An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Maintenance of lost weight and long-term management of obesity
Kevin d hall , ph.d., scott kahan , m.d., mph.
- Author information
- Copyright and License information
Corresponding Author: Kevin D. Hall, 12A South Drive, Room 4007, Bethesda, MD 20892, [email protected]
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. Obesity interventions typically result in early rapid weight loss followed by a weight plateau and progressive regain. This review describes our current understanding of the biological, behavioral, and environmental factors driving this near-ubiquitous body weight trajectory and the implications for long-term weight management. Treatment of obesity requires ongoing clinical attention and weight maintenance-specific counseling to support sustainable healthful behaviors and positive weight regulation.
Keywords: obesity treatment, weight loss, weight maintenance, behavioral counseling, appetite, physiology
Introduction
Robert is a 47 year old patient who initially weighed 270 pounds. He lost 85 pounds three years ago by carefully following your guidance to decrease his caloric intake to 1500 calories per day and exercise six days weekly. Today he comes in for his annual physical examination. You were excited to hear about his continued progress and see how much more he’s lost, but you felt immediately dejected to see that he had regained almost 60 pounds. “I don’t know what to do…the weight keeps coming back on. I keep trying, but there must be something wrong. I’m sure my metabolism is in the dumps. It feels like every moment of the day I can’t help but think about food – it was never like this before I lost the weight. And no matter how hard I try to stop eating after one serving, I just can’t seem to do it anymore.” Feeling defeated, he says “I don’t even know what’s the point of doing this anymore!” Frustrated, you remind him that he was able to do it just fine when he was losing weight initially, and he just needs to keep working hard at it. “I know it’s not easy, but I can’t help you unless you’re willing to help yourself. You just need to work harder and take control of this again.” You feel for him, but you know that you need to be stern to get him past this backsliding. Hoping to motivate him, you remind him how bad he will feel if he regains more weight, and you tell him to make a follow-up appointment for six months and warn him that if he doesn’t turn things around quickly he will have to restart his blood pressure medications .
Substantial weight loss is possible across a range of treatment modalities, but long-term sustenance of lost weight is much more challenging, and weight regain is typical 1 – 3 . In a meta-analysis of 29 long-term weight loss studies, more than half of the lost weight was regained within two years, and by five years more than 80% of lost weight was regained ( Figure 1 ) 4 . Indeed, previous failed attempts at achieving durable weight loss may have contributed to the recent decrease in the percentage of people with obesity who are trying to lose weight 5 and many now believe that weight loss is a futile endeavor 6 .

Average time course of weight regain after a weight loss intervention.
Data from Anderson JW, Konz EC, Frederich RC, et al. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74(5):579–584.
Here, we describe our current understanding of the factors contributing to weight gain, physiological responses that resist weight loss, behavioral correlates of successful maintenance of lost weight, as well as the implications and recommendations for long-term clinical management of patients with obesity.
Why is it so difficult to lose weight and keep it off?
The obesogenic environment.
Long term weight management is extremely challenging due to interactions between our biology, behavior, and the obesogenic environment. The rise in obesity prevalence over the past several decades has been mirrored by industrialization of the food system 7 involving increased production and marketing of inexpensive, highly-processed foods 8 – 10 with supernormal appetitive properties 11 , 12 . Ultraprocessed foods 13 now contribute the majority of calories consumed in America 14 and their overconsumption has been implicated as a causative factor in weight gain 15 . Such foods are typically more calorically dense and far less healthy than unprocessed foods such as fruits, vegetables, and fish 16 . Food has progressively become cheaper 17 , fewer people prepare meals at home 18 , 19 , and more food is consumed in restaurants 18 . In addition, changes in the physical activity environment have made it more challenging to be active throughout the day. Occupations have become more sedentary 20 and suburban sprawl necessitates vehicular transportation rather than walking to work or school as had been common in the past. Taken together, changes in the food and physical activity environments tend to drive individuals towards increased intake, decreased activity, and ultimately weight gain.
Physiological responses to weight loss
Outdated guidance to physicians and their patients gives the mistaken impression that relatively modest diet changes will consistently and progressively result in substantial weight loss at rate of one pound for every 3500 kcal of accumulated dietary calorie deficit 21 – 24 . For example, cutting just a couple of cans of soda (~300 kcal) from one’s daily diet was thought to lead to about 30 pounds of weight loss in a year, 60 pounds in 2 years, etc. Failure to achieve and maintain substantial weight loss over the long term is then simply attributed to poor adherence to the prescribed lifestyle changes, thereby potentially further stigmatizing the patient as lacking in willpower, motivation, or fortitude to lose weight 25 .
We now know that the simple calculations underlying the old weight loss guidelines are fatally flawed because they fail to consider declining energy expenditure with weight loss 26 . More realistic calculations of expected weight loss for a given change in energy intake or physical activity are provided by a web-based tool called NIH Body Weight Planner ( http://BWplanner.niddk.nih.gov ) that uses a mathematical model to account for dynamic changes in human energy balance 27 .
In addition to adaptations in energy expenditure with weight loss, body weight is regulated by negative feedback circuits that influence food intake 28 , 29 . Weight loss is accompanied by persistent endocrine adaptations 30 that increase appetite and decrease satiety 31 thereby resisting continued weight loss and conspiring against long-term weight maintenance.
Explaining the weight plateau
The overlapping physiological changes that occur with weight loss help explain the near-ubiquitous weight loss time course: early rapid weight loss that stalls after several months, followed by progressive weight regain 32 . Different interventions result in varying degrees of weight loss and regain, but the overall time courses are similar. As people progressively lose more and more weight, they fight an increasing battle against the biological responses that oppose further weight loss.
