]. UB221 is currently under investigation in phase I and phase II studies as a potential add-on therapy for patients with CSU (Table 1 ) (NCT03632291, NCT05298215). Other RTCs evaluating the pharmacodynamics, pharmacokinetics, safety, and efficacy of UB221 are also underway (NCT04175704, NCT04404023).
Bruton Tyrosine Kinase (BTK) inhibitors: Fenebrutinib, Remibrutinib, Rilzabrutinib
BTK is an enzyme involved in FcεRI-mediated mast cell activation, basophil signaling, and B-cell functioning. BTK inhibitors prohibit IgE-mediated responses and thus can be a useful therapy in CSU patients [ 8 , 55 ]. Fenebrutinib is a highly selective BTK inhibitor that is given orally and was recently evaluated in a phase II RTC for treatment of antihistamine-resistant CSU. By week 8, improvements in urticarial activity scores were seen in patients randomized to 200 mg twice daily and 150 mg daily but not 50 mg daily compared to placebo. This response was dose-dependent, with 39% of patients taking 200 mg twice daily achieving complete response at week 8 compared to 25% of those taking 150 mg daily and 4% of those taking the placebo [ 71 ]. Adverse effects were minimal and most commonly included headache, urticaria, and nasopharyngitis [ 71 ]. Remibrutinib, another potent oral BTK inhibitor, was also found to improve antihistamine-resistant CSU symptoms in a phase II RTC. Multiple doses were tested, and all were found to significantly reduce urticaria activity symptom scores. Improvement was observed as early as week 1 and maintained through the end of the study at week 12 [ 72 ]. This agent was also shown to improve the quality of life in CSU patients, with significant improvements in Dermatology Life Quality Index (DLQI) scores observed at week 12 compared to placebo [ 73 ]. The efficacy and safety of BTK inhibitor rilzabrutinib are currently being assessed in a phase II RTC (NCT05107115).
Anti-IL5 Monoclonal Antibodies: Mepolizumab, Reslizumab, Benralizumab
Cutaneous eosinophilic infiltration secondary to mast cell recruitment via IL-5 may contribute to the pathogenesis of CSU, for these patients have increased eosinophils in both lesional and nonlesional skin compared to controls [ 74 , 75 ]. Mepolizumab, an anti-IL5 monoclonal antibody, is approved for treatment of eosinophilic asthma and has been used off-label to treat CSU [ 76 ]. A current phase I open-label study is underway to assess the efficacy of 10 weeks of mepolizumab therapy in these patients (NCT03494881). Reslizumab, another monoclonal antibody that targets IL-5, led to sustained improvement in urticarial symptoms in a patient with angioedema-predominant CSU 4 weeks after starting treatment [ 77 ]. Furthermore, the anti-IL5 receptor antibody benralizumab has also been investigated as a potential therapy for CSU. Benralizumab was administered in 3 monthly injections to a cohort of 12 CSU patients who were refractory to antihistamines. After the three doses, the urticarial activity score decreased significantly by 15.7 points, and five patients achieved complete response at the end of the study [ 78 ]. In an ongoing phase II RTC, benralizumab induction and maintenance dosing regimens are being evaluated in a cohort of adult patients who were refractory to antihistamines (NCT04612725).
Tezepelumab
Thymic stromal lymphopoietin (TSLP) is a cytokine that promotes Th2 inflammation. TSLP expression was shown to be increased in lesional skin of patients with CSU, indicating it may play a role in the pathogenesis of the disease [ 79 ]. Therefore, blocking TSLP action with drugs such as the monoclonal antibody tezepelumab may be useful in reducing inflammation in CSU. The INCEPTION study, a phase II RTC evaluating the efficacy of tezepelumab in adult CSU patients compared to omalizumab and placebo, is currently underway but no results have been published (NCT04833855).
Lirentelimab
Sialic acid-binding, immunoglobulin-like lectin (Siglec)-8 is an inhibitory receptor selectively expressed on mast cells, basophils, and eosinophils (Fig. 1 ). Binding of monoclonal antibodies to this receptor leads to apoptosis of eosinophils and inhibition of FcεRI-mediated mast cell activation [ 80 ]. Due to its mechanism of action, lirentelimab, an anti-Siglec-8 monoclonal antibody, has been explored as a treatment for CSU. In a phase II study, both omalizumab-naïve and -resistant CSU patients were treated with intravenous infusions of lirentelimab. Patients experienced symptomatic improvement and decreases in urticaria activity scores from baseline at week 22. Furthermore, 95% of omalizumab-naïve and 46% of omalizumab-refractory CSU patients experienced complete response at week 22. The most common adverse events included infusion-related reactions, nasopharyngitis, and headache [ 81 ]. A study to assess subcutaneous liretenlimab in adult CSU patients is ongoing (NCT05528861).
Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) is a prostaglandin D2 (PGD2) receptor expressed on basophils, eosinophils, and some Th2 cells. By binding to CRTH2, PGD2—which is released by mast cells—leads to activation and chemotaxis of eosinophils and basophils [ 80 ]. Increased eosinophilic CRTH2 expression has been reported in allergic skin diseases, including chronic urticaria and atopic dermatitis [ 82 ]. However, Oliver et al. found decreased CRTH2 expression on blood basophils and eosinophils of CSU patients, which they speculated could be due to in vivo PGD2 activation [ 83 ]. The oral selective CRTH2 antagonist AZD1981 has been investigated in antihistamine-resistant CSU patients and was found to have a greater effect on weekly itch scores than on hives during a 4-week treatment period and into the washout period [ 84 ]. Treatment with AZD1981 was also found to increase circulating eosinophils and reduce PGD2-mediated eosinophil shape change [ 84 ]. More studies are needed to evaluate the long-term efficacy of this agent in CSU.
Barzolvolimab
The KIT receptor and its ligand stem cell factor (SCF) are important regulators of mast cell differentiation, maturation, and survival. Barzolvolimab is a monoclonal anti-KIT antibody and is a promising new treatment for CSU patients. A study in healthy human volunteers showed that barzolvolimab inhibits SCF-dependent mast cell activation and leads to systemic mast cell ablation [ 85 ]. A recently published abstract of an ongoing phase I clinical trial reported that CSU patients receiving IV barzolvolimab had dose-dependent improvements in urticarial activity score by week 8 of treatment [ 86 ]. A phase II study assessing this novel therapy in CSU patients is currently underway (NCT05368285).
Spleen tyrosine kinase (Syk) is involved in signal transduction in various immune cells and plays an important role in FcεRI signaling and activation of mast cells [ 80 ]. The selective, topical Syk inhibitor GSK2646264 has been shown to decrease histamine release from skin mast cells, indicating that this therapy could potentially be used in CSU patients [ 87 ]. A phase I study evaluating GSK2646264 in healthy volunteers, cold urticaria, and CSU patients found that the drug was well tolerated but no conclusions could be made about changes in urticaria activity score due to the low number of CSU patients recruited [ 88 ]. More studies are needed to evaluate the efficacy of GSK2646264 in these patients.
Limitations
The main limitation of this narrative review is that it does not directly compare the efficacy of different treatment modalities between studies. Therefore, we are unable to provide data on which therapies are the most effective new treatments for CSU.
Chronic spontaneous urticaria is a debilitating disease that can negatively impact the quality of life of patients. Although antihistamines and omalizumab are the recommended therapies for this condition, many patients continue to experience hives and pruritus despite the use of these treatments. Many novel therapies are currently being investigated for use in CSU, and the results of some clinical trials show great promise. Overall, more studies are needed to assess the efficacy and safety of these emerging drugs.
Lang DM. Chronic urticaria. N Engl J Med. 2022;387(9):824–31.
CAS PubMed Google Scholar
Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105(4):664–72.
Fricke J, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75(2):423–32.
PubMed Google Scholar
Wertenteil S, Strunk A, Garg A. Prevalence estimates for chronic urticaria in the United States: a sex-and age-adjusted population analysis. J Am Acad Dermatol. 2019;81(1):152–6.
Yosipovitch G, Ansari N, Goon A, Chan Y, Goh C. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br J Dermatol. 2002;147(1):32–6.
Dias GAC, et al. Impact of chronic urticaria on the quality of life of patients followed up at a university hospital. An Bras Dermatol. 2016;91:754–9.
PubMed PubMed Central Google Scholar
Özkan M, et al. Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2007;99(1):29–33.
Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(6):1772-1781.e1.
He L, Yi W, Huang X, Long H, Lu Q. Chronic urticaria: advances in understanding of the disease and clinical management. Clin Rev Allergy Immunol. 2021;1–25.
Dabas G, Thakur V, Bishnoi A, Parsad D, Kumar A, Kumaran MS. Causal relationship between D-dimers and disease status in chronic spontaneous urticaria and adjuvant effect of oral tranexamic acid. Indian Dermatol Online J. 2021;12(5):726.
Ghazanfar MN, Thomsen SF. D-dimer as a potential blood biomarker for disease activity and treatment response in chronic urticaria: a focused review. Eur J Dermatol. 2018;28:731–5.
Zuberbier T, Bernstein JA, Maurer M. Chronic spontaneous urticaria guidelines: what is new? J Allergy Clin Immunol. 2022;150(6):1249–55.
Jáuregui I, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17(Suppl 2):41–52.
Guillen-Aguinaga S, Jáuregui Presa I, Aguinaga-Ontoso E, Guillen-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br J Dermatol. 2016;175(6):1153–65.
Podder I, Dhabal A, Chakraborty SS. Efficacy and safety of up-dosed second-generation antihistamines in uncontrolled chronic spontaneous urticaria: a review. J Clin Aesthet Dermatol. 2023;16(3):44–50.
Gabrielli S, et al. Chronic urticaria in children can be controlled effectively with updosing second-generation antihistamines. J Am Acad Dermatol. 2020;82(6):1535–7.
Novák Z, et al. Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol. 2016;27(5):493–8.
Potter P, et al. Rupatadine is effective in the treatment of chronic spontaneous urticaria in children aged 2–11 years. Pediatr Allergy Immunol. 2016;27(1):55–61.
Hon KL, Leung AK, Ng WG, Loo SK. Chronic urticaria: an overview of treatment and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):27–37.
CAS PubMed PubMed Central Google Scholar
Akenroye AT, McEwan C, Saini SS. Montelukast reduces symptom severity and frequency in patients with angioedema-predominant chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2018;6(4):1403–5.
Zhao Z-T, et al. Omalizumab for the treatment of chronic spontaneous urticaria: a meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137(6):1742-1750.e4.
Urgert MC, van den Elzen MT, Knulst AC, Fedorowicz Z, van Zuuren EJ. Omalizumab in patients with chronic spontaneous urticaria: a systematic review and GRADE assessment. Br J Dermatol. 2015;173(2):404–15.
Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128(1):202-209.e5.
Metz M, Vadasz Z, Kocatürk E, Giménez-Arnau AM. Omalizumab updosing in chronic spontaneous urticaria: an overview of real-world evidence. Clin Rev Allergy Immunol. 2020;59(1):38–45.
Licari A, et al. Biologic drugs in chronic spontaneous urticaria. Acta Bio-medica Atenei Parmensis. 2021;92(S7):e2021527–e2021527.
Lieberman PL, Jones I, Rajwanshi R, Rosén K, Umetsu DT. Anaphylaxis associated with omalizumab administration: risk factors and patient characteristics. J Allergy Clin Immunol. 2017;140(6):1734-1736.e4.
Murphy KR, Winders T, Smith B, Millette L, Chipps BE. Identifying patients for self-administration of omalizumab. Adv Ther. 2023;40(1):19–24.
Kulthanan K, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6(2):586–99.
Savic S, et al. Retrospective case note review of chronic spontaneous urticaria outcomes and adverse effects in patients treated with omalizumab or ciclosporin in UK secondary care. Allergy Asthma Clin Immunol. 2015;11:1–11.
CAS Google Scholar
Endo T, et al. Identification of biomarkers for predicting the response to cyclosporine A therapy in patients with chronic spontaneous urticaria. Allergol Int. 2019;68(2):270–3.
Sharma VK, Singh S, Ramam M, Kumawat M, Kumar R. A randomized placebo-controlled double-blind pilot study of methotrexate in the treatment of H1 antihistamine-resistant chronic spontaneous urticaria. Indian J Dermatol Venereol Leprol. 2014;80(2):122–8. https://doi.org/10.4103/0378-6323.129382 .
Article PubMed Google Scholar
Leducq S, et al. Efficacy and safety of methotrexate versus placebo as add-on therapy to H1 antihistamines for patients with difficult-to-treat chronic spontaneous urticaria: a randomized, controlled trial. J Am Acad Dermatol. 2020;82(1):240–3. https://doi.org/10.1016/j.jaad.2019.07.097 .
Article CAS PubMed Google Scholar
Patil AD, Bingewar G, Goldust M. Efficacy of methotrexate as add on therapy to H1 antihistamine in difficult to treat chronic urticaria: a systematic review and meta-analysis of randomized clinical trials. Dermatol Ther. 2020;33(6): e14077. https://doi.org/10.1111/dth.14077 .
Tal Y, Toker O, Agmon-Levin N, Shalit M. Azathioprine as a therapeutic alternative for refractory chronic urticaria. Int J Dermatol. 2015;54(3):367–9.
Pathania YS, Bishnoi A, Parsad D, Kumar A, Kumaran MS. Comparing azathioprine with cyclosporine in the treatment of antihistamine refractory chronic spontaneous urticaria: a randomized prospective active-controlled non-inferiority study. World Allergy Organ J. 2019;12(5): 100033.
Zimmerman AB, Berger EM, Elmariah SB, Soter NA. The use of mycophenolate mofetil for the treatment of autoimmune and chronic idiopathic urticaria: experience in 19 patients. J Am Acad Dermatol. 2012;66(5):767–70.
Shahar E, Bergman R, Guttman-Yassky E, Pollack S. Treatment of severe chronic idiopathic urticaria with oral mycophenolate mofetil in patients not responding to antihistamines and/or corticosteroids. Int J Dermatol. 2006;45(10):1224–7.
Cassano N, D’Argento V, Filotico R, Vena GA. Low-dose dapsone in chronic idiopathic urticaria: preliminary results of an open study. Acta Derm Venereol. 2005;1(1):1–1.
Google Scholar
Noda S, Asano Y, Sato S. Long-term complete resolution of severe chronic idiopathic urticaria after dapsone treatment. J Dermatol. 2012;39(5):496–7.
Morgan M, Cooke A, Rogers L, Adams-Huet B, Khan DA. Double-blind placebo-controlled trial of dapsone in antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2(5):601–6.
Liang SE, Hoffmann R, Peterson E, Soter NA. Use of dapsone in the treatment of chronic idiopathic and autoimmune urticaria. JAMA Dermatol. 2019;155(1):90–5.
Watanabe J, Shimamoto J, Kotani K. The effects of antibiotics for Helicobacter pylori eradication or dapsone on chronic spontaneous urticaria: a systematic review and meta-analysis. Antibiotics. 2021;10(2):156.
Boonpiyathad T, Sangasapaviliya A. Hydroxychloroquine in the treatment of anti-histamine refractory chronic spontaneous urticaria, randomized single-blinded placebo-controlled trial and an open label comparison study. Eur Ann Allergy Clin Immunol. 2017;49(5):220–4.
Reeves G, Boyle M, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34(4):182–6.
Khan N, Epstein TG, DuBuske I, Strobel M, Bernstein DI. Effectiveness of hydroxychloroquine and omalizumab in chronic spontaneous urticaria: a real-world study. J Allergy Clin Immunol Pract. 2022;10(12):3300–5.
Mitzel-Kaoukhov H, Staubach P, Müller-Brenne T. Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2010;104(3):253–8.
Odonnell B, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138(1):101–6.
Pereira C, et al. Low-dose intravenous gammaglobulin in the treatment of severe autoimmune urticaria. Eur Ann Allergy Clin Immunol. 2007;39(7):237–42.
Yu L, et al. Immunological effects and potential mechanisms of action of autologous serum therapy in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2019;33(9):1747–54.
Majid I, Shah S, Hassan A, Aleem S, Aziz K. How effective is autologous serum therapy in chronic autoimmune urticaria. Indian J Dermatol. 2015;60(1):102.
Godse KV, Nadkarni N, Patil S, Mehta A. Subcutaneous autologous serum therapy in chronic spontaneous urticaria. Indian J Dermatol. 2017;62(5):505.
Datta A, Chandra S, Saha A, Sil A, Das NK. Exploring the safety and effectiveness of subcutaneous autologous serum therapy versus conventional intramuscular autologous serum therapy in chronic urticaria: an observer-blind, randomized, controlled study. Indian J Dermatol Venereol Leprol. 2020;86:632.
Abadeh A, Lee JK. Long-term follow-up of patients treated with dupilumab for chronic spontaneous urticaria: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221117702.
Maurer M, et al. Dupilumab significantly reduces itch and hives in patients with chronic spontaneous urticaria: results from a phase 3 trial (LIBERTY-CSU CUPID Study A). J Allergy Clin Immunol. 2022;149(2):AB312.
Kolkhir P, Altrichter S, Munoz M, Hawro T, Maurer M. New treatments for chronic urticaria. Ann Allergy Asthma Immunol. 2020;124(1):2–12.
Chakravarty SD, Yee AF, Paget SA. Rituximab successfully treats refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J Allergy Clin Immunol. 2011;128(6):1354–5. https://doi.org/10.1016/j.jaci.2011.08.023 .
Arkwright PD. Anti-CD20 or anti-IgE therapy for severe chronic autoimmune urticaria. J Allergy Clin Immunol. 2009;123(2):510–1. https://doi.org/10.1016/j.jaci.2008.11.043 . ( author reply 511 ).
Combalia A, Losno RA, Prieto-Gonzalez S, Mascaro JM. Rituximab in refractory chronic spontaneous urticaria: an encouraging therapeutic approach. Skin Pharmacol Physiol. 2018;31(4):184–7. https://doi.org/10.1159/000487402 .
Piconi S, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002;128(1):59–66. https://doi.org/10.1159/000058004 .
Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol. 1999;103(2 Pt 1):307–14. https://doi.org/10.1016/s0091-6749(99)70506-3 .
Sharma P, Sharma PK, Chitkara A, Rani S. To evaluate the role and relevance of cytokines IL-17, IL-18, IL-23 and TNF-alpha and their correlation with disease severity in chronic urticaria. Indian Dermatol Online J. 2020;11(4):594–7. https://doi.org/10.4103/idoj.IDOJ_396_19 .
