Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates

How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
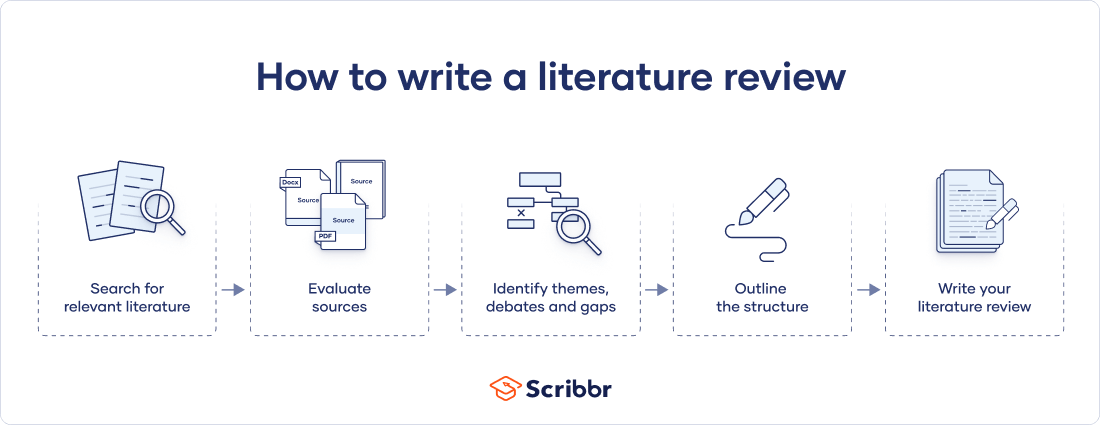
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved April 15, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, what is your plagiarism score.
Research Methods
- Getting Started
- Literature Review Research
- Research Design
- Research Design By Discipline
- SAGE Research Methods
- Teaching with SAGE Research Methods
Literature Review
- What is a Literature Review?
- What is NOT a Literature Review?
- Purposes of a Literature Review
- Types of Literature Reviews
- Literature Reviews vs. Systematic Reviews
- Systematic vs. Meta-Analysis
Literature Review is a comprehensive survey of the works published in a particular field of study or line of research, usually over a specific period of time, in the form of an in-depth, critical bibliographic essay or annotated list in which attention is drawn to the most significant works.
Also, we can define a literature review as the collected body of scholarly works related to a topic:
- Summarizes and analyzes previous research relevant to a topic
- Includes scholarly books and articles published in academic journals
- Can be an specific scholarly paper or a section in a research paper
The objective of a Literature Review is to find previous published scholarly works relevant to an specific topic
- Help gather ideas or information
- Keep up to date in current trends and findings
- Help develop new questions
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Helps focus your own research questions or problems
- Discovers relationships between research studies/ideas.
- Suggests unexplored ideas or populations
- Identifies major themes, concepts, and researchers on a topic.
- Tests assumptions; may help counter preconceived ideas and remove unconscious bias.
- Identifies critical gaps, points of disagreement, or potentially flawed methodology or theoretical approaches.
- Indicates potential directions for future research.
All content in this section is from Literature Review Research from Old Dominion University
Keep in mind the following, a literature review is NOT:
Not an essay
Not an annotated bibliography in which you summarize each article that you have reviewed. A literature review goes beyond basic summarizing to focus on the critical analysis of the reviewed works and their relationship to your research question.
Not a research paper where you select resources to support one side of an issue versus another. A lit review should explain and consider all sides of an argument in order to avoid bias, and areas of agreement and disagreement should be highlighted.
A literature review serves several purposes. For example, it
- provides thorough knowledge of previous studies; introduces seminal works.
- helps focus one’s own research topic.
- identifies a conceptual framework for one’s own research questions or problems; indicates potential directions for future research.
- suggests previously unused or underused methodologies, designs, quantitative and qualitative strategies.
- identifies gaps in previous studies; identifies flawed methodologies and/or theoretical approaches; avoids replication of mistakes.
- helps the researcher avoid repetition of earlier research.
- suggests unexplored populations.