Appetite changes likely play a more important role than slowing metabolism in explaining the weight loss plateau since the feedback circuit controlling long-term calorie intake has greater overall strength than the feedback circuit controlling calorie expenditure. Specifically, it has been estimated that for each kilogram of lost weight, calorie expenditure decreases by about 20–30 kcal/d whereas appetite increases by about 100 kcal/d above the baseline level prior to weight loss 31 . Despite these predictable physiologic phenomena, the typical response of the patient is to blame themselves as lazy or lacking in willpower, sentiments that are often reinforced by healthcare providers, as in the example of Robert, above.
Using a validated mathematical model of human energy balance dynamics 27 , 31 , Figure 2 illustrates the energy balance dynamics underlying the weight loss time courses of two example 90 kg women who either regain (blue curves) or maintain (orange curves) much of their lost weight after reaching a plateau within the first year of a diet intervention. In both women, large decreases in calorie intake at the start of the intervention result in rapid loss of weight and body fat leading to a modest decrease in calorie expenditure that contributes to slowing weight loss. However, the exponential rise in calorie intake from its initially reduced value is the primary factor that halts weight loss within the first year. In contrast to the modest drop in calorie expenditure of less than 200 kcal/d at the weight plateau, appetite has risen by 400–600 kcal/d and energy intake has increased by 600–700 kcal/d since the start of the intervention.

Mathematical model simulations of body weight, fat mass, energy intake, energy expenditure, appetite, and effort for two hypothetical women participating in a weight loss program. The curves in blue depict the typical weight loss, plateau and regain trajectory whereas the orange curves show successful weight loss maintenance.
These mathematical model results contrast with patients’ reports of eating approximately the same diet after the weight plateau that was previously successful during the initial phases of weight loss 33 . While self-reported diet measurements are notoriously inaccurate and imprecise 34 – 36 , it may be possible to reconcile such data with objectively quantified increases in calorie intake. It is entirely possible that patients truly believe they are sticking with their diet despite not losing any more weight or even regaining weight.
The patient’s perception of ongoing diet maintenance despite no further weight loss may arise because the physiological regulation of appetite occurs in brain regions that operate below the patient’s conscious awareness 37 . Thus, signals to the brain that increase appetite with weight loss could introduce subconscious biases such as portion sizes creeping upwards over time. Such a slow drift upwards in energy intake would be difficult to detect given the large 20–30% fluctuations in energy intake from day to day 38 , 39 . Furthermore, a relatively persistent effort is required to avoid overeating to match the increased appetite that grows in proportion to the weight lost 31 . For example, the model-calculated intervention effort for the simulated patient who experiences the weight plateau at six months followed by weight regain ( Figure 2 , blue curves) maintains more than ~70% of their initial intervention effort until the plateau. Perhaps self-reported diet maintenance before and after the weight plateau is more representative of the patients’ relatively persistent effort to avoid overeating in response to their increased appetite 31 . New technologies using repeated weight monitoring can be used calculate changes in calorie intake and effort over time 40 and help guide individuals participating in a weight loss intervention 41 – 44 .
Weight regain versus maintenance
From a purely calorie balance perspective, a patient who maintains lost weight after the first year of an intervention ( Figure 2 , orange curves) may be eating only about 100 kcal/d fewer than a patient who experiences long-term weight regain ( Figure 2 , blue curves). However, such a small difference in food intake behavior is somewhat misleading considering that prevention of weight regain requires about 300–500 kcal/d of increased persistent effort to counter the ongoing slowing of metabolism and increased appetite associated with the lost weight. The more typical pattern of long-term weight regain is characterized by a waning effort to sustain the intervention.
There are likely many factors that account for the ability of some patients to achieve and maintain large weight losses over the long term whereas others experience substantial weight regain. Unravelling the biological, psychosocial, educational, and environmental determinants of such individual variability will be an active area of obesity research for the foreseeable future 45 .
The role of diet composition
The laws of thermodynamics dictate that the energy derived from macronutrients being oxidized via the intricate biochemical pathways of oxidative phosphorylation inside cells can be equated to the values measured by combusting these fuels in a bomb calorimeter. However, this equivalence does not necessarily imply that “a calorie is a calorie” when it comes to diets with different macronutrient proportions differentially impacting weight loss.
Altering dietary macronutrient composition could theoretically influence overall calorie intake or expenditure resulting in a corresponding change in body weight. Alternatively, manipulation of diet composition can result in differences in the endocrine status in a way that could theoretically influence the propensity to accumulate body fat or affect subjective hunger or satiety. These possibilities do not necessarily violate the laws of thermodynamics since any change in the body’s overall energy stores (i.e. fat mass) must be accompanied by changes in calorie intake or expenditure. Therefore, it is theoretically possible that a particular diet could result in an advantageous endocrine or metabolic state that promotes weight loss. This promise provides fodder for the diet industry and false hope to the patient with obesity since it implies that if they simply choose the right diet then weight loss can be easily achieved.