Article PubMed PubMed Central Google Scholar
Sand FL, Thomsen SF. Off-label use of TNF-alpha inhibitors in a dermatological university department: retrospective evaluation of 118 patients. Dermatol Ther. 2015;28(3):158–65. https://doi.org/10.1111/dth.12222 .
Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfa inhibitors. J Am Acad Dermatol. 2011;64(6):1221–2. https://doi.org/10.1016/j.jaad.2009.10.043 .
Atwa M, Emara A, Youssef N, Bayoumy N. Serum concentration of IL-17, IL-23 and TNF-α among patients with chronic spontaneous urticaria: association with disease activity and autologous serum skin test. J Eur Acad Dermatol Venereol. 2014;28(4):469–74.
Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017;7(1):17797.
Sabag D, et al. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin Exp Allergy. 2020;50(7):799–804.
Arm JP, et al. Pharmacokinetics, pharmacodynamics and safety of QGE 031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–85.
Maurer M, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381(14):1321–32.
Maurer M, et al. Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: a one-year extension study. Allergy. 2022;77(7):2175–84.
Kuo B-S, et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J Clin Investig. 2022;132(15): e157765.
Metz M, et al. Fenebrutinib in H1 antihistamine-refractory chronic spontaneous urticaria: a randomized phase 2 trial. Nat Med. 2021;27(11):1961–9.
Maurer M, et al. Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;150(6):1498-1506.e2.
Maurer M, et al. Remibrutinib treatment improves quality of life in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;149(2):AB179.
Kay A, et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br J Dermatol. 2014;171(3):505–11.
Altrichter S, et al. The role of eosinophils in chronic spontaneous urticaria. J Allergy Clin Immunol. 2020;145(6):1510–6.
Magerl M, et al. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. JDDG Journal der Deutschen Dermatologischen Gesellschaft. 2018;16(4):477–8.
Maurer M, Altrichter S, Metz M, Zuberbier T, Church M, Bergmann K. Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J Eur Acad Dermatol Venereol JEADV. 2017;32(3):e112–3.
Bernstein JA, Singh U, Rao MB, Berendts K, Zhang X, Mutasim D. Benralizumab for chronic spontaneous urticaria. N Engl J Med. 2020;383(14):1389–91.
Kay A, Clark P, Maurer M, Ying S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br J Dermatol. 2015;172(5):1294–302.
Johal KJ, Saini SS. Current and emerging treatments for chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2020;125(4):380–7.
Altrichter S, et al. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J Allergy Clin Immunol. 2022;149(5):1683-1690.e7.
Yahara H, Satoh T, Miyagishi C, Yokozeki H. Increased expression of CRTH2 on eosinophils in allergic skin diseases. J Eur Acad Dermatol Venereol JEADV. 2009;24(1):75–6.
Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS. Altered expression of chemoattractant receptor–homologous molecule expressed on TH2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2016;137(1):304-306.e1.
Oliver ET, et al. Effects of an oral CRTh2 antagonist (AZD1981) on eosinophil activity and symptoms in chronic spontaneous urticaria. Int Arch Allergy Immunol. 2019;179(1):21–30.
Alvarado D, et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy. 2022;77(8):2393–403.
Maurer M, et al. Safety and clinical activity of multiple doses of barzolvolimab, an anti-KIT antibody, in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2023;151(2):AB133.
Ramirez Molina C, Falkencrone S, Skov PS, Hooper-Greenhill E, Barker M, Dickson MC. GSK2646264, a spleen tyrosine kinase inhibitor, attenuates the release of histamine in ex vivo human skin. Br J Pharmacol. 2019;176(8):1135–42.
Dickson MC, et al. Effects of a topical treatment with spleen tyrosine kinase inhibitor in healthy subjects and patients with cold urticaria or chronic spontaneous urticaria: results of a phase 1a/b randomised double-blind placebo-controlled study. Br J Clin Pharmacol. 2021;87(12):4797–808.
Download references
Acknowledgements
No funding or sponsorship was received for this study or publication of this article.
Medical Writing and Editorial Assistance
No medical writing or editorial assistance was used for this article.
Author Contributions
Georgia Biazus Soares- writing and editing of the manuscript, creation of the figure; Omar Mahmoud- writing of the manuscript; Gil Yosipovitch- concept design, reviewing and editing of the manuscript.
Disclosures
Georgia Biazus Soares and Omar Mahmoud have nothing to disclose. Dr. Gil Yosipovitch serves as an advisory board member for Abbvie, Arcutis, BMS, Cara Therapuetics, GSK. Escient Health, Eli Lilly, Galderma, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Pierre Fabre, Regeneron Pharmaceuticals, Inc., Sanofi, TreviTherapeutics, and Vifor; Dr. Gil Yosipovitch receives grants/research funding from Eli Lilly, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Galderma, Escient, Sanofi Regeneron, and Celldex; Dr. Gil Yosipovitch Received Pathways grants as Investigator for Regeneron Pharmaceuticals, Inc. Sanofi.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and affiliations.
Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, Miami Itch Center, University of Miami Miller School of Medicine, 5555 Ponce de Leon, Coral Gables, FL, 33146, USA
Gil Yosipovitch, Georgia Biazus Soares & Omar Mahmoud
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gil Yosipovitch .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Yosipovitch, G., Biazus Soares, G. & Mahmoud, O. Current and Emerging Therapies for Chronic Spontaneous Urticaria: A Narrative Review. Dermatol Ther (Heidelb) 13 , 1647–1660 (2023). https://doi.org/10.1007/s13555-023-00972-6
Download citation
Received : 15 May 2023
Accepted : 20 June 2023
Published : 29 June 2023
Issue Date : August 2023
DOI : https://doi.org/10.1007/s13555-023-00972-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic spontaneous urticaria
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Sign in through your institution
- Advance Articles
- BAD Guidelines
- Cover Archive
- Plain Language Summaries
- Supplements
- Trending Articles
- Virtual Issues
- Author Guidelines
- Reviewer Guidelines
- Submission Site
- Open Access Options
- Reasons to Publish
- Self-Archiving Policy
- About British Journal of Dermatology
- About British Association of Dermatologists
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Purpose and scope, methodology, summary of recommendations, appropriate investigations, interventions, considerations, recommended audit points, stakeholder involvement and peer review, limitations of the guideline, plans for guideline revision, acknowledgements.
- < Previous
British Association of Dermatologists guidelines for the management of people with chronic urticaria 2021 *
The author affiliations can be found in the Appendix.
Conflicts of interest The following interests were declared over the duration of the guideline development. C.E.H.G.: honoraria and research investigator – Novartis – specific. M.R.A.‐J.: (1) sponsorship to conferences – Allergy Therapeutics, Novartis, Celgene – specific; (2) grant/research support – AbbVie, Emblation, Unilever – nonspecific. A.B.: (1) consultant, AbbVie, Almirall, Eli Lilly, LEO Pharma, Galderma, Novartis, Pierre Fabre, Janssen Pharmaceuticals, UCB – specific. C.F.: sponsorship to attend a conference – Novartis – specific; (2) travel grants from LEO Pharma, Almirall, Novartis – specific; (3) investigator‐initiated studies from Janssen and LEO Pharma – nonspecific. T.A.L.: advisory board – Galderma, La Roche‐Posay, Novartis – specific. A.M.M.: (1) consultant and advisory board – Novartis – specific; (2) invited speaker – Novartis – specific. G.O.: advisory board – Novartis – specific. R.A.S., F.L., L.C., W.A.C.S., M.H. L.S.E., M.F.M.M. and M.C.E. declare they have no conflicts of interest.
Produced in 2001 by the British Association of Dermatologists. Reviewed and updated 2010, 2021
This is an updated guideline prepared for the British Association of Dermatologists (BAD) Clinical Standards Unit, which includes the Therapy & Guidelines Subcommittee (T&G). Members of the Clinical Standards Unit who have been involved are N.J. Levell (Chair, T&G), S.L. Chua, P. Laws, H. Frow, A. Bardhan, A. Daunton, G. Petrof, M. Hashme (BAD Information Scientist), L.S. Exton (BAD Senior Guideline Research Fellow), M.C. Ezejimofor (BAD Guideline Research Fellow) and M.F. Mohd Mustapa (BAD Director of Clinical Standards).
NICE has renewed accreditation of the process used by the British Association of Dermatologists to produce clinical guidelines. The renewed accreditation is valid until 31 May 2026 and applies to guidance produced using the processes described in the updated guidance for writing a British Association of Dermatologists clinical guideline – the adoption of the GRADE methodology 2016. The original accreditation term began on 12 May 2010. More information on accreditation can be viewed at www.nice.org.uk/accreditation .
* Plain language summary available online
- Article contents
- Figures & tables
- Supplementary Data
R.A. Sabroe, F. Lawlor, C.E.H. Grattan, M.R. Ardern‐Jones, A. Bewley, L. Campbell, C. Flohr, T.A. Leslie, A.M. Marsland, G. Ogg, W.A.C. Sewell, M. Hashme, L.S. Exton, M.F. Mohd Mustapa, M.C. Ezejimofor, on behalf of the British Association of Dermatologists’ Clinical Standards Unit, British Association of Dermatologists guidelines for the management of people with chronic urticaria 2021, British Journal of Dermatology , Volume 186, Issue 3, 1 March 2022, Pages 398–413, https://doi.org/10.1111/bjd.20892
- Permissions Icon Permissions

Plain language summary available online
offer an appraisal of all relevant literature up to March 2020, focusing on any key developments,
address important, practical clinical questions relating to the primary guideline objective, and
provide guideline recommendations and if appropriate research recommendations.
The guideline is presented as a detailed review with highlighted recommendations for practical use in primary, secondary and tertiary care, in addition to an updated patient information leaflet (PIL; available on the BAD Skin Health Information website, https://www.skinhealthinfo.org.uk/a‐z‐conditions‐treatments ).
Other than providing background information, the guideline does not cover angio‐oedema without weals [other than idiopathic, which is now classified as part of chronic spontaneous urticaria (CSU)], hereditary angio‐oedema, autoinflammatory syndromes or differential diagnosis. Additionally, the guideline focuses on chronic rather than acute urticaria.
This set of guidelines has been developed using the BAD’s recommended methodology. 1 Further information can be found in Appendix J (see Supporting Information) with reference to the AGREE II instrument ( www.agreetrust.org ) 2 and GRADE. 3 The recommendations were developed for implementation in the UK National Health Service (NHS).
The Guideline Development Group (GDG), which consisted of eight consultant dermatologists managing adults, children and young people; a consultant immunologist; a consultant psychodermatologist; a drug allergy specialist; two patient representatives and a technical team (consisting of an information scientist, guideline research fellows and a project manager providing methodological and technical support), established a number of clinical questions pertinent to the scope of the guideline and two sets of outcome measures of importance to patients, ranked according to the GRADE methodology (section 2.1; and Appendix A ; see Supporting Information).
A systematic literature search of the PubMed, MEDLINE, Embase and Cochrane databases was conducted to identify key articles on urticaria from January 2007 to March 2020. An additional, targeted literature search for the antihistamines acrivastine and bilastine was also carried out (from January 1980 to March 2020). Subsequently published papers known to the GDG were included. The final literature searches were run ahead of journal submission in 2021 to ensure currency. The search terms and strategies are detailed in Appendix K (see Supporting Information). Additional references relevant to the topic were also isolated from citations in the reviewed literature and the previous version of the guideline. 4 Evidence from the included studies was graded according to the GRADE system (high, moderate, low or very low certainty).
The recommendations are based on evidence drawn from systematic reviews of the literature pertaining to the clinical questions identified, following discussions with the entire GDG and factoring in all four factors that would affect their strength rating according to the GRADE approach (i.e. balance between desirable and undesirable effects, quality of evidence, patient values and preferences, and resource allocation). All GDG members contributed towards drafting and/or reviewing the narratives and information in the guideline and Supporting Information documents. When there was insufficient evidence from the literature, informal consensus was reached based on the experience of medical and patient GDG members.
The summary of findings with forest plots (Appendix B ), tables Linking the Evidence To the Recommendations (LETR; Appendix C ), GRADE evidence profiles indicating the quality of evidence (Appendix D ), summary of included studies (Appendix E ), narrative findings for noncomparative studies (Appendix F ), PRISMA flow diagram (Appendix G ), list of excluded studies (Appendix H ) and AMSTAR 2 appraisal of the included systematic reviews (Appendix I ) are detailed in the Supporting Information.
The strength of recommendation is expressed by the wording and symbols shown in Table 1 .
Strength of recommendation ratings
| Strength | Wording | Symbol | Definition |
| Strong recommendation the use of an intervention | ‘Offer’ (or similar, e.g. ‘use’, ‘provide’, ‘take’, ‘investigate’ etc.) | ↑↑ | Benefits of the intervention outweigh the risks; most patients would choose the intervention whilst only a small proportion would not; for clinicians, most of their patients would receive the intervention; for policymakers, it would be a useful performance indicator |
| Weak recommendation the use of an intervention | ‘Consider’ | ↑ | Risks and benefits of the intervention are finely balanced; most patients would choose the intervention but many would not; clinicians would need to consider the pros and cons for the patient in the context of the evidence; for policymakers it would be a poor performance indicator where variability in practice is expected |
| No recommendation | Θ | Insufficient evidence to support any recommendation | |
| Strong recommendation the use of an intervention | ‘Do not offer’ | ↓↓ | Risks of the intervention outweigh the benefits; most patients would choose the intervention whilst only a small proportion would; for clinicians, most of their patients would receive the intervention |
| Strength | Wording | Symbol | Definition |
| Strong recommendation the use of an intervention | ‘Offer’ (or similar, e.g. ‘use’, ‘provide’, ‘take’, ‘investigate’ etc.) | ↑↑ | Benefits of the intervention outweigh the risks; most patients would choose the intervention whilst only a small proportion would not; for clinicians, most of their patients would receive the intervention; for policymakers, it would be a useful performance indicator |
| Weak recommendation the use of an intervention | ‘Consider’ | ↑ | Risks and benefits of the intervention are finely balanced; most patients would choose the intervention but many would not; clinicians would need to consider the pros and cons for the patient in the context of the evidence; for policymakers it would be a poor performance indicator where variability in practice is expected |
| No recommendation | Θ | Insufficient evidence to support any recommendation | |
| Strong recommendation the use of an intervention | ‘Do not offer’ | ↓↓ | Risks of the intervention outweigh the benefits; most patients would choose the intervention whilst only a small proportion would; for clinicians, most of their patients would receive the intervention |
Clinical questions and outcomes
The GDG established the following clinical questions pertinent to the scope of the guideline.
Review question 1: investigation
Do tests, such as blood tests and the autologous serum skin test, alter the management of urticaria?
Review question 2: treatment
What is the clinical effectiveness of any H 1 ‐antihistamine compared with another for the treatment of urticaria?
Review question 3: treatment
Would changing from one H 1 ‐antihistamine to another lead to benefit in the treatment of urticaria?
Review question 4: treatment
Would adding an H 2 ‐antihistamine to an H 1 ‐antihistamine lead to benefit in the treatment of urticaria?
Review question 5: treatment
What is the effectiveness of leukotriene receptor antagonists in the treatment of urticaria?
Review question 6: treatment
What are the effectiveness and safety of increasing doses of H 1 ‐antihistamines?
Review question 7: treatment
Would adding other therapies to an H 1 ‐antihistamine lead to benefit in the treatment of urticaria, including sulfasalazine, dapsone, thyroxine, tricyclic antidepressants, hydroxychloroquine, methotrexate, danazol, tranexamic acid, mycophenolate mofetil, intravenous immunoglobulins (IVIg) and anticoagulants?
Review question 8: treatment
What is the effectiveness of taking systemic corticosteroids for the treatment of urticaria?
Review question 9: treatment
What is the effectiveness of dietary exclusions or supplements for the treatment of urticaria?
Review question 10: treatment
What is the effectiveness of Helicobacter pylori eradication for the treatment of urticaria?
Review question 11: treatment
What is the effectiveness of avoiding nonsteroidal anti‐inflammatory drugs (NSAIDs) for the treatment of urticaria?
Review question 12: treatment
What is the effectiveness of ciclosporin for the treatment of urticaria and are there any long‐term benefits?
Review question 13: treatment
What is the effectiveness of omalizumab for the treatment of urticaria?
Review question 14: treatment
Is the response to treatment for inducible urticarias (e.g. symptomatic dermographism, delayed pressure urticaria, cold urticaria, cholinergic urticaria, vibratory angio‐oedema, localized heat urticaria) the same as for CSU?
Review question 15: treatment
Do any other specific interventions work for inducible urticarias, such as antibiotics in cold urticaria, sulfasalazine in delayed pressure urticaria, phototherapy in dermographism, plasmapheresis in solar urticaria and anticholinergics in cholinergic urticaria?
Review question 16: treatment
Which H 1 ‐antihistamines can be used in pregnancy?
Review question 17: treatment
Which H 1 ‐antihistamines can be used during breastfeeding?
Review question 18: treatment
What are the key differences in the diagnosis and management of paediatric compared with adult urticaria (if there are any)?
Review question 19: treatment
What is the clinical effectiveness of miscellaneous monotherapies compared with each other for the treatment of urticaria?
The GDG also established two sets of outcome measures of importance to patients (for treatment and investigation), ranked according to the GRADE methodology, 5 by the patient representatives (for the full review protocol see Appendix A in the Supporting Information). In the investigation protocol, the outcome is either ‘yes’ or ‘no’. The treatment outcomes (Table 2 ) use a nine‐point scale; outcomes ranked 9, 8 or 7 are critical for decision making; those ranked 6, 5 or 4 are important but not critical for decision making; and those ranked 3, 2 or 1 are the least important for decision making.
Outcome measures and ranking
| Disease control | 9 |
| Decrease in urticarial activity | 9 |
| Adverse effects | 9 |
| Quality of life | 9 |
| Time to clinical effect | 7 |
| Relapse | 6 |
| When to stop treatment | 3 |
| Disease control | 9 |
| Decrease in urticarial activity | 9 |
| Adverse effects | 9 |
| Quality of life | 9 |
| Time to clinical effect | 7 |
| Relapse | 6 |
| When to stop treatment | 3 |
The following recommendations and ratings were agreed upon unanimously by members of the GDG and patient representatives.
For further information on the wording used for recommendations and strength of recommendation ratings see Table 1 . The evidence on which the recommendations are based is featured and discussed in Appendices B–E (see Supporting Information). The GDG is aware of the lack of high‐quality evidence for many of these recommendations, therefore, strong recommendations with an asterisk (*) are based on available evidence, as well as informal consensus and specialist experience of medical and patient GDG members. Good practice point (GPP) recommendations are derived from informal consensus.
the recommendations are based on expert opinion as there is very little published evidence
there are additional notes in section 9.1 and Table 3 .