- determines whether past studies agree or disagree; identifies controversy in the literature.
- tests assumptions; may help counter preconceived ideas and remove unconscious bias.
As Kennedy (2007) notes*, it is important to think of knowledge in a given field as consisting of three layers. First, there are the primary studies that researchers conduct and publish. Second are the reviews of those studies that summarize and offer new interpretations built from and often extending beyond the original studies. Third, there are the perceptions, conclusions, opinion, and interpretations that are shared informally that become part of the lore of field. In composing a literature review, it is important to note that it is often this third layer of knowledge that is cited as "true" even though it often has only a loose relationship to the primary studies and secondary literature reviews.
Given this, while literature reviews are designed to provide an overview and synthesis of pertinent sources you have explored, there are several approaches to how they can be done, depending upon the type of analysis underpinning your study. Listed below are definitions of types of literature reviews:
Argumentative Review This form examines literature selectively in order to support or refute an argument, deeply imbedded assumption, or philosophical problem already established in the literature. The purpose is to develop a body of literature that establishes a contrarian viewpoint. Given the value-laden nature of some social science research [e.g., educational reform; immigration control], argumentative approaches to analyzing the literature can be a legitimate and important form of discourse. However, note that they can also introduce problems of bias when they are used to to make summary claims of the sort found in systematic reviews.
Integrative Review Considered a form of research that reviews, critiques, and synthesizes representative literature on a topic in an integrated way such that new frameworks and perspectives on the topic are generated. The body of literature includes all studies that address related or identical hypotheses. A well-done integrative review meets the same standards as primary research in regard to clarity, rigor, and replication.
Historical Review Few things rest in isolation from historical precedent. Historical reviews are focused on examining research throughout a period of time, often starting with the first time an issue, concept, theory, phenomena emerged in the literature, then tracing its evolution within the scholarship of a discipline. The purpose is to place research in a historical context to show familiarity with state-of-the-art developments and to identify the likely directions for future research.
Methodological Review A review does not always focus on what someone said [content], but how they said it [method of analysis]. This approach provides a framework of understanding at different levels (i.e. those of theory, substantive fields, research approaches and data collection and analysis techniques), enables researchers to draw on a wide variety of knowledge ranging from the conceptual level to practical documents for use in fieldwork in the areas of ontological and epistemological consideration, quantitative and qualitative integration, sampling, interviewing, data collection and data analysis, and helps highlight many ethical issues which we should be aware of and consider as we go through our study.
Systematic Review This form consists of an overview of existing evidence pertinent to a clearly formulated research question, which uses pre-specified and standardized methods to identify and critically appraise relevant research, and to collect, report, and analyse data from the studies that are included in the review. Typically it focuses on a very specific empirical question, often posed in a cause-and-effect form, such as "To what extent does A contribute to B?"
Theoretical Review The purpose of this form is to concretely examine the corpus of theory that has accumulated in regard to an issue, concept, theory, phenomena. The theoretical literature review help establish what theories already exist, the relationships between them, to what degree the existing theories have been investigated, and to develop new hypotheses to be tested. Often this form is used to help establish a lack of appropriate theories or reveal that current theories are inadequate for explaining new or emerging research problems. The unit of analysis can focus on a theoretical concept or a whole theory or framework.
* Kennedy, Mary M. "Defining a Literature." Educational Researcher 36 (April 2007): 139-147.
All content in this section is from The Literature Review created by Dr. Robert Larabee USC
Robinson, P. and Lowe, J. (2015), Literature reviews vs systematic reviews. Australian and New Zealand Journal of Public Health, 39: 103-103. doi: 10.1111/1753-6405.12393

What's in the name? The difference between a Systematic Review and a Literature Review, and why it matters . By Lynn Kysh from University of Southern California

Systematic review or meta-analysis?
A systematic review answers a defined research question by collecting and summarizing all empirical evidence that fits pre-specified eligibility criteria.
A meta-analysis is the use of statistical methods to summarize the results of these studies.