In recent years, there has been a reemergence of low-carbohydrate, high-fat diets as popular weight loss interventions. Such diets have been claimed to reverse the metabolic and endocrine derangements resulting from following advice to consume low-fat, high-carbohydrate diets that allegedly caused the obesity epidemic. Specifically, the so-called “carbohydrate-insulin model of obesity” posits that diets high in carbohydrates are particularly fattening because they increase the secretion insulin and thereby drive fat accumulation in adipose tissue and away from oxidation by metabolically active tissues, and this altered fat partitioning results in a state of “cellular starvation” leading to adaptive increases in hunger, and suppression of energy expenditure 46 . Therefore, the carbohydrate-insulin model implies that reversing these processes by eating a low-carbohydrate, high-fat diet should result in effortless weight loss 47 . Unfortunately, important aspects of the carbohydrate-insulin model have failed experimental interrogation 48 and, for all practical purposes, “a calorie is a calorie” when it comes to body fat and energy expenditure differences between controlled isocaloric diets varying in the ratio of carbohydrate to fat 49 . Nevertheless, low-carbohydrate, high-fat diets may lead to spontaneous reduction in calorie reduction and increased weight loss, especially over the short term 50 – 52 . Meta-analyses of long-term weight loss have suggested that low-fat weight loss diets are slightly, if statistically, inferior to low-carbohydrate diets 53 , but the average differences between diets is too small to be clinically significant 54 . Furthermore, the similarity of the mean weight loss patterns between diet groups in randomized weight loss trials strongly suggests that there is no generalizable advantage of one diet over another when it comes to long-term calorie intake or expenditure 33 .
In contrast to the near equivalency of dietary carbohydrate and fat, dietary protein is known to positively influence body composition during weight loss 55 , 56 and has a small positive effect on resting metabolism 57 . Diets with higher protein may also offer benefits for maintaining weight loss 58 , particularly when the overall diet has a low glycemic index 59 . This might be partially mediated by dietary protein’s greater effect on satiety compared to carbohydrate and fat 55 , 56 along with the possibility of increased overall energy expenditure 60 . More research is needed to better understand whether these potentially positive attributes of higher protein diets outweigh concerns that such diets mitigate improvements in insulin sensitivity that are typically achieved with weight loss using lower protein diets 61 .
Whereas long-term diet trails have not resulted in clear superiority of one diet over another with respect to average weight loss, within each diet group there is a high degree of individual variability and anecdotal success stories abound for a wide range of weight loss diets 33 . Some of this variability may be due to interactions between diet type and patient genetics 62 , 63 or baseline physiology such as insulin sensitivity 64 – 67 . Such interactions offer the promise of personalized diets that optimize the patient’s chances for long-term weight loss success 45 , 63 . Unfortunately, diet-biology interactions for weight loss have not always been reproducible 68 , 69 and likely explain only a fraction of the individual variability.
It is certainly possible that the patients who successfully lost weight on one diet would have been equally successful had they been assigned to an alternative diet. In other words, long-term success with a weight loss diet may have less to do with biology than factors such as the patient’s food environment, socioeconomics, medical comorbidities, and social support, as well as practical factors, such as developing cooking skills and managing job requirements. Such non-biological factors likely play a strong role in determining whether diet adherence is sustainable.
Clinical recommendations for long term weight management counseling
Given the physiologic and environmental obstacles to long-term maintenance of lost weight described above, we offer the following recommendations for clinical practice and then present an alternative preferable depiction of the opening case example.
Long term benefits require long term attention
Long term behavioral changes and obesity management require ongoing attention. Even the highest quality short-term interventions are unlikely to yield continued positive outcomes without persisting intervention and support. Several studies show that ongoing interaction with healthcare providers or in group settings significantly improves weight maintenance and long-term outcomes, compared with treatments that end after a short period of time ( Figure 3 ) 70 , 71 . The importance of long-term intervention has been codified in the obesity treatment guidelines, which state that weight loss interventions should include long term comprehensive weight loss maintenance programs that continue for at least 1 year 72 .
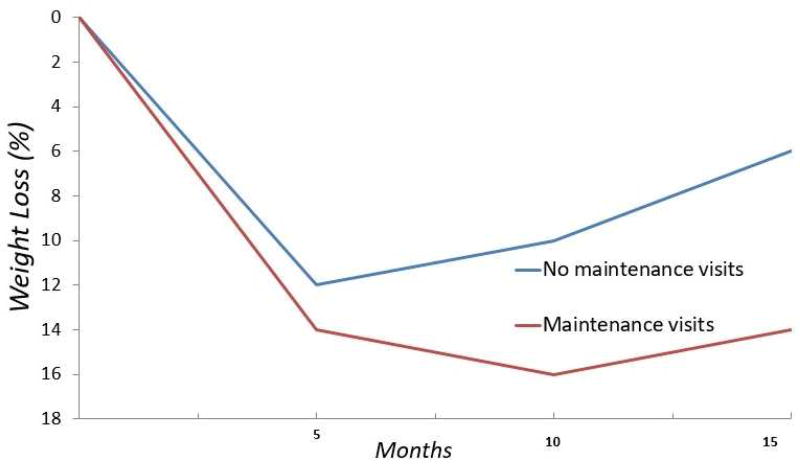
Weight management programs with a focus on maintenance of lost weight demonstrate improved long-term weight loss (red curve) compared to programs without maintenance visits (blue curve).
Adapted from Perri MG, McAllister DA, Gange JJ, et al. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol 1988;56(4):529–534; with permission.
With respect to the case study at the start of this paper, the physician should not expect ongoing weight loss without ongoing support and interaction. Rather than asking Robert to turn things around on his own, the physician has an opportunity to reengage with Robert to offer guidance and support in a more intensive and regular manner than sending him off on his own for six months, or if this is not realistic in a busy primary care practice, he could refer Robert to an obesity medicine specialist, registered dietitian, comprehensive weight management clinic, or recommend that he engage in a community weight management group, such as the Diabetes Prevention Program (now covered by Medicare for patients with prediabetes), or a commercial program, such as Weight Watchers.