First‐line antihistamines for chronic urticaria in children
| Drug | 1 month to 1 year | 1–2 years | 2–5 years | 6–11 years | 12–17 years | |
| 1 mg twice a day | x | x | x | x | ||
| 2 mg/5 mL oral solution | ||||||
| x | Unlicensed: 250 micrograms per kg twice a day (typically doses up to 2·5 mg twice a day) | 2·5 mg twice a day | 5 mg twice a day | 10 mg daily | ||
| 5 mg/5 mL oral solution | ||||||
| 10 mg tablets | ||||||
| x | 1·25 mg daily | 1·25 mg daily | 2·5 mg daily | 5 mg daily | ||
| 2·5 mg/5 mL oral solution | ||||||
| 5 mg tablets | ||||||
| x | x | < 31 kg: 5 mg daily | < 31 kg: 5 mg daily | > 31 kg: 10 mg daily | 10 mg daily | |
| 5 mg/5 mL oral solution | ||||||
| 10 mg tablets | ||||||
| x | x | x | Unlicensed: 30 mg twice daily | 180 mg daily | ||
| 30 mg/120 mg/180 mg tablets | ||||||
| Drug | 1 month to 1 year | 1–2 years | 2–5 years | 6–11 years | 12–17 years | |
| 1 mg twice a day | x | x | x | x | ||
| 2 mg/5 mL oral solution | ||||||
| x | Unlicensed: 250 micrograms per kg twice a day (typically doses up to 2·5 mg twice a day) | 2·5 mg twice a day | 5 mg twice a day | 10 mg daily | ||
| 5 mg/5 mL oral solution | ||||||
| 10 mg tablets | ||||||
| x | 1·25 mg daily | 1·25 mg daily | 2·5 mg daily | 5 mg daily | ||
| 2·5 mg/5 mL oral solution | ||||||
| 5 mg tablets | ||||||
| x | x | < 31 kg: 5 mg daily | < 31 kg: 5 mg daily | > 31 kg: 10 mg daily | 10 mg daily | |
| 5 mg/5 mL oral solution | ||||||
| 10 mg tablets | ||||||
| x | x | x | Unlicensed: 30 mg twice daily | 180 mg daily | ||
| 30 mg/120 mg/180 mg tablets | ||||||
Table 3 summarizes the antihistamines commonly used in the UK. Chlorphenamine is a sedating antihistamine and thus daytime drowsiness and reduced attention, visual memory and learning are very common. It also causes abnormal sleep at night with delayed and reduced REM sleep. For these reasons, it should be used with caution, particularly over longer periods. It is not recommended for use beyond 1 year when alternative low‐sedating antihistamines are available. Cetirizine can cause drowsiness, especially when used above the licensed dose. Hence it is to be used with caution in school‐aged children. However, if the child has been started on cetirizine with benefit, then this therapy can be continued. Loratadine and desloratadine may be less effective than cetirizine, but are less likely to cause drowsiness. Like chlorphenamine, they are metabolized in the liver, increasing the risks of drug accumulation and drug interactions. Fexofenadine is licensed for seasonal allergic rhinitis from the age of 6 years, and for urticaria from the age of 12 years. It can be considered for unlicensed use for urticaria from the age of 6 years. For all children with chronic urticaria who have an inadequate response to standard doses, the frequency of administration of nonsedating antihistamines can be increased up to a maximum of four times a day.
Licensing information, dosages and monitoring requirements for specific drugs are not included. However, of note, apart from H 1 ‐antihistamines, oral steroids and omalizumab, none of the other treatment options discussed are licensed in the UK for use in urticaria. Except where otherwise stated, we recommend adherence to published guidelines, for example by the manufacturer, the BAD or, in the UK, the British National Formulary ( www.bnf.org ). In particular, note the licensed dosages for people aged < 14 years (also see Table 3 ).
The recommendations relate to chronic spontaneous and inducible urticarias. Acute urticaria, angio‐oedema without weals (other than idiopathic, which is now classified as part of CSU), hereditary angio‐oedema and autoinflammatory diseases are not covered.
For clarity, we have divided the management options into sections (general treatment, first‐, second‐ and third‐line options). However, depending on disease severity, disease fluctuation, comorbidities and the national criteria for use of drugs, the order and combinations of treatment may vary and may change during the course of each person’s disease.
We note that, since submission of this article for publication, a new international guideline on the management of urticaria has been published. 6 Broadly, the recommendations are similar, except that the international guideline favours omalizumab over ciclosporin for CSU.
General management for people with all types of chronic urticaria
The most important step is to take a detailed clinical history, with examination supplemented by people’s own photographs. In most cases this will provide an accurate clinical diagnosis (section 5.2), which will guide management. Disease pathogenesis may also be important in management (section 5.3).
R1 (↑) Only consider baseline investigations if clinically indicated (see section 6.0).
R2 (GPP) Consider using appropriate validated scoring systems to assess disease activity and impact, for example Dermatology Life Quality Index (DLQI), weekly Urticaria Activity Score 7 (UAS7), Angioedema Activity Score (AAS) and/or Urticaria Control Test (UCT).
R3 (GPP) Provide educational material or a patient information leaflet on urticaria or angio‐oedema ( https://www.skinhealthinfo.org.uk/a‐z‐conditions‐treatments ).
R4 (GPP) Offer access to support and treatment for anxiety, depression and the psychosocial impact of the disease, where appropriate. The psychological impact can be assessed using, for example, the Hospital Anxiety and Depression Scale (HADS).
R5 (GPP) Consider topical antipruritic agents, such as a menthol‐containing emollient.
R6 (GPP) Advise avoidance of identified triggers or exacerbating factors, such as drugs, and in particular triggers for inducible urticarias.
R7 (↑↑) Stop angiotensin‐converting enzyme (ACE) inhibitors in people with angio‐oedema without weals.
General management for people with chronic spontaneous urticaria
R8 (↑↑) Avoid NSAIDs in people whose CSU appears to be exacerbated by this class of drugs.
R9 (↑) Consider switching NSAID treatment to a selective cyclooxygenase‐2 inhibitor, if tolerated and not contraindicated, when there is a history of acute exacerbation of CSU after NSAID intake for inflammation. However, evidence of benefit from switching low‐dose aspirin when taken as an antithrombotic to an alternative antiplatelet drug is lacking. Refer to the National Institute for Health and Care Excellence, 7 British Society of Allergy and Clinical Immunology (BSACI) 8 or European Academy of Allergy and Clinical Immunology (EAACI) guidance 9 if reactivity to NSAIDs is suspected.
R10 (GPP) Do not advise dietary exclusion routinely. If, from a detailed history, food appears to play a role, investigate appropriately.
Θ1 There is insufficient evidence to recommend routine screening for vitamin D deficiency.
Θ2 There is insufficient evidence to make a recommendation on dietary supplementation.
R11 (↓↓) Do not offer routine screening for Helicobacter pylori .
First‐line treatment options for people with chronic spontaneous urticaria
R12 (↑↑) Offer a second‐generation H 1 ‐antihistamine, using a regular daily licensed dose (Table 4 ).
Examples of first‐ and second‐generation H 1 ‐antihistamines
| Licensed oral dose for adults (see Table for children) | |
| Chlorphenamine | 4 mg every 4–6 h, maximum 24 mg per day (elderly maximum 12 mg per day) |
| Cyproheptadine | 4 mg three times per day (maximum 32 mg per day) |
| Hydroxyzine | Initially 10–25 mg at night, maximum 25 mg three to four times per day (elderly maximum 25 mg twice daily) |
| Promethazine | 10–20 mg two to three times per day |
| Acrivastine | 8 mg three times a day |
| Cetirizine | 10 mg once daily |
| Desloratadine | 5 mg once daily |
| Fexofenadine | 180 mg once daily |
| Loratadine | 10 mg once daily |
| Levocetirizine | 5 mg once daily |
| Mizolastine | 10 mg once daily |
| Licensed oral dose for adults (see Table for children) | |
| Chlorphenamine | 4 mg every 4–6 h, maximum 24 mg per day (elderly maximum 12 mg per day) |
| Cyproheptadine | 4 mg three times per day (maximum 32 mg per day) |
| Hydroxyzine | Initially 10–25 mg at night, maximum 25 mg three to four times per day (elderly maximum 25 mg twice daily) |
| Promethazine | 10–20 mg two to three times per day |
| Acrivastine | 8 mg three times a day |
| Cetirizine | 10 mg once daily |
| Desloratadine | 5 mg once daily |
| Fexofenadine | 180 mg once daily |
| Loratadine | 10 mg once daily |
| Levocetirizine | 5 mg once daily |
| Mizolastine | 10 mg once daily |
a From the British National Formulary.
R13 (↓↓) Do not offer first‐generation H 1 ‐antihistamines routinely, unless there is no alternative, due to concerns about their short‐ and long‐term effects on the central nervous system.
R14 (↑↑) Offer updosing (i.e. increasing the dose above the licensed dose) of a single second‐generation H 1 ‐antihistamine, by up to fourfold the licensed dose, to people whose symptoms are inadequately controlled by the standard licensed dose, provided it is tolerated and there is no caution or contraindication (see section 7.2 and Appendix C – LETR narratives). Attempt stepwise dose reduction following complete symptom control. There is no evidence to guide the optimum duration of updosing or speed of dose reduction.
R15 (↓↓) Do not updose mizolastine (see section 7.2).
R16 (GPP) Consider switching from one second‐generation H 1 ‐antihistamine to another in people whose symptoms do not respond adequately to, or who do not tolerate, the first drug at standard or increased doses.
Θ3 There is insufficient evidence to make a recommendation on using two different second‐generation H 1 ‐antihistamines at the same time.
R17 (↓↓) Do not updose first‐generation H 1 ‐antihistamines (see R13).
R18 (↑) Consider montelukast, in addition to a second‐generation H 1 ‐antihistamine, in people whose symptoms are inadequately controlled by standard and increased doses of second‐generation H 1 ‐antihistamines.
R19 (↑↑) Offer* progression of therapy, through first‐line treatment options (see R12–R18) every 2–4 weeks (every 2 weeks in severe treatment‐resistant disease).
Θ4 There is insufficient evidence to recommend routine addition of H 2 ‐antihistamines to second‐generation H 1 ‐antihistamines for people whose symptoms are inadequately controlled by the latter. However, they may be considered if urticaria is associated with dyspepsia, although dyspepsia should be investigated appropriately. See section 7.4.
R20 (↑) Consider oral prednisolone (e.g. 0·5 mg kg −1 ) for short, infrequent courses of a few days as rescue treatment to control severe exacerbations, in addition to continued use of a second‐generation H 1 ‐antihistamine.
R21 (↓↓) Do not offer* long‐term systemic corticosteroids unless there is no other option. Use the lowest effective dose for the shortest possible period. 10
Second‐line treatment options for people with chronic spontaneous urticaria
For people with CSU with an inadequate response to first‐line treatment, the following additional investigations may be relevant when considering the next treatment options.
R22 (↓↓) Do not offer autologous serum skin tests (ASSTs) or autologous plasma skin tests (APSTs) routinely.
R23 (↑) Consider measuring total IgE levels: a high total IgE level may indicate a higher probability of early disease responsiveness to omalizumab, whereas a normal total IgE level may indicate disease responsiveness to ciclosporin (section 6 and Appendix C – LETR narratives).
R24 (↑) If available, consider a basophil histamine release assay (BHRA), although it is not yet subject to a national quality assurance scheme. A positive BHRA may indicate a higher probability of disease responsiveness to ciclosporin and slower or delayed response to omalizumab, whereas a negative BHRA may indicate a higher probability of disease responsiveness to omalizumab (section 6 and Appendix C – LETR narratives).
Note: total IgE levels (R23) and BHRAs (R24) are only indicative and may not reflect actual clinical responsiveness in all patients.
R25 (↑↑) Offer omalizumab, in addition to a second‐generation H 1 ‐antihistamine, to people whose symptoms are inadequately controlled by first‐line options.
R26 (↑↑) Offer* ciclosporin for 3–6 months, in addition to a second‐generation H 1 ‐antihistamine, to people whose symptoms are inadequately controlled by first‐line options.
R27 (↑↑) Avoid long‐term use of ciclosporin where possible; if not, use at the lowest effective dose, interrupt treatment periodically to confirm continued requirement, and consider alternative agents (see R25, R28 and Θ5).
Third‐line treatment options for people with chronic spontaneous urticaria
azathioprine
doxepin (but there are concerns about central nervous system effects, as for first‐generation antihistamines)
hydroxychloroquine (particularly for urticaria occurring with systemic lupus erythematosus)
methotrexate
mycophenolate mofetil
narrowband ultraviolet (UV)B (typically a course of around 30 treatments, repeated after 12 months if necessary, but not for continual treatment)
oral tacrolimus
sulfasalazine
tranexamic acid (only if predominantly angio‐oedema).
cyclophosphamide
dipyridamole
interleukin‐1 antagonists (e.g. anakinra)
plasmapheresis
psychological interventions (although there is evidence that psychological interventions such as cognitive behavioural therapy, mindfulness and relaxation techniques are beneficial for general psychosocial wellbeing in patients with skin diseases)
tumour necrosis factor antagonists
Treatment options for inducible urticarias
There is much less evidence available than for CSU, but for people with all types of inducible urticaria the following are recommended (based mainly on small case series and anecdotal evidence).
First‐line treatment options for people with all types of inducible urticaria
R29 (↑↑) Offer* a second‐generation H 1 ‐antihistamine, using a regular daily licensed dose (Table 4 ).
R30 (↓↓) Do not offer* first‐generation H 1 ‐antihistamines routinely, unless there is no alternative, due to concerns about their short‐ and long‐term effects on the central nervous system.
R31 (↑↑) Offer* updosing of a single second‐generation H 1 ‐antihistamine by up to fourfold the licensed dose to people whose symptoms are inadequately controlled by the standard licensed dose, provided it is tolerated and there is no caution or contraindication (section 7.2 and Appendix C – LETR narratives). Attempt stepwise dose reduction following complete symptom control. There is no evidence to guide the optimum duration of updosing or speed of dose reduction.
R32 (↓↓) Do not updose mizolastine (see section 7.2).
R33 (GPP) Consider switching from one second‐generation H 1 ‐antihistamine to another in people whose symptoms do not respond adequately to, or who do not tolerate, the first drug at the standard or increased dose.
Θ6 There is insufficient evidence to make a recommendation on using two different second‐generation H 1 ‐antihistamines at the same time.
R34 (↓↓) Do not updose first‐generation H 1 ‐antihistamines (see R30).
Θ7 There is insufficient evidence to recommend routine use of montelukast, although there is some evidence to support its use in some subtypes of inducible urticaria.
Second‐line treatment options for people with all types of inducible urticaria
R35 (↑) Consider omalizumab, in addition to a second‐generation H 1 ‐antihistamine, in people whose symptoms are inadequately controlled by first‐line options, subject to licensing and funding.
R36 (GPP) Offer self‐injectable adrenaline, if appropriate, for those at risk of anaphylaxis, for example in association with cold or cholinergic urticaria.
Third‐line treatment options for people with all types of inducible urticaria
Consider the following options, in addition to second‐generation H 1 ‐antihistamines, in people with specific types of inducible urticaria, whose symptoms are inadequately responsive to first‐ and second‐line treatment options, or where the latter are contraindicated or inappropriate.
Cholinergic urticaria
R37 (GPP) Consider anticholinergic drugs (e.g. oxybutynin), or beta blockers (e.g. propranolol), or danazol, or possibly phototherapy.
Cold urticaria
R38 (GPP) Consider ciclosporin.
Θ8 There is insufficient evidence to recommend routine use of antibiotics (e.g. penicillin or tetracyclines).
R39 (GPP) Do not offer cold desensitization.
Delayed pressure urticaria
R40 (↑) Consider dapsone or sulfasalazine.
Solar urticaria
R41 (GPP) Offer advice about sun avoidance and sun protection.
R42 (↑) Consider UV prophylactic phototherapy using the wavelength of light relevant to the individual person, only following photoinvestigation and obtaining advice from a dermatologist at a specialist photodermatology centre.
Θ9 There is limited evidence to recommend plasmapheresis or IVIg for people with solar urticaria.
Symptomatic dermographism
R43 (↑) Consider narrowband UVB (typically a course of around 30 treatments, repeated after 12 months, if necessary, but not for continual treatment).
R44 (GPP) Consider psoralen plus UVA (similarly, not for continual treatment).
R45 (GPP) Consider narrowband UVB for other forms of inducible urticaria.
Θ10 There is insufficient evidence to make a recommendation about the safety of use of antihistamines during pregnancy and breastfeeding. However, in active disease and after counselling the female patient with any type of urticaria, where necessary, consider cetirizine or loratadine (see the individual drug summary of product characteristics 11 for information on safety during pregnancy) and the discussion in Appendix C (LETR narratives).
there is diagnostic doubt
the urticaria is not adequately controlled by first‐line treatment options
there are high levels of inflammatory markers
there are marked or persistent associated systemic symptoms, or if the person is systemically unwell
the urticaria is having a significant impact on quality of life, such as depression, anxiety, marked psychosocial impact, reduced work/school attendance or sleep disturbance
the person has angio‐oedema without weals, not controlled by first‐line treatment options.
Future research recommendations
The following list outlines future research recommendations (FRRs).
FRR1 Further investigation of the genetic predisposition and/or mechanistic factors that drive the development of all types of urticaria and/or angio‐oedema, including the new theory of IgE‐mediated ‘autoallergy’ and characterization of the roles of basophils, eosinophils and lymphocytes.
FRR2 Better characterization of, and comparisons between, basophil‐based assays as predictors of drug responses.
FRR3 Development of better biomarkers to predict responsiveness to anti‐IgE and other therapies.
FRR4 Utilizing the results from FRR1–3 to address the possibility of personalized therapy and whether new biological targets might offer new therapeutic options.
FRR5 Randomized controlled trials (RCTs) evaluating the safety and efficacy of updosing one second‐generation H 1 ‐antihistamine compared with using two different second‐generation H 1 ‐antihistamines at the same time in people with CSU.
FRR6 RCTs evaluating the safety and efficacy of omalizumab in people with all subtypes of inducible urticaria.
FRR7 RCTs evaluating the safety and efficacy of other treatment options (as featured in the treatment algorithm) in people with all subtypes of inducible urticaria.
FRR8 RCTs evaluating the safety and efficacy of emerging treatments for people with all types of urticaria, including the new high‐affinity, humanized monoclonal anti‐IgE antibody ligelizumab, and potential new treatment options such as tyrosine kinase inhibitors, dupilumab, histamine H 4 receptor antagonists, C5a receptor antagonists and drugs targeting inhibitory mast cell receptors (see section 7.8).