Systematic reviews, just like other research articles, can be of varying quality. They are a significant piece of work (the Centre for Reviews and Dissemination at York estimates that a team will take 9-24 months), and to be useful to other researchers and practitioners they should have:
- clearly stated objectives with pre-defined eligibility criteria for studies
- explicit, reproducible methodology
- a systematic search that attempts to identify all studies
- assessment of the validity of the findings of the included studies (e.g. risk of bias)
- systematic presentation, and synthesis, of the characteristics and findings of the included studies
Not all systematic reviews contain meta-analysis.
Meta-analysis is the use of statistical methods to summarize the results of independent studies. By combining information from all relevant studies, meta-analysis can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review. More information on meta-analyses can be found in Cochrane Handbook, Chapter 9 .
A meta-analysis goes beyond critique and integration and conducts secondary statistical analysis on the outcomes of similar studies. It is a systematic review that uses quantitative methods to synthesize and summarize the results.
An advantage of a meta-analysis is the ability to be completely objective in evaluating research findings. Not all topics, however, have sufficient research evidence to allow a meta-analysis to be conducted. In that case, an integrative review is an appropriate strategy.
Some of the content in this section is from Systematic reviews and meta-analyses: step by step guide created by Kate McAllister.
- << Previous: Getting Started
- Next: Research Design >>
- Last Updated: Aug 21, 2023 4:07 PM
- URL: https://guides.lib.udel.edu/researchmethods

Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy pp 1–15 Cite as
Methodological Approaches to Literature Review
- Dennis Thomas 2 ,
- Elida Zairina 3 &
- Johnson George 4
- Living reference work entry
- First Online: 09 May 2023
453 Accesses
The literature review can serve various functions in the contexts of education and research. It aids in identifying knowledge gaps, informing research methodology, and developing a theoretical framework during the planning stages of a research study or project, as well as reporting of review findings in the context of the existing literature. This chapter discusses the methodological approaches to conducting a literature review and offers an overview of different types of reviews. There are various types of reviews, including narrative reviews, scoping reviews, and systematic reviews with reporting strategies such as meta-analysis and meta-synthesis. Review authors should consider the scope of the literature review when selecting a type and method. Being focused is essential for a successful review; however, this must be balanced against the relevance of the review to a broad audience.
This is a preview of subscription content, log in via an institution .
Akobeng AK. Principles of evidence based medicine. Arch Dis Child. 2005;90(8):837–40.
Article CAS PubMed PubMed Central Google Scholar
Alharbi A, Stevenson M. Refining Boolean queries to identify relevant studies for systematic review updates. J Am Med Inform Assoc. 2020;27(11):1658–66.
Article PubMed PubMed Central Google Scholar
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Article Google Scholar
Aromataris E MZE. JBI manual for evidence synthesis. 2020.
Google Scholar
Aromataris E, Pearson A. The systematic review: an overview. Am J Nurs. 2014;114(3):53–8.
Article PubMed Google Scholar
Aromataris E, Riitano D. Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review. Am J Nurs. 2014;114(5):49–56.
Babineau J. Product review: covidence (systematic review software). J Canad Health Libr Assoc Canada. 2014;35(2):68–71.
Baker JD. The purpose, process, and methods of writing a literature review. AORN J. 2016;103(3):265–9.
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):1–12.
Brown D. A review of the PubMed PICO tool: using evidence-based practice in health education. Health Promot Pract. 2020;21(4):496–8.
Cargo M, Harris J, Pantoja T, et al. Cochrane qualitative and implementation methods group guidance series – paper 4: methods for assessing evidence on intervention implementation. J Clin Epidemiol. 2018;97:59–69.
Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80.
Article CAS PubMed Google Scholar
Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–7.
Cummings SR, Browner WS, Hulley SB. Conceiving the research question and developing the study plan. In: Cummings SR, Browner WS, Hulley SB, editors. Designing Clinical Research: An Epidemiological Approach. 4th ed. Philadelphia (PA): P Lippincott Williams & Wilkins; 2007. p. 14–22.
Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. JMLA. 2018;106(4):420.
Ferrari R. Writing narrative style literature reviews. Medical Writing. 2015;24(4):230–5.