Use weight maintenance-specific counseling/strategies
Behavioral strategies for initiation of weight loss are described elsewhere in this volume []. Weight-loss specific behaviors associated with long term success include: frequent self-monitoring and self-weighing, reduced calorie intake, smaller and more frequent meals/snacks throughout the day, increased physical activity, consistently eating breakfast, more frequent at-home meals compared with restaurant and fast-food meals, reducing screen time, and use of portion-controlled meals or meal substitutes 2 , 73 – 75 . Weight maintenance-specific behavioral skills and strategies help patients to build insight for long-term management, anticipate struggles and prepare contingency plans, moderate behavioral fatigue, and put into perspective the inevitable lapses and relapses of any long-term engagement.
Although the research is mixed, several studies show improved weight loss outcomes in patients receiving weight maintenance-specific training, compared with those who only receive traditional weight loss training 76 – 79 . Strategies are discussed below for weight maintenance-specific counseling.
Strengthen satisfaction with outcomes
People tend to focus on what they haven’t achieved, rather than what they’ve already accomplished. Unlike with weight loss, during which the external reward of watching the scale decrease and clinical measures (e.g., lipid levels) improve can increase motivation, the extended period of weight maintenance has fewer of these explicit rewards. To support motivation and make salient satisfaction with outcomes, call attention to patients’ progress, which often becomes overlooked. Providers can point to the magnitude of weight that has been kept off, putting it into context in terms of average expected weight loss (described below), as well as clinical improvements in risk factors, such as blood pressure and glycemic control. Additionally, showing patients “before and after” photographs of themselves and other tangible evidence of progress helps them to build awareness of and appreciate the benefits they have already achieved, which may improve long-term persistence with weight maintenance efforts.
Relapse prevention training
Anticipating and managing high-risk situations for “slips” and lapses helps patients minimize lapses, get back on track, and avoid giving up. This counseling often includes self-weighing and identifying weight thresholds that signal the need for reengaging with a support team or initiating contingency strategies; proactively developing plans and practicing strategies for managing and coping with lapses; problem solving to identify challenges, formulate solutions, and evaluate options; and building strategies for non-food activities and coping mechanisms, such as engaging in hobbies or mindfulness activities, to minimize counterproductive coping mechanisms, such as emotional eating.
Cognitive restructuring
Cycles of negative and maladaptive thoughts (e.g., “What’s the point…I failed again and I’ll never lose weight!”) and coping patterns (e.g., binge eating in response to gaining a few pounds) are counterproductive and demotivating. Helping patients to recognize and restructure the core beliefs and thought processes that underlie these patterns helps minimize behavioral fatigue and prevent or productively manage slips and lapses.
Developing cognitive flexibility
Many tendencies that promote initial weight loss are unrealistic over the long term. For example, many patients aim to make large, absolute changes in an “all-or-none” fashion via rigid rules, such as aiming for “no carbs” or very restrictive intake. Much as a sprinter can run all-out for a short race, but not for the entirety of a marathon, expecting strict, all-out efforts and clear-cut, black-and-white outcomes over the lifelong management of obesity is a recipe for frustration and failure. Instead, learning to accept that rigid expectations and “perfect” adherence to behavioral goals is unrealistic and building cognitive flexibility to take in stride when one’s plans do not go according to plan is a core competency for long term sustainable behavioral changes and weight management.

Appeal to patients’ deeper motivations
External, superficial rewards are unlikely to support the long term endurance needed for weight maintenance. For example, studies of financial rewards to incentivize behavioral changes, such as weight loss or tobacco cessation, yield initial benefits that invariably wane precipitously over time 80 , 81 . Whereas “white knuckling” and external, controlled motivations, such as directives from a spouse or healthcare provider, may lead to short-term weight loss, longer term sustained motivation is more likely when patients take ownership of their behavioral changes and goals, and engage in them because they are deeply meaningful or enjoyable 80 , 81 . As an example, compared with difficulty of sticking to a strict low-fat or low-carb diet, which are often arbitrarily prescribed and of little personal significance to the patient, and therefore difficult to maintain, countless millions throughout the world rigorously stick to comparably strict kosher, halal, or vegan eating patterns, which are aligned with their religious, ethical, or other deeply held beliefs and values. Similarly, prescribing daily gym visits to someone who hates the gym environment or gym activities is unlikely to be fruitful, whereas supporting patients to find more enjoyable physical activities, such as sports or group dance-exercise classes, increases the likelihood of continuing over time.
Manage expectations – both for patients and providers
Both patients and healthcare providers have wildly unrealistic expectations for weight loss outcomes. In one study, patients entering a diet and exercise program expected to lose 20–40% of their starting body weight - amounts that can only realistically be achieved by bariatric surgery 82 . Physician expectations are similarly inflated: in a survey of primary care physicians, acceptable behavioral weight loss was considered to be a loss of 21% of initial body weight 83 . In contrast, numerous studies show that diet, exercise, and behavioral counseling, in the best of cases, only leads to 5–10% average weight loss, and few patients with significantly elevated initial weights achieve and maintain an “ideal” body weight. From a cognitive psychology perspective, a waning intervention effort may be due to disappointment in the degree of weight loss actually achieved 82 leading the patient to conclude that the effort is not worth the achieved benefits 84 .