FRR9 Better characterization of the optimum duration of the various treatment options available to people with all types of urticaria.
FRR10 Investigations into disease incidence and prevalence, the predictive value of laboratory investigations (such as total IgE levels or basophil‐based assays), and the safety and efficacy of the various current and potential future treatment options in children and young people with urticaria and/or angio‐oedema of all types.
The recommendations, discussions in the LETRs (Appendix C ) and consensus specialist experience were used to produce management pathways for adults with chronic urticaria – see Figure 1 .

Patient management pathway for urticaria. For clarity we have divided management options into sections (general treatment, and first‐, second‐ and third‐line options). However, depending on disease severity, disease fluctuation, comorbidities and national criteria for use of drugs, the order and combinations of treatment may vary and change during each person’s disease. AAS, Angioedema Activity Score; ACE, angiotensin‐converting enzyme; BHRA, basophil histamine release assay; DLQI, Dermatology Life Quality Index; IVIg, intravenous immunoglobulin; NB‐UVB, narrowband ultraviolet B; PIL, patient information leaflet; PUVA, psoralen plus ultraviolet A; UAS7, Urticaria Activity Score summed over 7 days; UCT, Urticaria Control Test.
Definition and introduction
Urticaria consists of transient weals, angio‐oedema or both. Weals are usually itchy, whereas the swellings of angio‐oedema are often not. Depending on the disease subtype, angio‐oedema or weals may be painful. Urticaria is usually divided into acute and chronic forms, becoming chronic when daily or almost daily weals continue for 6 weeks or more, although many attacks of acute urticaria settle much more quickly. Some forms of urticaria may be accompanied by systemic symptoms, such as arthralgia, gastrointestinal disturbance, malaise, lethargy, or wheeze and/or mucosal involvement. Acute urticaria may be a presenting sign of anaphylaxis.
The reported lifetime prevalence rate of urticaria varies from 8% to 24%, with a lifetime prevalence rate of about 1–2% for chronic urticaria. 12–14 The point prevalence of chronic urticaria varies from 0·1% in North America to 1·4% in Asia. 15 The disease is slightly more common in females, except in young children.
People suffer greatly if they have any form of urticaria, and chronic disease may have a significant effect on quality of life. 16 , 17
Even though the initial treatment for many types of urticaria is similar, there are some important exceptions. Therefore, accurate clinical categorization, based on a detailed history and examination (section 5.2) and an understanding of disease pathogenesis (section 5.3), are essential to guide investigation and management. Of note, different forms of urticaria commonly occur together.
Clinical classification of urticaria, including diseases presenting with urticaria‐like rashes (Table 5 )
Spontaneous urticaria.
No cause is identified in more than 50% of people with acute urticaria (causes when identified include drugs, infections including COVID‐19, and type 1 hypersensitivity reactions) and in many of those with CSU (see section 5.3.2. for pathogenesis of CSU). Weals generally last for up to 24 h, but the swellings of angio‐oedema may last for up to 72 h.
Clinical classification of urticaria, including diseases presenting with urticaria‐like rashes 4
| mutations) gene) |
| mutations) gene) |
Inducible urticarias
These urticarias are usually chronic. Weals are reproducibly induced by the same physical stimulus. Weals usually appear within a few minutes of the stimulus and last for < 2 h, the exception being delayed pressure urticaria, where weals may take 30 min to 12 h to develop, and then last for a few days. The shape and size of the weals may aid diagnosis, for example linear weals in dermographism, or papular weals surrounded by a red flare in cholinergic or aquagenic urticarias. Some inducible urticarias present as a spectrum of symptoms from pruritus, urticaria and angio‐oedema to anaphylaxis. Inducible urticarias can be confirmed on provocation testing (see section 6.0). Disease severity may be reduced through the avoidance of triggers, although this can be difficult and disabling. Inducible urticarias tend to be underdiagnosed.
Angio‐oedema without weals
Usually no cause is identified. However, it is important not to miss uncommon cases of drug‐induced angio‐oedema where the culprit drug must be withdrawn (especially ACE inhibitors, where the angio‐oedema may occur soon or many years after drug initiation), 18 , 19 or rare cases of C1 esterase inhibitor deficiency (see section 6.4). Both may cause life‐threatening airway swelling and neither respond to the usual treatment for angio‐oedema. Angio‐oedema of the gastrointestinal tract is common in C1 esterase inhibitor deficiency.
Contact urticaria
Like inducible urticarias, this is characterized by a weal‐and‐flare response at the site of contact of a trigger, anaphylaxis may occur, the onset is rapid (within minutes) and reactions usually last for < 2 h. However, the disease is acute, not chronic, and the trigger is not physical but instead may be any of a large variety of substances, for example food, plants, animals, fragrances or preservatives.
Urticarial vasculitis
Weals are usually of prolonged duration, may be painful rather than itchy and sometimes leave residual bruising or postinflammatory change. It can be difficult to differentiate urticarial vasculitis from delayed pressure urticaria. However, in urticarial vasculitis, there are often marked systemic symptoms, there may be joint or renal involvement, an association with other underlying diseases and high inflammatory markers. A skin biopsy is needed to confirm the diagnosis (see section 6.5). 20
Autoinflammatory syndromes
Autoinflammatory syndromes presenting with urticaria‐like rashes include cryopyrin‐associated periodic syndrome (CAPS) (usually with onset in childhood, although late‐onset acquired disease is recognized) and Schnitzler syndrome (acquired with adult onset). CAPS consists of three overlapping conditions: familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal‐onset multisystem inflammatory disorder. These diseases are rare (for pathogenesis see section 5.3.3). They differ in associated organ involvement, but are all usually accompanied by fever, malaise and high levels of inflammatory markers. 21 , 22
Pathogenesis and aetiology
Some cases of acute or episodic urticaria and some cases of contact urticaria are due to mast cell degranulation caused by allergens crosslinking antigen‐specific IgE (type 1 hypersensitivity).
Autoimmune urticaria
Approximately 33% of people with CSU have functional, histamine‐releasing IgG autoantibodies. These either directly crosslink high‐affinity IgE receptors (FcεRI) or bind IgE. 23
A new theory is emerging of type 1 autoimmunity or ‘autoallergy’, in which IgE antibodies are directed at an element of self. Antigens may then crosslink IgE on mast cells or basophils, causing degranulation. It has been observed that there are fast and slow responders to treatment with omalizumab (an anti‐IgE antibody). This has led to the proposal that the rapid response may be due to omalizumab rapidly binding free IgE autoantibodies against autoallergens, whilst the slow responses may be due to the slower loss of mast cell (or basophil) membrane‐bound IgE and downregulation of FcεRI, thus reducing IgG‐mediated activation. 24 The functional importance of IgE ‘autoallergic’ autoantibodies is under investigation. Thus far, there is some evidence that IgE anti‐thyroid peroxidase antibodies and possibly IgE anti‐interleukin‐24 antibodies may play a role in some patients with CSU. 25 , 26
These are characterized by dysregulation of innate immunity. Persistent uncontrolled inflammation occurs in the absence of triggers and is mediated by excessive cytokine production. Most cases of CAPS are due to autosomal dominant or de novo mutations in the NLRP3 gene, resulting in increased activity of the NLRP‐3 inflammasome and increased secretion of interleukin‐1β. 27 , 28 Late‐onset CAPS is now thought to be due to acquired somatic (mosaic) mutations in NLRP3 , 29 but these have not been identified in Schnitzler syndrome, even though the clinical presentation is identical. 30 Instead, Schnitzler syndrome is defined by the presence of a monoclonal gammopathy of unknown significance (MGUS), usually IgM, but sometimes IgG. 30
Associations
Many people with CSU find that nonspecific factors including heat, alcohol, infections and stress exacerbate or trigger their urticaria, but the underlying mechanisms are poorly understood. Drugs may precipitate urticaria by various mechanisms (Table 6 ).
Pathogenesis and aetiology of urticaria 4
| Adaptive immune system Innate immune system |
| Adaptive immune system Innate immune system |
An urticaria‐like rash may also occur as a prodrome of bullous pemphigoid or be a presenting feature of progesterone‐induced dermatosis. Urticaria, and particularly urticarial vasculitis, may occur with other diseases, such as systemic lupus erythematosus.
There is an association between CSU and thyroid autoimmunity. 31 There is some evidence that Helicobacter pylori infection may be associated with an increased risk of CSU. There is conflicting evidence as to whether eradiation of H . pylori alleviates CSU, although identified H . pylori infection should, in any case, be treated appropriately (see Appendix C – LETR narratives for question 10). Given the association between chronic urticaria and autoimmunity, including coeliac disease in children 32 and adults, 33 , 34 clinical suspicion of coexisting autoimmune diseases should remain high, but screening in the absence of associated features is not suggested.
The most important part of assessment is a thorough clinical history and examination. This will usually lead to accurate clinical classification. In many cases, especially in acute and mild chronic spontaneous or inducible urticarias, responsive to H 1 ‐antihistamines, there is no need for further investigation.
Urticaria can have a significant effect on people’s lives. Therefore, assessments should be made as to the effect the disease is having on the person’s quality of life (e.g. using DLQI); anxiety, depression or associated psychological issues (e.g. using the HADS); 35 sleep and attendance at school or work. The activity of the disease should be measured (e.g. using UAS7, AAS or UCT) 36 , 37 before embarking on second‐line treatment options. 38 , 39
Acute or episodic spontaneous urticaria
Skin prick tests and/or blood tests for allergen‐specific IgE may help to confirm type 1 hypersensitivity as a cause, if suspected. These tests are usually not helpful in chronic urticaria. A full blood count (FBC) and inflammatory markers [C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR)] may be helpful in identifying infective causes.
Chronic spontaneous urticaria
A FBC may be useful to identify the minority of cases with an underlying cause. An eosinophilia may suggest a drug‐induced rash, pre‐bullous pemphigoid urticaria or a parasitic infection. Leucocytosis may be present in infection and sometimes in urticarial vasculitis or autoinflammatory disease, or, conversely, leucopenia may suggest systemic lupus erythematosus.
Inflammatory markers (CRP and ESR) are often normal in CSU, so elevated levels may prompt investigations for urticarial vasculitis, autoinflammatory disease or unrelated causes. Due to the association between CSU and thyroid autoimmunity (section 5.4), testing for thyroid‐stimulating hormone (TSH) and thyroid antibodies may be useful.
Thus, performing a small number of tests (FBC, ESR, CRP, TSH and thyroid antibodies) at presentation, may be of benefit in CSU.
In treatment‐resistant cases, total IgE levels and tests for IgG histamine‐releasing autoantibodies (if available) may help to inform the choice between the second‐line treatment agents omalizumab (or in the future perhaps new treatments such as the monoclonal anti‐IgE antibody ligelizumab) and ciclosporin (see R23 and R24). 40–42 Functional tests may also be useful in understanding disease pathogenesis and in providing an explanation to the person with urticaria.
Total IgE assays are widely available, low cost and well characterized. The BHRA is the most established test for functional autoantibodies; however, it is complex to perform. Some laboratories may prefer measuring basophil activation by other validated means. No functional test is well characterized or has been subject to national quality assurance schemes. Such tests are not always readily accessible or available. Further research is needed to determine the comparative utility of functional tests in predicting treatment responses in CSU. For more details see Appendix C (LETR narratives).
These should be confirmed by history and appropriate provocation tests. 43
In cold contact urticaria, cryoglobulins may be measured, although they are rarely present and, if so, are usually associated with infection or haematological disease. If they are measured, scrupulous attention to temperature‐controlled sampling, transport and processing is required (blood must be kept at 37°C). If weals follow the cold trigger after a delay of a few hours, are associated with systemic symptoms or start in early childhood, and/or there is a family history, investigations should be undertaken for familial cold autoinflammatory syndrome (section 6.6).
In solar urticaria, antinuclear antibodies and porphyrins should be checked. If the diagnosis is unclear, or if the disease is poorly responsive to treatment, referral to a specialist centre for an opinion and, if appropriate, phototesting may help.
In aquagenic pruritus, an annual FBC is recommended, as this condition may be associated with polycythaemia rubra vera and other haematological disorders.
C1 esterase inhibitor deficiency is characterized by low C4 levels, both between and during attacks. 44 If C4 levels are low, C1 esterase inhibitor level and function should then be checked. In about 85% of cases of hereditary angio‐oedema both are reduced (type I), but in the remainder only functional activity is reduced (type II). Reduced C1q levels are characteristic of (but not specific for) acquired C1 esterase inhibitor deficiency. All forms of C1 esterase inhibitor deficiency should be referred to immunology services for further investigation and management, in line with national and international consensus guidelines.
A skin biopsy showing a leucocytoclastic vasculitis is required to confirm the diagnosis, but the histological changes are often subtle. Possible histological features include fragmentation of leucocytes with nuclear debris (leucocytoclasia), endothelial cell swelling or damage, red cell extravasation and rarely fibrin deposition.
Inflammatory markers (CRP, ESR) are often raised. A full vasculitis screen should be checked to investigate for underlying causes, such as connective tissue disease or infection. Low C3 and C4, and positive anti‐C1q antibodies may indicate hypocomplementaemic urticarial vasculitis, a more severe disease with a greater potential for associated systemic disease, particularly systemic lupus erythematosus, and internal organ involvement. 20 , 45
Inflammatory markers (CRP and ESR) are usually elevated, as is serum amyloid A, which should be measured. Immunoglobulins and serum protein electrophoresis should be checked to investigate for Schnitzler syndrome in late‐onset disease, although a low‐level IgM paraprotein can be difficult to detect. Genetic tests should be arranged if CAPS is suspected. In England, patients should be referred to departments approved by NHS England for investigation and treatment.
Largely, these are as listed in the recommendations (section 3.0). However, a few important points are discussed below. Details of the supporting evidence for each recommendation can be found in Appendix C (LETR narratives).
The effectiveness of H 1 ‐antihistamines compared with each other
No first‐generation H 1 ‐antihistamine was found to stand out as being more effective than others in the Cochrane review. 46 However, the GDG generally considered loratadine and desloratadine to be slightly less effective, a position supported by in vivo suppression of weal‐and‐flare responses by different H 1 ‐antihistamines. 47 , 48 See Appendix C , question 2/3 for further information, including a network meta‐analysis published in 2021 that graded antihistamines in order of efficacy and acceptability, but acknowledged that this was based on low‐quality evidence. 49
Updosing of H 1 ‐antihistamines
If licensed doses of H 1 ‐antihistamines show inadequate response, the GDG agreed that evidence on efficacy supported the updosing of second‐generation H 1 ‐antihistamines for CSU, where tolerated and in the absence of contraindications. Efficacy gains were particularly evident for pruritus and quality of life, but the need for further research was noted. 50 The GDG recommended considering a switch from one second‐generation H 1 ‐antihistamine to another in people with CSU who do not respond adequately to the first drug, or if side‐effects develop. The GDG does not recommend routinely offering combinations of two different second‐generation H 1 ‐antihistamines at the same time to people with CSU, although it was noted that some people may benefit. 51 The safety of giving two H 1 ‐antihistamines at lower dosages has not been investigated and there is no published evidence on using such combination treatment. The GDG does not recommend updosing first‐generation H 1 ‐antihistamines in people with CSU.
In general, evidence supported the good safety profile of up to fourfold updosing of second‐generation H 1 ‐antihistamines, where tolerated, and in the absence of contraindications. However, the following should be considered before proceeding. Firstly, some studies have suggested that a proportion of people may develop increased side‐effects, such as sedation, on updosing second‐generation H 1 ‐antihistamines. 52 , 53 The possibility of sedation after updosing second‐generation H 1 ‐antihistamines should be discussed with people with urticaria. Secondly, the summary of product characteristics on www.medicines.org.uk provides specific information on the cautions and contraindications of individual antihistamines and these should be considered before updosing. These include closed‐angle glaucoma, prostatism, interactions with other drugs (e.g. cytochrome P450 modulators, drugs with associated sedation), foods (e.g. grapefruit) and alcohol, renal and liver impairment, epilepsy, elderly people and heart disease. Thirdly, the potential to prolong the electrocardiogram QTc interval should also be considered for all H 1 ‐antihistamines. 54 For example, among other contraindications, mizolastine is contraindicated in people with known or suspected QT prolongation or with electrolyte imbalance, in particular hypokalaemia or hypomagnesaemia; in clinically significant bradycardia; and where used with medicinal products known to prolong the QT interval, such as class I and III antiarrhythmics. The GDG does not recommend updosing mizolastine.
The GDG noted that most studies on updosing have shown significant heterogeneity in terms of study design, doses, specific antihistamines and responses, and recommended that large‐scale, high‐quality studies be undertaken.
Subsets of urticaria in which montelukast may be of benefit
There is published evidence that montelukast in combination with a second‐generation H 1 ‐antihistamine may be more effective than taking a second‐generation H 1 ‐antihistamine alone for people with CSU. 55 Much less data are available for inducible urticarias, although there is some evidence for efficacy in delayed pressure urticaria, 56 and several members of the GDG try montelukast in people with various inducible urticarias.
There may also be specific circumstances when this combination may be beneficial, such as when urticaria is exacerbated by salicylates, or where angio‐oedema is the predominant symptom. 57 , 58
Montelukast can be prescribed in primary care without monitoring of blood tests, but a recent Medicines and Healthcare products Regulatory Agency (MHRA) warning reminds prescribers of the neuropsychiatric side‐effects that may occur in a minority of people, particularly in children. People given montelukast should be counselled so as to be vigilant for these symptoms.
H 2 ‐antihistamines
There is insufficient evidence to recommend routine addition of H 2 ‐antihistamines to second‐generation H 1 ‐antihistamines for people with chronic urticaria whose symptoms are inadequately controlled by the latter alone. 59 However, some people may benefit, especially if urticaria is associated with dyspepsia. However, dyspepsia should be investigated appropriately. If an H 2 ‐antihistamine is used, famotidine (at the dose licensed for dyspepsia) is preferable to cimetidine, because the latter has a higher risk of adverse effects and interacts with cytochrome p450 modulators. Indeed, cimetidine interacts with some first‐generation H 1 ‐antihistamines, raising their plasma concentration, which may explain some of the beneficial effects of combination therapy demonstrated many years ago. 60 However, there are very few data on the use of famotidine in urticaria.