Flemming K, Booth A, Hannes K, Cargo M, Noyes J. Cochrane qualitative and implementation methods group guidance series – paper 6: reporting guidelines for qualitative, implementation, and process evaluation evidence syntheses. J Clin Epidemiol. 2018;97:79–85.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108.
Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5(3):101–17.
Gregory AT, Denniss AR. An introduction to writing narrative and systematic reviews; tasks, tips and traps for aspiring authors. Heart Lung Circ. 2018;27(7):893–8.
Harden A, Thomas J, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 5: methods for integrating qualitative and implementation evidence within intervention effectiveness reviews. J Clin Epidemiol. 2018;97:70–8.
Harris JL, Booth A, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 2: methods for question formulation, searching, and protocol development for qualitative evidence synthesis. J Clin Epidemiol. 2018;97:39–48.
Higgins J, Thomas J. In: Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3, updated February 2022). Available from www.training.cochrane.org/handbook.: Cochrane; 2022.
International prospective register of systematic reviews (PROSPERO). Available from https://www.crd.york.ac.uk/prospero/ .
Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Landhuis E. Scientific literature: information overload. Nature. 2016;535(7612):457–8.
Lockwood C, Porritt K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, Loveday H, Carrier J, Stannard D. Chapter 2: Systematic reviews of qualitative evidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global . https://doi.org/10.46658/JBIMES-20-03 .
Chapter Google Scholar
Lorenzetti DL, Topfer L-A, Dennett L, Clement F. Value of databases other than medline for rapid health technology assessments. Int J Technol Assess Health Care. 2014;30(2):173–8.
Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for (SR) and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;6:264–9.
Mulrow CD. Systematic reviews: rationale for systematic reviews. BMJ. 1994;309(6954):597–9.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
Munthe-Kaas HM, Glenton C, Booth A, Noyes J, Lewin S. Systematic mapping of existing tools to appraise methodological strengths and limitations of qualitative research: first stage in the development of the CAMELOT tool. BMC Med Res Methodol. 2019;19(1):1–13.
Murphy CM. Writing an effective review article. J Med Toxicol. 2012;8(2):89–90.
NHMRC. Guidelines for guidelines: assessing risk of bias. Available at https://nhmrc.gov.au/guidelinesforguidelines/develop/assessing-risk-bias . Last published 29 August 2019. Accessed 29 Aug 2022.
Noyes J, Booth A, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 1: introduction. J Clin Epidemiol. 2018b;97:35–8.
Noyes J, Booth A, Flemming K, et al. Cochrane qualitative and implementation methods group guidance series – paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018a;97:49–58.
Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Glob Health. 2019;4(Suppl 1):e000893.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Healthcare. 2015;13(3):141–6.
Polanin JR, Pigott TD, Espelage DL, Grotpeter JK. Best practice guidelines for abstract screening large-evidence systematic reviews and meta-analyses. Res Synth Methods. 2019;10(3):330–42.
Article PubMed Central Google Scholar
Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):1–7.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Brit Med J. 2017;358
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12.
Tawfik GM, Dila KAS, Mohamed MYF, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2019;47(1):1–9.
The Critical Appraisal Program. Critical appraisal skills program. Available at https://casp-uk.net/ . 2022. Accessed 29 Aug 2022.
The University of Melbourne. Writing a literature review in Research Techniques 2022. Available at https://students.unimelb.edu.au/academic-skills/explore-our-resources/research-techniques/reviewing-the-literature . Accessed 29 Aug 2022.
The Writing Center University of Winconsin-Madison. Learn how to write a literature review in The Writer’s Handbook – Academic Professional Writing. 2022. Available at https://writing.wisc.edu/handbook/assignments/reviewofliterature/ . Accessed 29 Aug 2022.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16(1):15.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Yoneoka D, Henmi M. Clinical heterogeneity in random-effect meta-analysis: between-study boundary estimate problem. Stat Med. 2019;38(21):4131–45.
Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104(5):1086–92.
Download references
Author information
Authors and affiliations.