Although the published data is mixed on whether unrealistic outcomes will deter weight loss success, it stands to reason that excessive discrepancies between expectations and actual outcomes would be demoralizing and increase negative thoughts and self-blame (which itself is associated with numerous negative health outcomes 85 ), and may diminish long term persistence for continued behavioral change and weight loss maintenance. We recommend advising patients about the physiologic challenges of long term weight loss and the degree of weight loss that can be realistically expected from behavioral interventions. At minimum, there’s no known harm of offering this insight and being frank with patients about expectations, and it may help them navigate the minefield of unscrupulous diet programs and promises that promise miraculous outcomes.
Nonetheless, positive outcomes of behavioral counseling extend beyond weight loss. Despite the modest weight losses associated with behavioral interventions, small weight losses can lead to impressive health improvements and risk factor reductions. In the Diabetes Prevention Program, 7% weight loss over six months led to 58% reduction in development of diabetes, despite half the weight being regained over three years 86 . In the Look Ahead trial, 6% weight loss over eight years yielded improvements in a range of cardiovascular risk factors, including glycemic control and lipids, as well as less medication usage, and reduced hospitalizations and healthcare costs 87 , 88 .
While losing weight is important for improved health, people’s motivations for seeing the scale go down is all-too-often driven by cultural norms for thinness and healthcare provider-imposed weight loss directives. These external motivations can move the weight loss needle in the short-run, but they rarely lead to long-lasting determination. As described in the section above, long term management is improved when motivations are aligned with personal values and preferences. Helping patients shift their locus of motivation from weight loss alone to intrinsically meaningful areas, such as health improvement, can improve long term weight and behavioral outcomes 89 .
Escalate treatment as needed
For patients that do not achieve sufficient weight loss or health improvements with basic counseling in primary care settings, there are several opportunities to intensify therapy. Consider referral to a registered dietitian, obesity medicine physician, or comprehensive weight management clinic, as well as targeted specialists (such as a behavioral psychologist for patients with binge eating disorder or body dysmorphia). For patients with BMI greater than 30 kg/m 2 (or 27–30 kg/m 2 with obesity-related comorbid conditions), obesity pharmacotherapy leads to as much as 15% weight loss in responders, with weight loss being maintained in several studies for several years 90 – 92 . For patients with BMI greater than 40 kg/m 2 (or 35–40 kg/m 2 with comorbidities), bariatric surgery is a well-studied and valuable option that leads to large, sustainable weight losses in most patients 93 .
Using the principles discussed above, a more productive encounter in response to Robert’s presentation might go like this:
Physician: “I understand, and I know it’s challenging. It sounds like you’re feeling frustrated because you’ve worked so hard and you feel like you’ve got nothing to show for it.” He nods and says, “Exactly. What’s the point of doing this anymore.” Physician: “From my view, the evidence we have shows something different: You’re actually doing quite well in the scheme of things. I actually see quite a lot of progress for your efforts. You’re down 25 lbs, right? That’s almost 10% down from where you started…that’s impressive. Few people lose that much weight and keep it off for three years. Studies show that even under the best of circumstances with aggressive counseling, average weight loss is between 5–10% of starting body weight – so you’re doing better than most! You’ve been able to get off several blood pressure medications and you no longer take the pain medicine for your back and knees. And, we know from studies that losing just 7%, even if part of it is regained over the years, lowers the risk of diabetes by 60%!” His eyes widen. “Weight goes up and down, and our bodies fight back against weight loss, so this is never easy. Some regain and relapse is inevitable – just like in other areas of life.” He takes a deep breath and clearly seems more engaged and hopeful. ”So let’s figure out how we can move forward and keep getting the benefits, and I’ll be here with you to help along the way. Let’s agree on a couple of next steps, and we’ll meet again in a few weeks to see how it’s going. If we need, we can also consider additional strategies or treatments.”
The degree of weight loss and its maintenance should not be the sole metric of obesity treatment success. Rather, physicians should support and encourage patients to make sustainable improvements in their diet quality and physical activities if these behaviors fail to meet national guidelines 94 , 95 . Such lifestyle changes over the long-term will likely improve the health of patients even in the absence of major weight loss 96 .
Key points.
Long-term maintenance of lost weight is the primary challenge of obesity treatment.
Biological, behavioral, and environmental factors conspire to resist weight loss and promote regain.
Treatment of obesity requires ongoing attention and support, and weight maintenance-specific counseling, to improve long-term weight management.
Realistic long-term weight loss magnitude is significantly lower than patient and healthcare provider expectations. However, even small amounts of sustained weight loss lead to clinical health improvements and risk factor reductions.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes & Digestive & Kidney Diseases.
KDH has received funding from the Nutrition Science Initiative to investigate the effects of ketogenic diets on human energy expenditure. KDH also has a patent on a method of personalized dynamic feedback control of body weight (US Patent No 9,569,483; assigned to the National Institutes of Health).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: SK has no relevant disclosures.