It is of note that we no longer recommend ranitidine as it has been withdrawn by the manufacturers due to possible contamination with a known carcinogen. 61

Oral corticosteroids for inducible urticarias
There are very few published data on the use of systemic corticosteroids in people with inducible urticarias. As for CSU, a short course of oral prednisolone may be considered for severe exacerbations, although it may not be as effective. 62
Avoidance of triggers in inducible urticarias
Avoidance of triggers may be helpful, especially where disease control is difficult. Avoidance can be disabling. However, where there is a risk of anaphylaxis, people should be warned to avoid particularly dangerous situations. For example, in cold urticaria, swimming in cold water (especially in the sea) or rock climbing could lead to a drop in core temperature or cooling of much of the body surface, resulting in anaphylaxis, potentially with fatal outcome.
These usually respond very rapidly (usually within 24 h) to agents that inhibit the actions of interleukin‐1, such as the interleukin‐1 receptor antagonist anakinra. Early treatment may prevent (and possibly reverse) disease complications. A therapeutic trial can be used as a diagnostic tool. 63
Potential new treatments for chronic spontaneous urticaria
A number of drugs are under investigation and/or in clinical trials for CSU. 64 Biosimilars for omalizumab are being developed. Ligelizumab, a new anti‐IgE monoclonal antibody, may be more efficacious and need less frequent administration than omalizumab. Dupilumab (an anti‐interleukin‐4 receptor antibody that inhibits the interleukin‐4 and interleukin‐13 pathways), monoclonal antibodies targeting the interleukin‐5 pathway (such as mepolizumab, reslizumab and benralizumab) and Bruton’s tyrosine kinase inhibitors are all being investigated for CSU. Other potential therapeutic targets include siglec‐8 (an inhibitory receptor on mast cells, basophils and eosinophils), prostaglandin receptors, the H 4 receptor and the C5a receptor.
Approximately 45% of people with CSU respond to H 1 ‐antihistamines at licensed doses, 65 and reports estimate that up to two‐thirds of the residual may respond to updosing. 50 About two‐thirds of those unresponsive to H 1 ‐antihistamines respond to omalizumab, 66 and a similar, probably different but overlapping, proportion to ciclosporin, although there is less evidence for the latter. 67 Of note, the response rates given include partial and complete responses, so full disease suppression may not be achieved in many people with CSU with these treatments alone.
Approximately 50% of people with CSU go into remission after 6 months to 5 years (longer if there is angio‐oedema), but about 20% still have active disease after 10 years, and 10% after 20 years. 65 , 68
In some people, urticaria can be very long lasting, difficult to treat and disabling. This is perhaps particularly so for people with severe inducible urticarias, where there are fewer options and less data to support treatment.
Children and young people
Consider an underlying autoinflammatory disease if a child is systemically unwell and/or has raised inflammatory markers. In England, children who are thought to have autoinflammatory disease should be referred to a rheumatologist with a specialist interest in autoinflammatory syndromes.
A parasitic infection may be responsible for chronic urticaria. At‐risk children should be screened and, if parasitic infection is present, treated accordingly. Indicators of a potential parasitic infection include eosinophilia, gastrointestinal symptoms and recent foreign travel.
The same proportion of children and young people with CSU have a positive BHRA, but there is a lack of evidence that this influences treatment response or disease remission.
H 1 ‐antihistamines currently authorized for use in children aged 2–11 years (Table 3 ) include cetirizine, levocetirizine, loratadine and rupatadine. 70 When needed, these antihistamines can also be used below age 2 years, despite the licensing age cutoff, given their overall good safety profile. Desloratadine is licensed from age 1 year and chlorphenamine from age 1 month (the latter is licensed in the UK according to the British National Formulary).
Second‐generation H 1 ‐antihistamines can be updosed with care (section 7.2) as for adults, taking into consideration cautions and contraindications, and proportionate to the manufacturers’ recommendations for age and weight (Table 3 ). 71
As for adults, do not offer first‐generation H 1 ‐antihistamines routinely, unless there is no alternative, due to concerns about their short‐ and long‐term effects on the central nervous system.
Children may be more likely than adults to have neuropsychiatric side‐effects from montelukast, including dysphemia (described as ‘stuttering’), nightmares or night terrors, aggression and behavioural changes.
Although not licensed in the UK, omalizumab has been successfully used in children below the age of 12 years with CSU and inducible urticarias, typically at the lower dose of 150 mg every 4 weeks and down to age 6 years, but very occasionally at even younger ages. 72 The same applies to ciclosporin (typically 3–4 mg kg −1 per day). 73–75
As in adults, self‐injectable adrenaline should be considered in children with inducible urticarias where there is a risk of anaphylaxis following a significant trigger (those with cold urticaria must not immerse themselves in cold water), especially in children with a history of systemic symptoms.
There is very little published evidence for treatment interventions in children under 12 years, or for phototherapy for children of any age, with any subtype of urticaria.
Pregnancy and breastfeeding
There is insufficient evidence to make a recommendation about the safety of using most drugs during pregnancy and breastfeeding. Refer to the manufacturer’s summary of product characteristics, 11 or other published guidelines.
However, in active disease and after counselling the person with any type of urticaria, where necessary consider cetirizine or loratadine (see Appendix C – LETR narratives – for available information on antihistamines).
Similarly, to date there is no evidence of harm to the fetus or mother from the use of omalizumab in pregnancy, or whilst breastfeeding, although again the person should be fully informed of the lack of available evidence.
The clinical subtype(s) of urticaria.
Provision of educational material.
Advice on avoidance of triggers.
Assessment of disease impact, for example DLQI (at a minimum, prior to commencement of second‐line agents).
Assessment of disease severity, for example UAS7, AAS or UCT (at a minimum, prior to commencement of second‐line agents).
Use of a second‐generation H 1 ‐antihistamine at the licensed dosage, as a first‐line agent for all types of chronic urticaria.
Use of a second‐generation H 1 ‐antihistamine above the manufacturer’s recommended dose for all types of urticaria, if a licensed dose fails to adequately control symptoms, unless there are contraindications.
The addition of montelukast to a second‐generation H 1 ‐antihistamine, in people with CSU whose symptoms are not adequately responsive to H 1 ‐antihistamines alone.
The addition of omalizumab (or ciclosporin in CSU) to a second‐generation H 1 ‐antihistamine, if symptoms are not adequately controlled by first‐line agents.
Use of oral corticosteroids limited to short courses, if applicable.
Avoidance of first‐generation H 1 ‐antihistamines as first‐line treatment.
The audit recommendation of 20 cases per department is to reduce variation in the results due to a single person and allow benchmarking between different units. However, departments unable to achieve this recommendation may choose to audit all cases seen in the preceding 12 months. See Appendix L (Supporting Information) for the set of audit standards, data items and data collection methodology.
The draft document and Supporting Information were made available to the BAD membership, British Dermatological Nursing Group (BDNG), Primary Care Dermatological Society (PCDS), British Society for Allergy & Clinical Immunology (BSACI) and the Royal College of Pathologists’ Immunology Specialist Advisory Committee for comments, which were actively considered by the GDG. Following further review, the finalized version was sent for peer review by the Clinical Standards Unit of the BAD, made up of the T&G subcommittee, prior to submission for publication.
This document has been prepared on behalf of the BAD and is based on the best data available when the document was prepared. It is recognized that under certain conditions it may be necessary to deviate from the guidelines and that the results of future studies may require some of the recommendations herein to be changed. Failure to adhere to these guidelines should not necessarily be considered negligent, nor should adherence to these recommendations constitute a defence against a claim of negligence. Limiting the review to English‐language references was a pragmatic decision, but the authors recognize this may exclude some important information published in other languages.
The proposed revision date for this set of recommendations is scheduled for 2026; where necessary, important interim changes will be updated on the BAD website.
We are very grateful to Dr Frances Humphreys (formerly South Warwickshire NHS Foundation Trust), who led this guideline updating project initially; both of the patient representatives including Ms Linda Campbell for their input in formulating the clinical questions, ranking of the outcomes, reviewing the evidence and formulating the recommendations; and all those who commented on the draft during the consultation period.
Mohd Mustapa MF , Exton LS , Bell HK et al . Updated guidance for writing a British Association of Dermatologists clinical guideline: the adoption of the GRADE methodology 2016 . Br J Dermatol 2017 ; 176 : 44 – 51 .
Google Scholar
Brouwers MC , Kho ME , Browman GP et al . AGREE II: advancing guideline development, reporting and evaluation in health care . CMAJ 2010 ; 182 : E839 – 42 .
GRADEpro Guideline Development Tool . Hamilton, OH, Canada : McMaster University (developed by Evidence Prime, Inc.) , 2020 .
Google Preview
Grattan CE , Humphreys F . Guidelines for evaluation and management of urticaria in adults and children . Br J Dermatol 2007 ; 157 : 1116 – 23 .
Guyatt GH , Oxman AD , Vist GE et al . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations . BMJ 2008 ; 336 : 924 – 6 .
Zuberbier T , Abdul Latiff AH , Abuzakouk M et al . The international EAACI/GA 2 LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis and management of urticaria . Allergy 2022 . https://doi.org/10.1111/all.15090 .
National Institute for Health and Care Excellence . NSAIDs – prescribing issues . Available at: https://cks.nice.org.uk/topics/nsaids‐prescribing‐issues (last accessed 15 November 2021).
British Society for Allergy & Clinical Immunology (BSACI) . Non‐steroidal anti‐inflammatory drugs (NSAIDS) . Available at: https://www.bsaci.org/professional‐resources/allergy‐management/drug‐allergy/non‐steroidal‐anti‐inflammatory‐drugs‐nsaids (last accessed 15 November 2021).
Kowalski ML , Asero R , Bavbek S et al . Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti‐inflammatory drugs . Allergy 2013 ; 68 : 1219 – 32 .
National Institute for Health and Care Excellence . Corticosteroids – oral . Available at: https://cks.nice.org.uk/topics/corticosteroids‐oral (last accessed 15 November 2021).
emc . Latest medicine updates. Available at: https://www.medicines.org.uk/emc/latest‐medicines‐updates (last accessed 15 November 2021).
Sánchez J , Amaya E , Acevedo A et al . Prevalence of inducible urticaria in patients with chronic spontaneous urticaria: associated risk factors . J Allergy Clin Immunol 2017 ; 5 : 464 – 70 .
Zuberbier T , Balke M , Worm M et al . Epidemiology of urticaria: a representative cross‐sectional population survey . Clin Exp Dermatol 2010 ; 35 : 869 – 73 .
Kim BR , Yang S , Choi JW et al . Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population‐based study . J Dermatol 2018 ; 45 : 10 – 16 .
Fricke J , Ávila G , Keller T et al . Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta‐analysis . Allergy 2020 ; 75 : 423 – 32 .
Maurer M , Abuzakouk M , Bérard F et al . The burden of chronic spontaneous urticaria is substantial: real‐world evidence from ASSURE‐CSU . Allergy 2017 ; 72 : 2005 – 16 .
Guillet G , Bécherel P‐A , Pralong P et al . The burden of chronic urticaria: French baseline data from the international real‐life AWARE study . Eur J Dermatol 2019 ; 29 : 49 – 54 .
Bezalel S , Mahlab‐Guri K , Asher I et al . Angiotensin‐converting enzyme inhibitor‐induced angioedema . Am J Med 2015 ; 128 : 120 – 5 .
Stone C , Brown NJ . Angiotensin‐converting enzyme inhibitor and other drug‐associated angioedema . Immunol Allergy Clin 2017 ; 37 : 483 – 95 .
Mehregan DR , Hall MJ , Gibson LE . Urticarial vasculitis: a histopathologic and clinical review of 72 cases . J Am Acad Dermatol 1992 ; 26 : 441 – 8 .
Gusdorf L , Asli B , Barbarot S et al . Schnitzler syndrome: validation and applicability of diagnostic criteria in real‐life patients . Allergy 2017 ; 72 : 177 – 82 .
Kuemmerle‐Deschner JB , Ozen S , Tyrrell PN et al . Diagnostic criteria for cryopyrin‐associated periodic syndrome (CAPS) . Ann Rheum Dis 2017 ; 76 : 942 – 7 .
Niimi N , Francis DM , Kermani F et al . Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria . J Invest Dermatol 1996 ; 106 : 1001 – 6 .
Kolkhir P , Church MK , Weller K et al . Autoimmune chronic spontaneous urticaria: what we know and what we do not know . J Allergy Clin Immunol 2017 ; 139 : 1772 – 81 .
Sánchez J , Sánchez A , Cardona R . Causal relationship between anti‐TPO IgE and chronic urticaria by in vitro and in vivo tests . Allergy Asthma Immunol Res 2019 ; 11 : 29 – 42 .
Schmetzer O , Lakin E , Topal FA et al . IL‐24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria . J Allergy Clin Immunol 2018 ; 142 : 876 – 82 .
Hoffman HM , Mueller JL , Broide DH et al . Mutation of a new gene encoding a putative pyrin‐like protein causes familial cold autoinflammatory syndrome and Muckle‐Wells syndrome . Nat Genet 2001 ; 29 : 301 – 5 .
Aksentijevich I , Nowak M , Mallah M et al . De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal‐onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin‐associated autoinflammatory diseases . Arthritis Rheum 2002 ; 46 : 3340 – 8 .
Rowczenio DM , Gomes SM , Aróstegui JI et al . Late‐onset cryopyrin‐associated periodic syndromes caused by somatic NLRP3 mosaicism – UK single center experience . Front Immunol 2017 ; 8 : 1410 .
Rowczenio DM , Pathak S , Arostegui JI et al . Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome . Blood 2018 ; 131 : 974 – 81 .
Leznoff A , Sussman GL . Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients . J Allergy Clin Immunol 1989 ; 84 : 66 – 71 .
Caminiti L , Passalacqua G , Magazzu G et al . Chronic urticaria and associated coeliac disease in children: a case–control study . Pediatr Allergy Immunol Pulmonol 2005 ; 16 : 428 – 32 .
Confino‐Cohen R , Chodick G , Shalev V et al . Chronic urticaria and autoimmunity: associations found in a large population study . J Allergy Clin Immunol 2012 ; 129 : 1307 – 13 .
Lebwohl B , Söderling J , Roelstraete B et al . Risk of skin disorders in patients with celiac disease: a population‐based cohort study . J Am Acad Dermatol 2022 ; 85 : 1456 – 64 .
Zigmond AS , Snaith RP . The hospital anxiety and depression scale . Acta Psychiatr Scand 1983 ; 67 : 361 – 70 .
Weller K , Groffik A , Church MK et al . Development and validation of the Urticaria Control Test: a patient‐reported outcome instrument for assessing urticaria control . J Allergy Clin Immunol 2014 ; 133 : 1365 – 72 .
Weller K , Groffik A , Magerl M et al . Development, validation, and initial results of the Angioedema Activity Score . Allergy 2013 ; 68 : 1185 – 92 .
Jáuregui I , Ortiz de Frutos F , Ferrer M et al . Assessment of severity and quality of life in chronic urticaria . J Investig Allergol Clin Immunol 2014 ; 24 : 80 – 6 .
Młynek A , Zalewska‐Janowska A , Martus P et al . How to assess disease activity in patients with chronic urticaria? Allergy 2008 ; 63 : 777 – 80 .
Ertas R , Ozyurt K , Atasoy M et al . The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change . Allergy 2018 ; 73 : 705 – 12 .
Iqbal K , Bhargava K , Skov PS et al . A positive serum basophil histamine release assay is a marker for ciclosporin‐responsiveness in patients with chronic spontaneous urticaria . Clin Transl Allergy 2012 ; 2 : 19 .
Maurer M , Giménez‐Arnau AM , Sussman G et al . Ligelizumab for chronic spontaneous urticaria . N Engl J Med 2019 ; 381 : 1321 – 32 .
Magerl M , Altrichter S , Borzova E et al . The definition, diagnostic testing, and management of chronic inducible urticarias – the EAACI/GA 2 LEN/EDF/UNEV consensus recommendations 2016 update and revision . Allergy 2016 ; 71 : 780 – 802 .
Gompels M , Lock R , Abinun M et al . C1 inhibitor deficiency: consensus document . Clin Exp Immunol 2005 ; 139 : 379 – 94 .
Sjöwall C , Mandl T , Skattum L et al . Epidemiology of hypocomplementaemic urticarial vasculitis (anti‐C1q vasculitis) . Rheumatology 2018 ; 57 : 1400 – 7 .
Sharma M , Bennett C , Cohen SN , Carter B . H 1 ‐antihistamines for chronic spontaneous urticaria . Cochrane Database Syst Rev 2014 ; 2014 : CD006137 .
Purohit A , Melac M , Pauli G et al . Twenty‐four‐hour activity and consistency of activity of levocetirizine and desloratadine in the skin . Br J Clin Pharmacol 2003 ; 56 : 388 – 94 .
Lever L , Hill S , Marks R et al . Effects of cetirizine, loratadine and terfenadine on histamine weal and flare reactions . Skin Pharmacol Physiol 1992 ; 5 : 29 – 33 .
Phinyo P , Koompawichit P , Nochaiwong S et al . Comparative efficacy and acceptability of licensed dose second‐generation antihistamines in chronic spontaneous urticaria: a network meta‐analysis . J Allergy Clin Immunol 2021 ; 9 : 956 – 70 .
Guillen‐Aguinaga S , Jauregui Presa I , Aguinaga‐Ontoso E et al . Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta‐analysis . Br J Dermatol 2016 ; 175 : 1153 – 65 .
Maurer M , Church M , Gonçalo M et al . Management and treatment of chronic urticaria (CU) . J Eur Acad Dermatol Venereol 2015 ; 29 : 16 – 32 .
Casale TB , Blaiss MS , Gelfand E et al . First do no harm: managing antihistamine impairment in patients with allergic rhinitis . J Allergy Clin Immunol 2003 ; 111 : S835 – 42 .
Weller K , Ziege C , Staubach P et al . H1‐antihistamine up‐dosing in chronic spontaneous urticaria: patients’ perspective of effectiveness and side effects – a retrospective survey study . PLOS ONE 2011 ; 6 :e23931.
Cataldi M , Maurer M , Taglialatela M et al . Cardiac safety of second‐generation H 1 ‐antihistamines when updosed in chronic spontaneous urticaria . Clin Exp Allergy 2019 ; 49 : 1615 – 23 .
de Silva NL , Damayanthi H , Rajapakse AC et al . Leukotriene receptor antagonists for chronic urticaria: a systematic review . Allergy Asthma Clin Immunol 2014 ; 10 : 24 .
Nettis E , Colanardi M , Soccio A et al . Desloratadine in combination with montelukast suppresses the dermographometer challenge test papule, and is effective in the treatment of delayed pressure urticaria: a randomized, double‐blind, placebo‐controlled study . Br J Dermatol 2006 ; 155 : 1279 – 82 .