- 1. Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health technology assessment (Winchester, England) 2011;15(2):1–182. doi: 10.3310/hta15020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10(3):313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Snook KR, Hansen AR, Duke CH, Finch KC, Hackney AA, Zhang J. Change in Percentages of Adults With Overweight or Obesity Trying to Lose Weight, 1988–2014. JAMA. 2017;317(9):971–973. doi: 10.1001/jama.2016.20036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. The American psychologist. 2007;62(3):220–233. doi: 10.1037/0003-066X.62.3.220. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS medicine. 2012;9(6):e1001235. doi: 10.1371/journal.pmed.1001235. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Blatt H. America's food: What you don't know about what you eat. Cambridge: The MIT press; 2008. [ Google Scholar ]
- 10. Roberts P. The end of food. New York: Houghton Mifflin Harcourt Publishing Company; 2008. [ Google Scholar ]
- 11. Kessler DA. The end of overeating: controling the insatiable American appetite. Rodale Inc.; 2009. [ Google Scholar ]
- 12. Moss M. Salt, sugar, fat: how the food giants hooked us. New York: Random House; 2013. [ Google Scholar ]
- 13. Monteiro CA, Levy RB, Claro RM, Castro IR, Cannon G. A new classification of foods based on the extent and purpose of their processing. Cadernos de saude publica. 2010;26(11):2039–2049. doi: 10.1590/s0102-311x2010001100005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultraprocessed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ open. 2016;6(3):e009892. doi: 10.1136/bmjopen-2015-009892. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Mendonca RD, Pimenta AM, Gea A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Kahan S, Cheskin LJ. Obesity and eating behaviors and behavior change. In: Kahan S, Gielen AC, Fagen PJ, Green LW, editors. Health Behavior Change in Populations. Baltimore: Johns Hopkins University Press; 2014. [ Google Scholar ]
- 17. Putnam J. Major trends in the U.S. food supply, 1909–99. Food Review. 2000;23(1):8–15. [ Google Scholar ]
- 18. Lin BH, Guthrie J. Nutritional Quality of Food Prepared at Home and Away From Home. U.S. Department of Agriculture; [December 2012]. 2012. pp. EIB–105. [ Google Scholar ]
- 19. Smith LP, Ng SW, Popkin BM. Trends in US home food preparation and consumption: analysis of national nutrition surveys and time use studies from 1965–1966 to 2007–2008. Nutrition journal. 2013;12:45. doi: 10.1186/1475-2891-12-45. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Guth E. JAMA patient page. Healthy weight loss. JAMA. 2014;312(9):974. doi: 10.1001/jama.2014.10929. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. NHLBI. Aim for a Healthy Weight. National Institutes of Health, National Heart, Lung and Blood Institute; [August 2005]. 2005. pp. 05–5213. [ Google Scholar ]
- 23. NHLBI Obesity Education Initiative Expert Panel on the Identification E, Treatment of Overweight and Obesity in Adults. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Heart, Lung, and Blood Institute; 2000. [ Google Scholar ]
- 24. NHS. Your Weight Your Health: How to take control of your weight. London: National Health Service, Department of Health; [April 2006]. 2006. p. 274537. [ Google Scholar ]
- 25. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–912. doi: 10.1093/ajcn/88.4.906. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond) 2015;39(8):1188–1196. doi: 10.1038/ijo.2015.59. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Ochner CN, Tsai AG, Kushner RF, Wadden TA. Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015;3(4):232–234. doi: 10.1016/S2213-8587(15)00009-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Polidori D, Sanghvi A, Seeley RJ, Hall KD. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity (Silver Spring) 2016;24(11):2289–2295. doi: 10.1002/oby.21653. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Freedhoff Y, Hall KD. Weight loss diet studies: we need help not hype. Lancet. 2016;388(10047):849–851. doi: 10.1016/S0140-6736(16)31338-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Dhurandhar NV, Schoeller DA, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2014 doi: 10.1038/ijo.2014.199. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48(10):373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6(3):199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Berthoud HR, Munzberg H, Morrison CD. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology. 2017;152(7):1728–1738. doi: 10.1053/j.gastro.2016.12.050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Chow CC, Hall KD. Short and long-term energy intake patterns and their implications for human body weight regulation. Physiol Behav. 2014;134:60–65. doi: 10.1016/j.physbeh.2014.02.044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Kim WW, Kelsay JL, Judd JT, Marshall MW, Mertz W, Prather ES. Evaluation of long-term dietary intakes of adults consuming self-selected diets. Am J Clin Nutr. 1984;40(6 Suppl):1327–1332. doi: 10.1093/ajcn/40.6.1327. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Sanghvi A, Redman LA, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.111070. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Brady I, Hall KD. Dispatch from the field: is mathematical modeling applicable to obesity treatment in the real world? Obesity (Silver Spring) 2014;22(9):1939–1941. doi: 10.1002/oby.20804. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Hall KD Inventor; National Institutes of Health, assignee. Personalized dynamic feedback control of body weight. 9,569,483. [02/14/2017];US patent. 2013
- 43. Martin CK, Gilmore LA, Apolzan JW, Myers CA, Thomas DM, Redman LM. Smartloss: A Personalized Mobile Health Intervention for Weight Management and Health Promotion. JMIR mHealth and uHealth. 2016;4(1):e18. doi: 10.2196/mhealth.5027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Martin CK, Miller AC, Thomas DM, Champagne CM, Han H, Church T. Efficacy of SmartLoss, a smartphone-based weight loss intervention: results from a randomized controlled trial. Obesity (Silver Spring) 2015;23(5):935–942. doi: 10.1002/oby.21063. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. MacLean PS, Wing RR, Davidson T, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23(1):7–15. doi: 10.1002/oby.20967. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167–2168. doi: 10.1001/jama.2014.4133. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Ludwig DS. Always hungry? Conquer cravings, retrain your fat cells and lose weight permanetly. New York: Grand Central Life & Style; 2016. [ Google Scholar ]