Pacor M , Di Lorenzo G , Corrocher R . Efficacy of leukotriene receptor antagonist in chronic urticaria. A double‐blind, placebo‐controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid . Clin Exp Allergy 2001 ; 31 : 1607 – 14 .
Akenroye AT , McEwan C , Saini SS . Montelukast reduces symptom severity and frequency in patients with angioedema‐predominant chronic spontaneous urticaria . J Allergy Clin Immunol 2018 ; 6 : 1403 – 5 .
Fedorowicz Z , van Zuuren EJ , Hu N . Histamine H 2 ‐receptor antagonists for urticaria . Cochrane Database Syst Rev 2012 ; 2012 : CD008596 .
Bleehen S , Thomas S , Greaves M et al . Cimetidine and chlorpheniramine in the treatment of chronic idiopathic urticaria: a multi‐centre randomized double‐blind study . Br J Dermatol 1987 ; 117 : 81 – 8 .
GOV.UK. Ranitidine – MHRA drug alert issued for Teva UK recall . Available at: https://www.gov.uk/government/news/ranitidine‐mhra‐drug‐alert‐issued‐for‐teva‐uk‐recall (last accessed 15 November 2021).
Zuberbier T , Aberer W , Asero R et al . The EAACI/GA 2 LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria . Allergy 2018 ; 73 : 1393 – 414 .
Kone‐Paut I , Piram M . Targeting interleukin‐1β in CAPS (cryopyrin‐associated periodic) syndromes: what did we learn? Autoimmun Rev 2012 ; 12 : 77 – 80 .
Kolkhir P , Altrichter S , Munoz M et al . New treatments for chronic urticaria . Ann Allergy Asthma Immunol 2020 ; 124 : 2 – 12 .
Humphreys F , Hunter JA . The characteristics of urticaria in 390 patients . Br J Dermatol 1998 ; 138 : 635 – 8 .
Tharp MD , Bernstein JA , Kavati A et al . Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta‐analysis of ‘real‐world’ evidence . JAMA Dermatol 2019 ; 155 : 29 – 38 .
Kulthanan K , Chaweekulrat P , Komoltri C et al . Cyclosporine for chronic spontaneous urticaria: a meta‐analysis and systematic review . J Allergy Clin Immunol 2018 ; 6 : 586 – 99 .
Champion RH , Roberts SO , Carpenter RG et al . Urticaria and angio‐oedema. A review of 554 patients . Br J Dermatol 1969 ; 81 : 588 – 97 .
Netchiporouk E , Sasseville D , Moreau L et al . Evaluating comorbidities, natural history, and predictors of early resolution in a cohort of children with chronic urticaria . JAMA Dermatol 2017 ; 153 : 1236 – 42 .
Fitzsimons R , van der Poel LA , Thornhill W et al . Antihistamine use in children . Arch Dis Child Educ Pract Ed 2015 ; 100 : 122 – 31 .
Gabrielli S , Le M , Netchiporouk E et al . Chronic urticaria in children can be controlled effectively with updosing second‐generation antihistamines . J Am Acad Dermatol 2020 ; 82 : 1535 – 7 .
Al‐Shaikhly T , Rosenthal JA , Ayars AG et al . Omalizumab for chronic urticaria in children younger than 12 years . Ann Allergy Asthma Immunol 2019 ; 123 : 208 – 10 .
Doshi DR , Weinberger MM . Experience with cyclosporine in children with chronic idiopathic urticaria . Pediatr Dermatol 2009 ; 26 : 409 – 13 .
Giuliodori K , Ganzetti G , Campanati A et al . A non‐responsive chronic autoimmune urticaria in a 12‐year‐old autistic girl treated with cyclosporin . J Eur Acad Dermatol Venereol 2009 ; 23 : 619 – 20 .
Neverman L , Weinberger M . Treatment of chronic urticaria in children with antihistamines and cyclosporine . J Allergy Clin Immunol Pract 2014 ; 2 : 434 – 8 .
Author notes
Supplementary data.
Appendix A Systematic review protocols and clinical questions.
Appendix B Forest plots for comparative studies.
Appendix C Linking the Evidence To the Recommendations (LETR).
Appendix D GRADE evidence tables.
Appendix E Summary of included comparative studies.
Appendix F Narrative findings for noncomparative studies.
Appendix G PRISMA diagram – study selection.
Appendix H Papers excluded from quantitative analysis.
Appendix I AMSTAR 2 critical appraisal of the included systematic reviews.
Appendix J Methodology.
Appendix K Search strategy.
Appendix L Audit standards, data items and data collection methodology.
Powerpoint S1 Journal Club Slide Set.
| Month: | Total Views: |
|---|---|
| November 2022 | 1 |
| January 2023 | 30 |
| February 2023 | 253 |
| March 2023 | 517 |
| April 2023 | 141 |
| May 2023 | 486 |
| June 2023 | 395 |
| July 2023 | 400 |
| August 2023 | 402 |
| September 2023 | 426 |
| October 2023 | 379 |
| November 2023 | 384 |
| December 2023 | 281 |
| January 2024 | 394 |
| February 2024 | 323 |
| March 2024 | 354 |
| April 2024 | 298 |
| May 2024 | 406 |
| June 2024 | 390 |
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1365-2133
- Print ISSN 0007-0963
- Copyright © 2024 British Association of Dermatologists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Introduction
- Article Information
eMethods. Supplemental Methods
Data Sharing Statement
- Error in Table 1 and Results JAMA Network Open Correction April 5, 2023
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Duperrex O , Tommasini F , Muller YD. Incidence of Chronic Spontaneous Urticaria Following Receipt of the COVID-19 Vaccine Booster in Switzerland. JAMA Netw Open. 2023;6(2):e2254298. doi:10.1001/jamanetworkopen.2022.54298
Manage citations:
© 2024
- Permissions
Incidence of Chronic Spontaneous Urticaria Following Receipt of the COVID-19 Vaccine Booster in Switzerland
- 1 Center for Primary Care and Public Health (Unisanté), Lausanne, Switzerland
- 2 Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
- Correction Error in Table 1 and Results JAMA Network Open
The messenger RNA (mRNA)–based vaccines Spikevax (Moderna) and Comirnaty (Pfizer-BioNTech) are the most widely distributed vaccines in Switzerland. 1 Many chronic spontaneous urticaria (CSU) cases (recurrent wheals, angioedema, or both for more than 6 weeks 2 ) have been observed after the booster dose. 3 To assess whether a temporal association exists between COVID-19 vaccines and new-onset CSU, we compared the incidence rates of vaccine-related CSU in the canton of Vaud (CSU-Vaud) with all of Switzerland (CSU-Swiss).
Sixteen local allergists helped identify eligible patients, who were then contacted through the Lausanne University Hospital. Patients were sent an online questionnaire link between April 14 and August 8, 2022. Because local allergists were eligible to identify but not include patients for this study, written informed consent was not required. The Commission Cantonale d’Éthique de la Recherche sur l’Être Humain approved the study. This study followed the STROBE reporting guideline.
We obtained the number of first booster doses given to the CSU-Vaud population (n = 298 813) and the CSU-Swiss population (n = 3 278 808) between December 1, 2021, and August 31, 2022, by brand. We calculated the crude incidence risk ratio of CSU per 100 000 persons having received a first booster dose and estimated the relative risk of CSU after the Moderna vs Pfizer-BioNTech booster (eMethods in Supplement 1 ).
Among 97 patients, 80 (56 [70%] female; median [IQR] age, 41 [35-49] years) agreed to participate and were assigned to the CSU-Vaud cohort. The CSU-Swiss cohort included 782 patients (446 [58%] female; median [IQR] age, 39 [33-48] years). In 72 patients (90%) in the CSU-Vaud cohort and 636 (81%) in the CSU-Swiss cohort, CSU started after the booster. The median (IQR) time between vaccination and CSU onset was 10 (8-12) days in the CSU-Vaud cohort and 11 (9-13) days in the CSU-Swiss cohort. Seventy-four cases (92%) in the CSU-Vaud and 727 (93%) in the CSU-Swiss cohort were associated with the Moderna vaccine ( Table 1 ).
In the CSU-Vaud cohort, 76 participants (95%) reported taking antihistamines (taken daily in 60). Of the 80 CSU-Vaud participants, 11 (14%) reported previous urticaria, 23 (29%) reported hay fever, and 9 (11%) reported drug allergies. At data collection, 25 patients (31%) reported a diagnosis of COVID-19 infection after vaccination, with a median (IQR) delay of 51 (18-89) days.
The overall crude incidence rate of CSU after a COVID-19 booster per 100 000 persons immunized with a booster was similar in the CSU-Vaud (n = 24) and CSU-Swiss (n = 19) cohorts ( Table 2 ). Compared with the Pfizer-BioNTech vaccine, the relative risk of developing CSU after the Moderna vaccine was 20.8 (95% CI, 6.5-66.0) in the CSU-Vaud cohort and 16.1 (10.8-24.0) in the CSU-Swiss cohort ( Table 2 ).
The results of this cohort study suggest an association between the booster dose of mainly the Moderna vaccine and new-onset CSU. However, this study has limitations. First, there is a selection bias for patients with CSU in relation to COVID-19 vaccines. Baseline data on the incidence of CSU, independent of COVID-19 vaccines, in the general population are not available in Switzerland. Second, a confounding association with the Omicron variant wave is also possible, although only 31% reported a confirmed infection in the CSU-Vaud cohort. Third, we could not adjust incidence rates because individual data on vaccination by brand, age, and sex were not available.
As a potential contributing mechanism warranting further investigations, our group previously showed that the Moderna vaccine had a greater association with positive skin and basophil activation tests results compared with the Pfizer-BioNTech vaccine. 4 Alternatively, with the Moderna vaccines containing a higher dose of mRNA and being more immunogenic than the Pfizer-BioNTech vaccine, 5 one could speculate that the booster nonspecifically triggered CSU in predisposed individuals.
These data should not discourage patients from being vaccinated. However, guidelines defining the eligibility and dosing for upcoming mRNA-based boosters are needed for patients with CSU after an mRNA-based COVID-19 vaccine.
Accepted for Publication: December 15, 2022.
Published: February 1, 2023. doi:10.1001/jamanetworkopen.2022.54298
Correction: This article was corrected on April 5, 2023, to fix the number of participants in the CSU-Swiss cohort who received the Moderna vaccine, which was erroneously written in Table 1 and the Results as 77 (94%); the correct value is 727 (93%).
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Duperrex O et al. JAMA Network Open .
Corresponding Author: Yannick Daniel Muller, MD, PhD, Lausanne University Hospital and University of Lausanne, Rue du Bugnon 46, CH-1011 Lausanne, Switzerland ( [email protected] ).
Author Contributions: Drs Duperrex and Muller had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Muller.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Duperrex, Muller.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: All authors.
Administrative, technical, or material support: Tommasini.
Supervision: Muller.
Conflict of Interest Disclosures: None reported.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Davide Mercuri, Lausanne University Hospital and University of Lausanne, Lausanne Switzerland, assisted in the coordination of the study. We thank the many allergists who helped in the identification of patients with CSU, in particular Olivier Estoppey, DrMed, private practice, Nyon, Switzerland. They were not compensated for their work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
A Guide To Urticaria
Home / Clinical Trials / A Guide to Urticaria and Clinical Trials
Match to Urticaria Clinical Trials
What is urticaria.
Urticaria is a common skin disorder also known as hives.
It can present in two main forms: Chronic Spontaneous Urticaria (CSU) describes a condition where hives are present for most days of the week for at least six weeks. Acute Urticaria is a similar condition, but it presents for fewer than six weeks.
Physical urticaria happens only when an irritating stimulus is applied to the skin; the hives fade when the stimulus is withdrawn. It is understood as an allergic reaction.
CSU and acute urticaria are known collectively as spontaneous urticaria and may require medical care.
What Conditions Are Associated With Urticaria?
Both CSU and acute urticaria can be debilitating due to the itching and pain the hives cause. The disorder is considered uncommonly difficult to treat among dermatological conditions. It is also a more emotionally demanding condition, which can cause great mental distress.
Flare-ups of urticaria are sudden and occur without warning. Itchy, burning, rash-like lesions can appear virtually anywhere on the skin. Individual lesions are called wheals. One characteristic of urticaria is the very short duration of the wheals, each fading within 24 hours.
Urticaria Recovery and Lifestyle Changes
The various forms of urticaria can be difficult to diagnose. This is because there can be several causes for any patient to develop wheals similar to urticaria. A complete diagnosis requires identifying any environmental factors that might contribute to the symptoms.
Some very effective treatments are available, but must be prescribed and used correctly. Most treatments center around non-sedating antihistamines. Antihistamines interfere with some of the body’s inflammatory responses and are also used to treat certain allergies.
Although the exact source of urticaria is not yet understood, doctors do know certain physical stimuli can lead to flare-ups. These include things like alcoholic drinks, over-the-counter NSAID medications like aspirin and ibuprofen, exercise, and extreme temperatures (both hot and cold.)
Patients can improve their symptom management by noticing which stimuli most often lead to their own flare-ups. By reducing or avoiding contact with these factors, many can substantially reduce the number of flare-ups they experience.
Recent Urticaria Medical Research
Nearly half of all cases of urticaria are associated with autoimmune dysfunction. The immune system can become too responsive to benign stimuli, leading to inflammation with no protective purpose. The course of treatment varies based on whether immune function is involved.
With that in mind, most medical research on urticaria is focused on finding targeted ways to counteract overactive immune function. Urticaria clinical studies also strive to uncover the other factors that might be involved in spontaneous urticaria, such as genetic factors.
Why Are Further Urticaria Clinical Studies Necessary?
Urticaria is unusual among dermatological conditions and has received plenty of attention. While urticaria clinical research has been successful so far, physicians have not been able to answer why some people get urticaria or isolate the risk factors involved.
Both acute and chronic spontaneous urticaria clinical trials are necessary if doctors are to fully understand these diseases and develop better treatments. Recent urticaria medical research led to promising findings, with several novel medications under development.
Current Urticaria Clinical Studies
The following list includes all urticaria clinical studies registered with ClinicalStudies.gov. To submit urticaria clinical studies to our list, please contact our team.
Although there are several treatments for spontaneous urticaria, many patients receive few benefits or have adverse reactions. Likewise, some specific forms of the disorder have few effective treatment options. Urticaria clinical studies are an essential piece in broadening the horizons of urticaria medical research and improving patients’ quality of life.
- https://www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/urticaria-acute-and-chronic-spontaneous-urticaria/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4622315/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3479225/
- https://www.webmd.com/skin-problems-and-treatments/chronic-skin-rash
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6557779/
Other Conditions
- Acute Aortic Dissection
- Ankylosing Spondylitis
- Alzheimer’s
- Auditory Loss and Deafness
- Autoimmune Hepatitis
- Bronchiectasis
- Breast Cancer
- Esophageal Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Compensation
- COPD Treatments
- Coronavirus
- Crohn’s Disease
- Diet And Nutrition
- Endometriosis
- Fibromyalgia
- Hepatitis B
- Hepatitis C
- Mindfulness
- Multiple Sclerosis
- Myasthenia Gravis
- Ophthalmology
- Osteoarthritis
- Parkinson’s Disease
- PBC and PSC
- Plant Based Diet
- Plastic Surgery
- Pulmonary Fibrosis
- Ulcerative Colitis
- Urinary Tract Infections
- Weight Loss
Paid Vaccine Trial For Women 16-17 Years Old
CMVictory: a vaccine trial for women between 16-17 years old.
Paid Clinical Trials Nationwide
Nationwide clinical trials offering up to $3,000 for participation and treatment.
Paid Vaccine Study For Teens
Vaccine study that aims to protect teens ages 12–17 against Epstein-Barr virus (EBV) and infectious mononucleosis (mono).
Paid Clinical Studies Nationwide
Sign up to receive email notifications on clinical trials for individual conditions in your local area.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- AN AUDIENCE WITH
- 27 June 2024
When chronic and rare disease worlds converge
- Asher Mullard
You can also search for this author in PubMed Google Scholar
Sharon Barr was delighted when AstraZeneca acquired rare-disease specialists Alexion in 2021. For Barr, then head of research and product development at Alexion, the US$39 billion deal was set to give her team access to a broader range of technologies and expertise to solve the many problems faced by patients with rare diseases. “We have been able to reach more patients than ever, and that is fantastic. That's all that I ever wanted to do,” says Barr. Fittingly, therefore, this acquisition has also catapulted Barr into the world of chronic diseases, which affect some 2 billion people worldwide. Last year AstraZeneca promoted Barr to head up BioPharmaceuticals R&D, overseeing the company’s portfolio of drugs for complex and prevalent conditions.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41573-024-00114-3
Interviewed by Asher Mullard
Questions and answers have been edited for length and clarity.
Full-Time Faculty Member in Molecular Agrobiology at Peking University
Faculty positions in molecular agrobiology, including plant (crop) molecular biology, crop genomics and agrobiotechnology and etc.
Beijing, China
School of Advanced Agricultural Sciences, Peking University
Research Postdoctoral Fellow - MD
Houston, Texas (US)
Baylor College of Medicine (BCM)
Assistant Professor (Tenure Track) of Biomolecular Engineering for Health
The Department of Biosystems Science and Engineering (D-BSSE) (www.bsse.ethz.ch) at ETH Zurich invites applications for the above-mentioned position
Zurich, Switzerland
Suzhou Institute of Systems Medicine Seeking High-level Talents
Full Professor, Associate Professor, Assistant Professor
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)
Faculty Positions in Westlake University
Founded in 2018, Westlake University is a new type of non-profit research-oriented university in Hangzhou, China, supported by public a...
Hangzhou, Zhejiang, China
Westlake University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Chronic Spontaneous Urticaria Market Forecast 2032: Clinical Trials, Epidemiology, FDA Approvals, Therapies and Companies
Press release from: delveinsight business research llp.

Chronic Spontaneous Urticaria Pipeline
Permanent link to this press release:
You can edit or delete your press release Chronic Spontaneous Urticaria Market Forecast 2032: Clinical Trials, Epidemiology, FDA Approvals, Therapies and Companies here
Delete press release Edit press release
More Releases from DelveInsight Business Research LLP

All 5 Releases
More Releases for Chronic
- Categories Advertising, Media Consulting, Marketing Research Arts & Culture Associations & Organizations Business, Economy, Finances, Banking & Insurance Energy & Environment Fashion, Lifestyle, Trends Health & Medicine Industry, Real Estate & Construction IT, New Media & Software Leisure, Entertainment, Miscellaneous Logistics & Transport Media & Telecommunications Politics, Law & Society Science & Education Sports Tourism, Cars, Traffic RSS-Newsfeeds
- Order Credits
- About Us About / FAQ Newsletter Terms & Conditions Privacy Policy Imprint
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases
Affiliations.