- 48. Hall KD. A review of the carbohydrate-insulin model of obesity. European journal of clinical investigation. 2017 doi: 10.1038/ejcn.2016.260. In press. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Hall KD, Guo J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology. 2017;152(7):1718–1727. e1713. doi: 10.1053/j.gastro.2017.01.052. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. Jama. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat vs. other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. The Lancet Diabetes & Endocrinology. 2015;3(12):968–979. doi: 10.1016/S2213-8587(15)00367-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Hall KD. Prescribing low-fat diets: useless for long-term weight loss? Lancet Diabetes Endocrinol. 2015;3(12):920–921. doi: 10.1016/S2213-8587(15)00413-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S–1329S. doi: 10.3945/ajcn.114.084038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–1298. doi: 10.3945/ajcn.112.044321. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28(1):57–64. doi: 10.1038/sj.ijo.0802461. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–2113. doi: 10.1056/NEJMoa1007137. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. Jama. 2012;307(24):2627–2634. doi: 10.1001/jama.2012.6607. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Smith GI, Yoshino J, Kelly SC, et al. High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell reports. 2016;17(3):849–861. doi: 10.1016/j.celrep.2016.09.047. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Bray GA, Siri-Tarino PW. The Role of Macronutrient Content in the Diet for Weight Management. Endocrinology and metabolism clinics of North America. 2016;45(3):581–604. doi: 10.1016/j.ecl.2016.04.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Bray MS, Loos RJ, McCaffery JM, et al. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity (Silver Spring) 2016;24(1):14–22. doi: 10.1002/oby.21381. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Cornier MA, Donahoo WT, Pereira R, et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13(4):703–709. doi: 10.1038/oby.2005.79. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–2102. doi: 10.1001/jama.297.19.2092. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. McClain AD, Otten JJ, Hekler EB, Gardner CD. Adherence to a low-fat vs. low-carbohydrate diet differs by insulin resistance status. Diabetes Obes Metab. 2013;15(1):87–90. doi: 10.1111/j.1463-1326.2012.01668.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28(12):2939–2941. doi: 10.2337/diacare.28.12.2939. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Gardner CD, Hauser M, Del Gobbo L, et al. EPI | Lifestyle Scientific Sessions. Portland, OR: 2017. Neither Insulin Secretion nor Genotype Pattern Modify 12-Month Weight Loss Effects of Healthy Low-Fat vs. Healthy Low-Carbohydrate Diets Among Adults with Obesity. [ Google Scholar ]
- 69. Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. Weight loss on low-fat vs. low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: A randomized pilot trial. Obesity (Silver Spring) 2016;24(1):79–86. doi: 10.1002/oby.21331. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. Journal of consulting and clinical psychology. 1988;56(4):529–534. doi: 10.1037//0022-006x.56.4.529. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev. 2012;13(6):509–517. doi: 10.1111/j.1467-789X.2011.00972.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. American journal of preventive medicine. 2014;46(1):17–23. doi: 10.1016/j.amepre.2013.08.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Archives of internal medicine. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. Jama. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Voils CI, Olsen MK, Gierisch JM, et al. Maintenance of Weight Loss After Initiation of Nutrition Training: A Randomized Trial. Ann Intern Med. 2017;166(7):463–471. doi: 10.7326/M16-2160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–2637. doi: 10.1001/jama.2008.804. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients' expectations and evaluations of obesity treatment outcomes. Journal of consulting and clinical psychology. 1997;65(1):79–85. doi: 10.1037//0022-006x.65.1.79. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Phelan S, Nallari M, Darroch FE, Wing RR. What do physicians recommend to their overweight and obese patients? Journal of the American Board of Family Medicine : JABFM. 2009;22(2):115–122. doi: 10.3122/jabfm.2009.02.080081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2000;19(1S):64–69. doi: 10.1037/0278-6133.19.suppl1.64. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Kahan S, Puhl RM. The damaging effects of weight bias internalization. Obesity (Silver Spring) 2017;25(2):280–281. doi: 10.1002/oby.21772. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–2556. doi: 10.2337/dc14-0093. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Silva MN, Vieira PN, Coutinho SR, et al. Using self-determination theory to promote physical activity and weight control: a randomized controlled trial in women. Journal of behavioral medicine. 2010;33(2):110–122. doi: 10.1007/s10865-009-9239-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Promotiion CfNPa. Dietary Guidelines for Americans. 2015. [Accessed 07/14/2017];2017 https://www.cnpp.usda.gov/2015-2020-dietary-guidelines-americans .
- 95. Promotion OoDPaH. Physical Activity Guidelines for Americans. 2008. [Accessed 7/14/2017];2017 https://health.gov/paguidelines/guidelines .
- 96. Matheson EM, King DE, Everett CJ. Healthy lifestyle habits and mortality in overweight and obese individuals. Journal of the American Board of Family Medicine : JABFM. 2012;25(1):9–15. doi: 10.3122/jabfm.2012.01.110164. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (632.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
"I tried so many diets, now I want to do it differently" - A single case study on coaching for weight loss
Affiliation.
- 1 Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen N, Denmark; [email protected].
- PMID: 26282867
- PMCID: PMC4539387
- DOI: 10.3402/qhw.v10.26925
- Corrigendum. [No authors listed] [No authors listed] Int J Qual Stud Health Well-being. 2017 Dec;12(1):1400264. doi: 10.1080/17482631.2017.1400264. Int J Qual Stud Health Well-being. 2017. PMID: 29130868 Free PMC article. No abstract available.
In this single case study, the author presented an in-depth description and analysis of a coaching intervention with focus on weight loss, conducted over 10 sessions in the course of 17 months. The client was a well-educated woman in her late 30s, who had tried many different forms of dieting over the years-with little and no lasting effect. In his coaching approach, the author went beyond a pure behavioural change model, that is, based on the Health Belief Model, and tried to take a whole-life perspective, where the client learned to link specific events and habits in her work life and everyday life with specific eating habits. In their collaborative practice, coach and coachee initiated changes both in regard to diet, physical activity, and healthy life style, in general. In a theoretical section, the change in understanding with regard to overeating was presented. Finally, an intra-active model-viewing the client as a self-reflective individual-was used as theoretical basis. A narrative analysis of the first session and a cross-session examination was presented to show, analyse, and understand the procedure of the coaching approach. Finally, the voice of the coachee was heard in regard to her personal experiences during the process. The data material was based on audio recordings of selected sessions, notes written by the coach from every session, and final written reflections by the coachee.