- 1 Department of Dermatology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan.
- 2 Department of Dermatology, Tufts Medical Center, Boston, MA, United States.
- 3 Department of Dermatology, Venereology and Allergology, University Medical Center Göttingen, Göttingen, Germany.
- 4 Lower Saxony Institute of Occupational Dermatology, University Medical Center Göttingen, Göttingen, Germany.
- 5 Center for Chronic Pruritus, Department of Dermatology, University Hospital Muenster, Muenster, Germany.
- 6 Department of Dermatology, University of Lübeck, Lübeck, Germany.
- 7 Institute for Allergology, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
- 8 Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Allergology and Immunology, Berlin, Germany.
- 9 Institute and Comprehensive Center for Inflammation Medicine, University of Lübeck, Lübeck, Germany.
- 10 Lübeck Institute of Experimental Dermatology and Center for Research on Inflammation of the Skin, University of Lübeck, Lübeck, Germany.
- 11 Division of Dermatology, Rush University Medical Center, Chicago, IL, United States.
- 12 Department of Internal Medicine, Rush University Medical Center, Chicago, IL, United States.
- 13 Department of Dermatology and Allergology, Philipps-Universität, Marburg, Germany.
- 14 Department of Rheumatology and Clinical Immunology, University of Lübeck, Lübeck, Germany.
- 15 Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 16 Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA, United States.
- 17 Department of Dermatology, Columbia University Medical Center, New York, NY, United States.
- 18 Psoriasis Research and Treatment Centre, Charité-Universitätsmedizin Berlin, Berlin, Germany.
- 19 Interdisciplinary Group Molecular Immunopathology, Dermatology/Medical Immunology, Charité-Universitätsmedizin Berlin, Berlin, Germany.
- 20 Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
- PMID: 35755063
- PMCID: PMC9218547
- DOI: 10.3389/fmed.2022.875492
An estimated 20-25% of the population is affected by chronic, non-communicable inflammatory skin diseases. Chronic skin inflammation has many causes. Among the most frequent chronic inflammatory skin diseases are atopic dermatitis, psoriasis, urticaria, lichen planus, and hidradenitis suppurativa, driven by a complex interplay of genetics and environmental factors. Autoimmunity is another important cause of chronic skin inflammation. The autoimmune response may be mainly T cell driven, such as in alopecia areata or vitiligo, or B cell driven in chronic spontaneous urticaria, pemphigus and pemphigoid diseases. Rare causes of chronic skin inflammation are autoinflammatory diseases, or rheumatic diseases, such as cutaneous lupus erythematosus or dermatomyositis. Whilst we have seen a significant improvement in diagnosis and treatment, several challenges remain. Especially for rarer causes of chronic skin inflammation, early diagnosis is often missed because of low awareness and lack of diagnostics. Systemic immunosuppression is the treatment of choice for almost all of these diseases. Adverse events due to immunosuppression, insufficient therapeutic responses and relapses remain a challenge. For atopic dermatitis and psoriasis, a broad spectrum of innovative treatments has been developed. However, treatment responses cannot be predicted so far. Hence, development of (bio)markers allowing selection of specific medications for individual patients is needed. Given the encouraging developments during the past years, we envision that many of these challenges in the diagnosis and treatment of chronic inflammatory skin diseases will be thoroughly addressed in the future.
Keywords: alopecia areata; atopic dermatitis; chronic spontaneous urticaria; hidradenitis suppurativa; inflammation; medical need; psoriasis; skin.
Copyright © 2022 Ujiie, Rosmarin, Schön, Ständer, Boch, Metz, Maurer, Thaci, Schmidt, Cole, Amber, Didona, Hertl, Recke, Graßhoff, Hackel, Schumann, Riemekasten, Bieber, Sprow, Dan, Zillikens, Sezin, Christiano, Wolk, Sabat, Kridin, Werth and Ludwig.
PubMed Disclaimer
Conflict of interest statement
HU has received research grants from JB, Otsuka, Taiho, Boehringer Ingelheim, Kyowa Kirin, Kaken, Sun Pharma, Shionogi, Teijin, Mitsubishi Tanabe, Nihon Zoki, Eisai, Torii and Tokiwa, consultant fees from Ono, Nihon-Pharmaceutical, Sun Pharma, argenx and Ishin Pharma, and speaker's fees from Nihon-Pharmaceutical, Maruho, Eli Lilly, Abbie, Eisai, Sanofi, Janssen, Kyowa Kirin, Ono, UCB, Novartis, Sun Pharma, Torii, Taiho, Mitsubishi Tanabe, and Boehringer Ingelheim during the last 3 years, DR has received honoraria or research support from AbbVie, Abcuro, AltruBio, Amgen, Boehringer-Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galdrema, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio during the last 3 years, MS has received consulting/speakers' fees, or grant support from AbbVie, Almirall, Amgen, Biogen, BMS, Janssen, Novartis, and UCB during the last 3 years, SS has received funding and personal fees from Celldex, Clexio, Dermasence, Galderma, GSK, Kiniksa, Menlo, Trevi, Novartis, Sanofi (investigator all), Abbvie, Almirall, Beiersdorf, Bellus Health, Benevolent, Bionorica, Cara, Celgene, CelloHealth, Clexio, DS Biopharma, Eli Lilly, Escient, Galderma, Grünenthal, Kiniksa, Klinge Pharma, Menlo, Sanofi, Sienna, Trevi, P.G. Unna Academy, Perrigo, Pfizer, Vanda, Vifor, WebMD (Consultancy/Advisory board) and Almirall, Eli Lilly, Sanofi, Galderma, Menlo, Omnicuris, Beiersdorf, Leo Pharma, Novartis, P. G. Unna Academy, Pfizer, Pierre Fabre (speaker) during the last 3 years, MMe has received honoraria as a speaker and/or consultant for Amgen, Aralez, argenx, Bayer, Beiersdorf, Celgene, Escient, Galderma, GSK, Menlo, Moxie, Novartis, Pharvaris, Pfizer, Roche, Sanofi, Siennabio, and Uriach, MMa was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach during the last 3 years, is a consultant, advisory board member, and/or investigator for AbbVie, Almirall, Amgen, Beiersdorf, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Janssen-Cilag, LEO Pharma, MorphoSys, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Samsung, Sandoz, Sanofi, Sun Pharma, and UCB, ES has received research grants from UCB, Incyte, Biotest, ArgenX, Dompe, Fresenius Medical Care, Bayer, AstraZenca, and Euroimmun and honoraria from Biotest, Thermo Fisher, ArgenX, Fresenius Medical care, Topas, Leo, Chugai, AstraZenca, and Almirall during the last 3 years, MH has received honoraria from Novartis, Sanofi, Celgene, and unrestricted grants from Biotest, Janssen Cilag and Topas durimg the last 3 years, KBi has received research funds from ArgenX during the last 3 years, DZ has received support for research and development work, lecturing and consulting from Euroimmun AG, UCB Pharma, ArgenX, Biotest, Abbvie, Janssen, Sanofi in the last 3 years, KW has received research grants, travel grants, consulting honoraria or lecturer's honoraria from Amgen, Bristol Myers Squibb, Celgene, Charité Research Organization, Flexopharm, Janssen-Cilag, Novartis Pharma, Sanofi-Aventis, and Trial Form Support during the last 3 years, RS has received research grants or honoraria for participation in advisory boards, clinical trials, or as speaker for one or more of the following: AbbVie, Amgen, Bayer, Boehringer Ingelheim Pharma, Bristol Myers Squibb, Celgene, Charité Research Organization, CSL Behring, Dr. Willmar Schwabe, Flexopharm, Incyte, Janssen-Cilag, La Roche-Posay Laboratoire Dermatologique, Novartis Pharma, Parexel International, Sanofi–Aventis, TFS, and UCB Biopharma during the last 3 years, VW has received grants from Celgene, Amgen, Janssen, Biogen, Gilead, Viela; Horizon therapeutics, Pfizer, Corbus, CSL Behring, consulted Astra-Zeneca, Pfizer, Biogen, Celgene, Resolve, Janssen, Gilead, Lilly, BMS, Nektar, Abbvie, Viela, GSK, EMD Serona, Sanofi, Anaptysbio, Amgen, Merck, Pfizer, Janssen, Neovacs, Idera, Octapharma, CSL Behring, Corbus, Novartis, Romefor during the last 3 years, RL has received honoraria for speaking or consulting or has obtained research grants from Novartis, Lilly, Bayer, Dompe, Synthon, Argen-X, and Incyte during the last 3 years. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Clinical images of patients with…
Clinical images of patients with chronic skin inflammatory diseases. (A) Blurry erythema and…
Similar articles
- Aberrant inflammasome activation as a driving force of human autoimmune skin disease. Fetter T, de Graaf DM, Claus I, Wenzel J. Fetter T, et al. Front Immunol. 2023 May 30;14:1190388. doi: 10.3389/fimmu.2023.1190388. eCollection 2023. Front Immunol. 2023. PMID: 37325658 Free PMC article. Review.
- Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Gibson F, Hanly A, Grbic N, Grunberg N, Wu M, Collard M, Alani RM. Gibson F, et al. Clin Rev Allergy Immunol. 2022 Dec;63(3):447-471. doi: 10.1007/s12016-022-08956-8. Epub 2022 Nov 8. Clin Rev Allergy Immunol. 2022. PMID: 36346551 Review.
- Multimorbidity and mortality risk in hospitalized adults with chronic inflammatory skin disease in the United States. Narla S, Silverberg JI. Narla S, et al. Arch Dermatol Res. 2020 Sep;312(7):507-512. doi: 10.1007/s00403-020-02043-8. Epub 2020 Feb 11. Arch Dermatol Res. 2020. PMID: 32047999
- Comorbidities in Dermatology: What's Real and What's Not. Qureshi A, Friedman A. Qureshi A, et al. Dermatol Clin. 2019 Jan;37(1):65-71. doi: 10.1016/j.det.2018.07.007. Epub 2018 Nov 1. Dermatol Clin. 2019. PMID: 30466689 Review.
- Dissecting susceptibility from exogenous triggers: the model of alopecia areata and associated inflammatory skin diseases. Garzorz N, Alsisi M, Todorova A, Atenhan A, Thomas J, Lauffer F, Ring J, Schmidt-Weber C, Biedermann T, Eyerich S, Eyerich K. Garzorz N, et al. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2429-35. doi: 10.1111/jdv.13325. Epub 2015 Sep 28. J Eur Acad Dermatol Venereol. 2015. PMID: 26416203
- Inhibition of interferon gamma impairs induction of experimental epidermolysis bullosa acquisita. Gross N, Marketon J, Mousavi S, Kalies K, Ludwig RJ, Bieber K. Gross N, et al. Front Immunol. 2024 May 10;15:1343299. doi: 10.3389/fimmu.2024.1343299. eCollection 2024. Front Immunol. 2024. PMID: 38799441 Free PMC article.
- Improving systemic therapy selection for inflammatory skin diseases: A clinical need survey. Brownstone ND, Farberg AS, Litchman GH, Quick AP, Siegel JJ, Hurton LV, Goldberg MS, Lio PA. Brownstone ND, et al. JAAD Int. 2024 Apr 6;16:49-56. doi: 10.1016/j.jdin.2024.03.019. eCollection 2024 Sep. JAAD Int. 2024. PMID: 38774343 Free PMC article.
- Incorporating Dermatologic Clinical Research Into Private Practice: A Review. Slater KN, Fivenson D. Slater KN, et al. Cureus. 2024 Apr 6;16(4):e57733. doi: 10.7759/cureus.57733. eCollection 2024 Apr. Cureus. 2024. PMID: 38711732 Free PMC article. Review.
- Patient-reported assessment of medical care for chronic inflammatory skin diseases: an enterprise-based survey. Wolk K, Schielein M, Maul JT, Widmayer F, Wanke K, Fischmann W, Nathan P, Sabat R. Wolk K, et al. Front Med (Lausanne). 2024 Apr 18;11:1384055. doi: 10.3389/fmed.2024.1384055. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38698787 Free PMC article.
- Blood MALT1 expression could help predict treatment outcomes in psoriasis patients, especially in those receiving biologics. Liu Q, Zhang Y, Xu B, Jin X, Yang T, Fan L. Liu Q, et al. Immun Inflamm Dis. 2024 Apr;12(4):e1235. doi: 10.1002/iid3.1235. Immun Inflamm Dis. 2024. PMID: 38578002 Free PMC article.
- Schmidt T, Solimani F, Pollmann R, Stein R, Schmidt A, Stulberg I, et al. . TH1/TH17 cell recognition of desmoglein 3 and bullous pemphigoid antigen 180 in patients with lichen planus. J Allergy Clin Immunol. (2018) 142:669–72.e7. 10.1016/j.jaci.2018.02.044 - DOI - PubMed
- Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, et al. . Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. (2015) 212:2203–12. 10.1084/jem.20151093 - DOI - PMC - PubMed
- Christophers E, van de Kerkhof PCM. Severity, heterogeneity and systemic inflammation in psoriasis. J Eur Acad Dermatol Venereol. (2019) 33:643–7. 10.1111/jdv.15339 - DOI - PubMed
- Meijer JM, Lamberts A, Luijendijk HJ, Diercks GFH, Pas HH, Zuidema SU, et al. . Prevalence of pemphigoid as a potentially unrecognized cause of pruritus in nursing home residents. JAMA Dermatol. (2019) 155:1423–4. 10.1001/jamadermatol.2019.3308 - DOI - PMC - PubMed
- Eyerich K, Eyerich S. Immune response patterns in non-communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol. (2018) 32:692–703. 10.1111/jdv.14673 - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- P30 AR069632/AR/NIAMS NIH HHS/United States
- P50 AR070588/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- Frontiers Media SA
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Chronic urticaria.
Dominique Dabija ; Prasanna Tadi ; Gerard N. Danosos .
Affiliations
Last Update: April 17, 2023 .
- Continuing Education Activity
Chronic spontaneous urticaria is defined as the occurrence of wheals and/or angioedema for a total duration of six weeks or more. Synonymous with "chronic urticaria" and "chronic idiopathic urticaria," it can be a debilitating condition with significant detriment on a patient's quality of life. This activity reviews the etiology, pathogenesis, and management of chronic spontaneous urticaria and highlights the role of the interprofessional team in improving care for patients with this condition.
- Identify the proposed etiology of chronic spontaneous urticaria.
- Summarize the pertinent points to explore in history in the evaluation of patients with chronic spontaneous urticaria.
- Outline the pharmacologic and non-pharmacologic treatment options available for chronic spontaneous urticaria.
- Discuss strategies for improving care coordination and communication among the interprofessional team to improve outcomes in the treatment of chronic spontaneous urticaria.
- Introduction
Chronic urticaria is a mast cell-mediated condition characterized by the recurrent occurrence of urticaria and/or angioedema.
The current definitions and classification stratify urticaria according to time course and etiology:
- Acute spontaneous urticaria : Spontaneous occurrence of wheals and/or angioedema for a total duration of fewer than six weeks.
- Chronic spontaneous urticaria (CSU) : Spontaneous occurrence of wheals and/or angioedema for a total duration of six weeks or more. This is synonymous with "chronic urticaria" and "chronic idiopathic urticaria."
- Chronic inducible urticaria (CIndU) : Occurrence of wheals for a total duration of six weeks or more, which is inducible by physical factors ( e.g., touch, pressure extremes) . This is synonymous with "physical urticaria." [1]
The etiology of CSU is yet to be fully established. The prevailing hypothesis is that it relates to autoimmune dysfunction involving autoantibodies targeting IgE and/or IgE receptors to activate histamine release from basophils and mast cells. Up to 40% of patients with CSU demonstrate a positive autologous serum skin test (ASST), whereby the patient’s serum injected into the dermis can induce urticaria. One-third of patients with CSU also has a positive basophil histamine release assay (BHRA), which tests for anti-FceRIa (an IgE receptor) or anti-IgE autoantibodies in the serum. [2] [3]
Further support is lent by the increased prevalence of autoimmune disorders amongst CSU patients. Of these, autoimmune hypothyroidism is the most common, observed in up to 9.8%. Other associated conditions include rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, celiac disease, and type-1 diabetes mellitus. [4]
Infections by a variety of organisms have also been associated with CSU. These include bacteria ( Helicobacter pylori, Streptococci, Staphylococci, Yersinia, Mycoplasma pneumoniae ), virus (H epatitis virus, Norovirus, Parvovirus B19 ), and parasites ( Giardia lamblia, Entamoeba spp., Anisakis simplex ). Causality remains unproven but may involve infection-mediated autoimmune response and molecular mimicry. [1] [5]
Foods and additives, while implicated in acute IgE-mediated food allergy, are rarely the cause of CSU. [6]
Evidence for the association between malignancy and CSU is conflicting as different studies, including retrospective studies and systematic reviews, demonstrate varying results. [7] [8]
- Epidemiology
The prevalence of CSU is estimated at between 0.23% and 1.8% of the population in the U.S. and internationally. There is a strong female predilection, affecting women twice as often as men. Both children and adults are affected, although the prevalence is highest amongst those 40 to 60 years of age. [9] [10]
- Pathophysiology
Urticaria and angioedema result from the activation of mast cells and basophils. Mast cell degranulation leads to the release of immune mediators. The primary mediator is histamine, which binds to H-receptors located on endothelial cells and sensory nerves. However, prostaglandin, leukotrienes, and a variety of cytokines and chemokines are also involved. Ultimately, this induces vasodilatation - increased permeability of vessels - and in turn, dermal edema and recruitment of inflammatory cells.
Activation of mast cells can be divided into immunological and non-immunological processes.
- Immunological activation is mediated by effectors of adaptive immunity binding receptors on mast cells. In type 1 hypersensitivity, crosslinking of allergen with IgE on sensitized mast cells leads to acute urticaria and anaphylaxis. In CSU, however, immunological activation of mast cells is thought to occur independently of allergen-IgE complexes and may involve IgG (type 2 hypersensitivity), circulating immune complexes (type 3 hypersensitivity), and T-cells (type 4 hypersensitivity).
- Non-immunological activation can be mediated by physical factors, as in CIndU, as well as food and drug molecules independent of adaptive immunity. In CSU, it may be that the threshold for activation by external stimuli is reduced. [11]
- Histopathology
The histopathological features of urticaria include interstitial edema involving the upper and mid-dermis, mixed inflammatory perivascular infiltrates, and dilated venules and lymphatic vessels that permit leakage of serum into the surrounding tissue.
Angioedema shares similar features, but they are observed deeper within the lower dermis and subcutaneous tissue.
A biopsy of involved skin for histology is not a routine aspect of the workup for CSU; however, it can be considered when the rash is atypical. Leukocytoclasis and vasculitis are not seen with CSU and must prompt consideration of alternate diagnoses. [12]
- History and Physical
Urticaria is characterized by pruritic pink-to-red papules and plaques typically with central pallor. These can vary in size and shape as the plaques coalesce together. Urticaria is fleeting, and each wheal remains for fewer than 24 hours without any residual ecchymosis or pigmentation. [1] There may be a predilection for pressure-prone areas such as the waistline, axilla, and groin, but urticaria can generally affect any part of the skin.
Secondary changes from scratching may be evident on examination of the skin, including excoriations, erosions, and hemorrhagic crust.
Concurrent angioedema occurs in up to 40%, characterized by subcutaneous or submucosal edema affecting non-dependent areas, most commonly lips, peri-orbital, genitals, and extremities. Patients complain of discomfort or pain rather than pruritus. This may take longer to resolve - up to 72 hours - and is the primary manifestation of CSU in 10% of patients. [1] [13]
A thorough history aims to identify potential triggers and exacerbating factors and to exclude differential diagnoses. This should explore time course and clinical features of urticarial rash, associated angioedema, associated systemic and infective symptoms, personal or family history of allergies and autoimmune diseases, social and occupational history, induction by physical factors, recent newly administered medications, relationship to foods, and any exacerbating factors. [1]
If the patient cannot recall the time course of wheals, drawing around an individual lesion with a skin marking pen is useful to document resolution within 24 hours. Where history is unrevealing, an external cause is highly unlikely to be identified for patients with CSU. [1]
Routine diagnostic work-up for CSU is limited to blood tests for complete blood count and inflammatory markers (C-reactive protein and/or erythrocyte sedimentation rate), mostly to rule out other potential diseases. Eosinophilia may be associated with atopy and parasitic infection. Elevated inflammatory markers should prompt consideration of associated systemic disease. Further investigations without clinical suspicion, as guided by history, are unlikely to yield any additional diagnoses. [14] Skin prick testing is not useful as CSU is rarely caused by type 1 allergy. [15] The utility and interpretation of autologous serum skin test as a screen for autoantibodies to IgE and IgE receptors is an area of active research. [16] [17]
A number of tools have been developed to assess disease activity ( e.g., urticaria activity score) , disease control ( e.g., urticaria control test ), and impacts on quality of life ( e.g., chronic urticaria quality of life index ). Baseline assessments should be performed to help guide treatment decisions and monitor progress. [1]
- Treatment / Management
The principles of management are to avoid exacerbating factors and to control symptoms as long as CSU persists. Pharmacological agents are directed at preventing mast cell degranulation and/or the effects of mast cell mediators released.
Second-generation H1-antihistamines ( e.g., cetirizine, loratadine, fexofenadine ), taken regularly, are the first-line pharmacological treatment. The dose can be up-titrated to 4 times standard dose if symptoms remain at 2 to 4-week intervals. Due to anticholinergic properties and the adverse effect profile on the central nervous system, the routine use of first-generation H1-antihistamines is no longer recommended. [1]
The primary difference in treatment between CSU and CIndU is that as needed, treatment may be used for the latter if the patient knows his trigger and is able to plan by taking an H1-antihistamine approximately two hours before being exposed to it. [1]
Omalizumab is a monoclonal antibody with a high affinity for free IgE. Given as a subcutaneous injection, the standard dosing regimen is 300 mg every 4 weeks. This has been shown to be efficacious as a second-line adjunct therapy for CSU that is unresponsive to H1-antihistamines. [18]
Ciclosporin is recommended as a third-line agent for CSU that remains refractory to the combination of H1-antihistamine and omalizumab. CSU is an off-label indication, and the dose and duration of ciclosporin should be minimized to avoid adverse events, including nephrotoxicity and hypertension. [19]
For acute flares of CSU, short courses only of systemic corticosteroids may help alleviate symptom severity and reduce the duration of the flare. [1] Topical corticosteroids do not have a role in CSU. [20]
There is limited low-quality evidence for alternate therapeutic options, including leukotriene antagonists, dapsone, methotrexate, sulfasalazine, plasmapheresis, phototherapy, and intravenous immunoglobulin. [1]
- Differential Diagnosis
- Urticarial vasculitis is a small vessel vasculitis characterized by wheals that persist for greater than 24 hours, associated with pain rather than an itch, and which resolve with residual ecchymosis and/or pigmentation.
- Papular urticaria is a persistent insect bite reaction characterized by clusters of pruritic papules, often with a central punctum, which persists for days to weeks.
- Mastocytosis encompasses a group of disorders resulting from the accumulation of clonal populations of mast cells in the skin and other organs. Darier sign, where a wheal can be induced by rubbing the affected skin, is pathognomonic.
- Auto-inflammatory syndromes such as cryopyrin-associated periodic syndromes (CAPS) and Schnitzler syndrome may present with urticarial eruptions in association with fever, arthralgia, and systemic symptoms.
- C1-esterase inhibitor deficiency, either hereditary or acquired, should be considered in patients who present with isolated angioedema in the absence of an urticarial rash.
- Bullous pemphigoid is an immunobullous disease affecting the elderly. The pre-bullous phase is characterized by pruritus and urticarial plaques, which evolve to tense bullae.
- Anaphylaxis is a potentially life-threatening type I hypersensitivity reaction that most often presents acutely with cutaneous symptoms of hives and angioedema.
CSU is typically self-limited, on average lasting 3 to 5 years. [21] The reported rate of remission in the first 12 months is as high as 80%. [22] However, up to 14% of patients may have persistent disease beyond 5 years. [23] Factors that portend a prolonged disease course include thyroid autoimmunity and concurrent angioedema. [21] [24]
- Complications
The pruritus associated with CSU can be of significant detriment to the patient’s quality of life and disrupt both activities of daily living and sleep. [25] Health status scores are also negatively affected by CSU. [1]
- Deterrence and Patient Education
Patients should be educated on treatment goals. Regular use of H1-antihistamines confers better symptom control than as-needed administration. [1] Avoidance and understanding of factors that can further aggravate urticaria and pruritus also play an important role. These include:
- Drugs: Non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin can cause non-allergic hypersensitivity reactions in a subset of patients. [26] Avoidance is recommended.
- Intercurrent illness: Urticaria may flare during bacterial and viral infections.
- Environmental factors: Tight clothing and heat ( e. g., hot showers) can often aggravate symptoms.
- Pearls and Other Issues
- Disease duration of less than and more than 6 weeks separates acute from chronic spontaneous urticaria.
- If the patient cannot recall the time course of urticarial lesions, drawing around an individual lesion with a skin marking pen is useful to document resolution or migration within 24 hours.
- Where history is unrevealing, an external cause is highly unlikely to be identified for patients with chronic spontaneous urticaria. Diagnostic workup is unrevealing.
- Skin prick test is not indicated in chronic spontaneous urticaria.
- Wheals that are painful rather than pruritic, that persist for greater than 24 hours, and/or resolve with ecchymosis or pigmentation should prompt consideration of urticarial vasculitis.
- The recommended stepwise approach to management begins with second-generation H1-antihistamines, followed by the addition of omalizumab, and subsequently ciclosporin.
- The rate of spontaneous remission in the first twelve months is as high as 80%.
- Enhancing Healthcare Team Outcomes
Patients in whom symptoms remain refractory to H1-antihistamines should be referred to a dermatologist and/or clinical immunologist for review and initiation of second and third-line therapeutic agents. CSU follows a fluctuant disease course and may resolve spontaneously. Accordingly, efficacy and need for continuation of treatment should be evaluated periodically at 3- to- 6-month intervals. [1] The psychological burden of CSU should not be underestimated, and where appropriate, counseling and psychotherapy may enhance overall health outcomes. [27]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Urticaria Pigmentosa DermNet New Zealand
Urticaria, Upper Arm DermNet New Zealand
Case of urticaria showing urticarial wheels Contributed by Ahmad Al Aboud, MD
Disclosure: Dominique Dabija declares no relevant financial relationships with ineligible companies.
Disclosure: Prasanna Tadi declares no relevant financial relationships with ineligible companies.
Disclosure: Gerard Danosos declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Dabija D, Tadi P, Danosos GN. Chronic Urticaria. [Updated 2023 Apr 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The Classification, Pathogenesis, Diagnostic Workup, and Management of Urticaria: An Update. [Handb Exp Pharmacol. 2022] The Classification, Pathogenesis, Diagnostic Workup, and Management of Urticaria: An Update. Maurer M, Zuberbier T, Metz M. Handb Exp Pharmacol. 2022; 268:117-133.
- Review Urticaria and Angioedema: an Update on Classification and Pathogenesis. [Clin Rev Allergy Immunol. 2018] Review Urticaria and Angioedema: an Update on Classification and Pathogenesis. Radonjic-Hoesli S, Hofmeier KS, Micaletto S, Schmid-Grendelmeier P, Bircher A, Simon D. Clin Rev Allergy Immunol. 2018 Feb; 54(1):88-101.
- A comparative analysis of chronic inducible urticaria in 423 patients: Clinical and laboratory features and comorbid conditions. [J Eur Acad Dermatol Venereol. ...] A comparative analysis of chronic inducible urticaria in 423 patients: Clinical and laboratory features and comorbid conditions. Ornek Ozdemir S, Kuteyla Can P, Degirmentepe EN, Cure K, Singer R, Kocaturk E. J Eur Acad Dermatol Venereol. 2024 Mar; 38(3):513-520. Epub 2023 Nov 22.
- Review [Angioedema and urticaria]. [Ann Dermatol Venereol. 2014] Review [Angioedema and urticaria]. Boccon-Gibod I, Bouillet L. Ann Dermatol Venereol. 2014 Nov; 141 Suppl 3:S586-95.
- Chronic spontaneous urticaria activity, impact and control as well as their changes are strongly linked, and these links are not affected by angioedema or comorbid inducible urticaria - Results from the validation of the Polish Urticaria Control Test. [World Allergy Organ J. 2022] Chronic spontaneous urticaria activity, impact and control as well as their changes are strongly linked, and these links are not affected by angioedema or comorbid inducible urticaria - Results from the validation of the Polish Urticaria Control Test. Brzoza Z, Badura-Brzoza K, Maurer M, Hawro T, Weller K. World Allergy Organ J. 2022 Mar; 15(3):100635. Epub 2022 Mar 21.
Recent Activity
- Chronic Urticaria - StatPearls Chronic Urticaria - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Published August 31, 2022. N Engl J Med 2022;387: 824 - 831. DOI: 10.1056/NEJMra2120166. VOL. 387 NO. 9. Chronic urticaria is defined as wheals (hives), angioedema (swelling), or both that have ...
Introduction. Chronic urticaria is characterized by wheals, angioedema, or both that occur continuously or sporadically for at least 6 weeks. The wheals are intensely pruritic, resolve in < 24 h, and tend to recur on a daily basis [].Chronic urticaria typically lasts for 2-5 years before spontaneously resolving [].The point prevalence of the disease ranges from 0.23 to 0.70% in different ...
Urticaria (hives) is a relatively common condition, with a point prevalence of about 0.5-1% 1. The peak incidence is in the range of 20-40 years. Urticaria is the general term for a cutaneous response characterized by wheals and swellings. A deeper localized swelling often associated with urticaria is called angioedema.
Urticaria is a heterogeneous inflammatory disorder that can be acute or chronic and is defined by the appearance of wheals, angioedema, or both. Very recently, the newest update and revision of the international European Academy of Allergy and Clinical Immunology/Global Allergy and Asthma European Network/European Dermatology Forum/Asia Pacific Association of Allergy Asthma Clinical Immunology ...
Chronic urticaria (CU) is a common, heterogeneous, and debilitating disease. Antihistamines and omalizumab are the mainstay therapies of CU. Additional treatment options are needed. Here, we review the off and beyond label use of licensed drugs, novel treatments that are currently under development, and promising new targets.
Conclusion. Chronic urticaria is significantly associated with poor quality of life and forms a major cause of economic burden for those who suffer with it for a long time. With the development of biologicals, management of AHs refractory urticaria has been eased with a good success rate in up to 80-90% patients.
Further research should focus on defining disease endotypes and their biomarkers, identifying new treatment targets and developing improved therapies. ... Chronic urticaria (CU) is either ...
Background: In the past 20 years, an increasing number of studies have advanced our understanding of the pathogenic mechanism of chronic urticaria (CU), providing new treatment options. Objectives: This bibliometric study aimed to evaluate published reports of CU-related studies from a number of different angles, review the research trends of the studies, and provide future perspectives of CU.
Chronic spontaneous urticaria (CSU) is a condition in which wheals, angioedema, and pruritus occur spontaneously and recurrently for at least 6 weeks. The etiology of this disease is partially dependent on production of autoantibodies that activate and recruit inflammatory cells. Although the wheals can resolve within 24 h, symptoms have a significant detrimental impact on the quality of life ...
Until 2014, only H1-antihistamines were approved for the treatment of chronic urticaria (CU); however, they do not sufficiently control symptoms in most patients. Approval of the anti-IgE mAb omalizumab in 2014 for chronic urticaria was a milestone. Up to 80% of patients are well controlled with this agent.
Objective: Chronic urticaria (CU) is a common, heterogeneous, and debilitating disease. Antihistamines and omalizumab are the mainstay therapies of CU. Additional treatment options are needed. Here, we review the off and beyond label use of licensed drugs, novel treatments that are currently under development, and promising new targets.
Chronic Urticaria*. Humans. Hypersensitivity*. Urticaria is a heterogeneous inflammatory disorder that can be acute or chronic and is defined by the appearance of wheals, angioedema, or both. Very recently, the newest update and revision of the international European Academy of Allergy and Clinical Immunology/Global Allergy and Asthma European ...
Bradykinin-mediated angioedema was not addressed in the review. This rare chronic hereditary urticaria mediated by bradykinin may be life-threatening, and treatments for this condition have been ...
A new research paper describes a stepped-care treatment to help improve the quality of life for patients with chronic uticaria. Locations: Abu Dhabi | Canada ... Chronic urticaria can profoundly impair quality of life, with random episodes of intense itching that disrupt sleep and interfere with physical, social, and emotional functioning. ...
828 n engl j med 387;9 nejm.org September 1, 2022 The new england journal of medicine has not been encouraged.1,13,17 For patients with chronic urticaria who have an unremarkable comprehensive ...
Chronic urticaria, including chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU), is characterized by the activation and degranulation of skin mast cells. Approximately 20% of patients do not respond to the currently approved and guideline-recommended treatments.
The reported lifetime prevalence rate of urticaria varies from 8% to 24%, with a lifetime prevalence rate of about 1-2% for chronic urticaria. 12-14 The point prevalence of chronic urticaria varies from 0·1% in North America to 1·4% in Asia. 15 The disease is slightly more common in females, except in young children.
Characteristics and Clinical Presentation. Urticaria is a systemic condition affecting the skin characterized by the development of wheals (hives) and/or angioedema, and is classified on the basis of its duration (acute or chronic) and its triggers (inducible [CIndU] or spontaneous [CSU]; Fig. 1 a) [1, 5].Wheals are pruritic, often described as itchy and/or burning, are of variable size and ...
The messenger RNA (mRNA)-based vaccines Spikevax (Moderna) and Comirnaty (Pfizer-BioNTech) are the most widely distributed vaccines in Switzerland. 1 Many chronic spontaneous urticaria (CSU) cases (recurrent wheals, angioedema, or both for more than 6 weeks 2) have been observed after the booster dose. 3 To assess whether a temporal association exists between COVID-19 vaccines and new-onset ...
Chronic spontaneous urticaria (CSU) is one of the common and frustrating diseases for both patients and physicians. It is a non-life-threatening disease though it has a major impact on quality of life (QOL) and may interfere with sleep and daily activities. 5 - 7 CU patients may suffer from considerable loss of energy, sleep disturbance ...
The New England Journal of Medicine has published results from a positive phase 3 study of Dupixent (dupilumab) in children aged ... received research grant funding from Regeneron. ... such as Dupixent for the treatment of chronic spontaneous urticaria, chronic pruritus of unknown origin, chronic obstructive pulmonary disease with evidence of ...
Phase 1 AK006 Study in Healthy Volunteers and Patients with Chronic Spontaneous Urticaria . AK006 is being studied in an ongoing Phase 1 single IV and subcutaneous (SC) ascending dose (SAD) and multiple IV ascending dose (MAD) trial that includes a randomized, double-blind, placebo-controlled CSU arm (NCT06072157).
This study is being conducted to evaluate the efficacy and safety of povorcitinib in adults with CSU that is inadequately controlled using SOC treatments. Gender: All. Ages: Between 18 years and 65 years. Trial Updated: 06/13/2024. Locations: Clinical Research Center of Alabama, Birmingham, Alabama +38 locations.
Sharon Barr, head of BioPharmaceuticals R&D at AstraZeneca, discusses her chronic disease research priorities, including the need for new diagnostic tools, fixed-dose drugs and a better ...
Press release - DelveInsight Business Research LLP - Chronic Spontaneous Urticaria Market Forecast 2032: Clinical Trials, Epidemiology, FDA Approvals, Therapies and Companies - published on openPR ...
10 Lübeck Institute of Experimental Dermatology and Center for Research on Inflammation of the Skin, University of Lübeck ... New York, NY, United States. 18 Psoriasis Research and Treatment Centre, Charité ... or B cell driven in chronic spontaneous urticaria, pemphigus and pemphigoid diseases. Rare causes of chronic skin inflammation are ...
The latest research on the prevalence of aspirin use to prevent cardiovascular disease suggests that in 2021, nearly a third of adults 60 or older without cardiovascular disease were still using ...
Here are the top six antihistamines myths—busted. Myth 1. Oral antihistamines are the best way to control hay fever symptoms. Wrong. In fact, the recommended first line medical treatment for ...
Chronic urticaria is a mast cell-mediated condition characterized by the recurrent occurrence of urticaria and/or angioedema. ... of autologous serum skin test as a screen for autoantibodies to IgE and IgE receptors is an area of active research. A number of tools have been developed to assess disease ... Urticaria, Upper Arm DermNet New Zealand .
At baseline, the mean AHI was 51.5 events per hour in trial 1 and 49.5 events per hour in trial 2, and the mean body-mass index (BMI, the weight in kilograms divided by the square of the height in ...