Keywords: Health coaching; case study; diet; life style change; meaning making; obesity; overweight; physical activity; weight loss.
PubMed Disclaimer
The intra-active individual.
Similar articles
- The contribution of lifestyle coaching of overweight patients in primary care to more autonomous motivation for physical activity and healthy dietary behaviour: results of a longitudinal study. Rutten GM, Meis JJ, Hendriks MR, Hamers FJ, Veenhof C, Kremers SP. Rutten GM, et al. Int J Behav Nutr Phys Act. 2014 Jul 16;11:86. doi: 10.1186/s12966-014-0086-z. Int J Behav Nutr Phys Act. 2014. PMID: 25027848 Free PMC article.
- The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, Giglia R, Smith R, Collins C. Spencer L, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):88-98. doi: 10.11124/jbisrir-2015-1812. JBI Database System Rev Implement Rep. 2015. PMID: 26447010
- Expert Coaching in Weight Loss: Retrospective Analysis. Painter SL, Ahmed R, Kushner RF, Hill JO, Lindquist R, Brunning S, Margulies A. Painter SL, et al. J Med Internet Res. 2018 Mar 13;20(3):e92. doi: 10.2196/jmir.9738. J Med Internet Res. 2018. PMID: 29535082 Free PMC article.
- Could habits hold the key to weight loss maintenance? A narrative review. Cleo G, Isenring E, Thomas R, Glasziou P. Cleo G, et al. J Hum Nutr Diet. 2017 Oct;30(5):655-664. doi: 10.1111/jhn.12456. Epub 2017 Feb 2. J Hum Nutr Diet. 2017. PMID: 28150402 Review.
- A new clinical perspective: Treating obesity with nutritional coaching versus energy-restricted diets. Dayan PH, Sforzo G, Boisseau N, Pereira-Lancha LO, Lancha AH Jr. Dayan PH, et al. Nutrition. 2019 Apr;60:147-151. doi: 10.1016/j.nut.2018.09.027. Epub 2018 Oct 6. Nutrition. 2019. PMID: 30586658 Review.
- Behavioral and Psychological Factors Affecting Weight Loss Success. Pigsborg K, Kalea AZ, De Dominicis S, Magkos F. Pigsborg K, et al. Curr Obes Rep. 2023 Sep;12(3):223-230. doi: 10.1007/s13679-023-00511-6. Epub 2023 Jun 19. Curr Obes Rep. 2023. PMID: 37335395 Review.
- Compendium of the Health and Wellness Coaching Literature. Sforzo GA, Kaye MP, Todorova I, Harenberg S, Costello K, Cobus-Kuo L, Faber A, Frates E, Moore M. Sforzo GA, et al. Am J Lifestyle Med. 2017 May 19;12(6):436-447. doi: 10.1177/1559827617708562. eCollection 2018 Nov-Dec. Am J Lifestyle Med. 2017. PMID: 30542254 Free PMC article.
- Editorial to the QHW thematic cluster "Health, Physical Activity and Lifestyle". Johnson U, Stambulova N. Johnson U, et al. Int J Qual Stud Health Well-being. 2015 Aug 14;10:29156. doi: 10.3402/qhw.v10.29156. eCollection 2015. Int J Qual Stud Health Well-being. 2015. PMID: 26282871 Free PMC article. No abstract available.
- Adams K. F, Schatzkin A, Harris T. B, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. New England Journal of Medicine. 2006;355:763–778. doi: 10.1056/NEJMoa055643. - DOI - PubMed
- Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice Hall; 1977.
- Bandura A. Self-efficacy. The exercise of control. New York: Freeman; 1997.
- Barad K. Meeting the universe halfway. London, UK: Duke University Press; 2007.
- Becker M. H, Rodenstock I. M. Comparing social learning theory and the health belief model. In: Ward W. B, editor. Advances in health education and promotion. Greenwich, CT: JAI Press; 1987. pp. 235–249.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
- Taylor & Francis
Other Literature Sources
- scite Smart Citations
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

COMMENTS
This case report describes the use of a system-oriented approach to weight loss, based on emphasizing “the importance of high-quality foods and phytonutrient diversity to address clinical imbalances and move individuals toward the highest expression of health.” 5 A 5 to 10 percent weight loss can delay or prevent some of the diseases ...
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. Obesity interventions typically result in early rapid weight loss followed by a weight plateau and progressive regain.
In this trial, we found that adults with obesity (or overweight with one or more weight-related coexisting conditions) and without diabetes had a mean weight loss of 14.9% from baseline with ...
A real-life weight loss story. A few years ago, I embarked on a personal weight loss journey. I had had two pregnancies back-to-back, and had gained considerable weight, to the point where my own body mass index was over 30 (obesity range). I was many months postpartum, and realized that the "baby weight" wasn't going anywhere.
Obesity is a major global public health challenge. 1 Weight loss by means of lifestyle modification has been documented to be the cornerstone of weight management. 2 Daily calorie restriction...
Abstract. In this single case study, the author presented an in-depth description and analysis of a coaching intervention with focus on weight loss, conducted over 10 sessions in the course of 17 months.