Centre of Excellence in Treatable Traits, College of Health, Medicine and Wellbeing, University of Newcastle, Hunter Medical Research Institute Asthma and Breathing Programme, Newcastle, NSW, Australia
Dennis Thomas
Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
Elida Zairina
Centre for Medicine Use and Safety, Monash Institute of Pharmaceutical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, VIC, Australia
Johnson George
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Johnson George .
Section Editor information
College of Pharmacy, Qatar University, Doha, Qatar
Derek Charles Stewart
Department of Pharmacy, University of Huddersfield, Huddersfield, United Kingdom
Zaheer-Ud-Din Babar
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry.
Thomas, D., Zairina, E., George, J. (2023). Methodological Approaches to Literature Review. In: Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy. Springer, Cham. https://doi.org/10.1007/978-3-030-50247-8_57-1
Download citation
DOI : https://doi.org/10.1007/978-3-030-50247-8_57-1
Received : 22 February 2023
Accepted : 22 February 2023
Published : 09 May 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-50247-8
Online ISBN : 978-3-030-50247-8
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 5. The Literature Review
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
A literature review surveys prior research published in books, scholarly articles, and any other sources relevant to a particular issue, area of research, or theory, and by so doing, provides a description, summary, and critical evaluation of these works in relation to the research problem being investigated. Literature reviews are designed to provide an overview of sources you have used in researching a particular topic and to demonstrate to your readers how your research fits within existing scholarship about the topic.
Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . Fourth edition. Thousand Oaks, CA: SAGE, 2014.
Importance of a Good Literature Review
A literature review may consist of simply a summary of key sources, but in the social sciences, a literature review usually has an organizational pattern and combines both summary and synthesis, often within specific conceptual categories . A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information in a way that informs how you are planning to investigate a research problem. The analytical features of a literature review might:
- Give a new interpretation of old material or combine new with old interpretations,
- Trace the intellectual progression of the field, including major debates,
- Depending on the situation, evaluate the sources and advise the reader on the most pertinent or relevant research, or
- Usually in the conclusion of a literature review, identify where gaps exist in how a problem has been researched to date.
Given this, the purpose of a literature review is to:
- Place each work in the context of its contribution to understanding the research problem being studied.
- Describe the relationship of each work to the others under consideration.
- Identify new ways to interpret prior research.
- Reveal any gaps that exist in the literature.
- Resolve conflicts amongst seemingly contradictory previous studies.
- Identify areas of prior scholarship to prevent duplication of effort.
- Point the way in fulfilling a need for additional research.
- Locate your own research within the context of existing literature [very important].
Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper. 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Jesson, Jill. Doing Your Literature Review: Traditional and Systematic Techniques . Los Angeles, CA: SAGE, 2011; Knopf, Jeffrey W. "Doing a Literature Review." PS: Political Science and Politics 39 (January 2006): 127-132; Ridley, Diana. The Literature Review: A Step-by-Step Guide for Students . 2nd ed. Los Angeles, CA: SAGE, 2012.
Types of Literature Reviews
It is important to think of knowledge in a given field as consisting of three layers. First, there are the primary studies that researchers conduct and publish. Second are the reviews of those studies that summarize and offer new interpretations built from and often extending beyond the primary studies. Third, there are the perceptions, conclusions, opinion, and interpretations that are shared informally among scholars that become part of the body of epistemological traditions within the field.
In composing a literature review, it is important to note that it is often this third layer of knowledge that is cited as "true" even though it often has only a loose relationship to the primary studies and secondary literature reviews. Given this, while literature reviews are designed to provide an overview and synthesis of pertinent sources you have explored, there are a number of approaches you could adopt depending upon the type of analysis underpinning your study.
Argumentative Review This form examines literature selectively in order to support or refute an argument, deeply embedded assumption, or philosophical problem already established in the literature. The purpose is to develop a body of literature that establishes a contrarian viewpoint. Given the value-laden nature of some social science research [e.g., educational reform; immigration control], argumentative approaches to analyzing the literature can be a legitimate and important form of discourse. However, note that they can also introduce problems of bias when they are used to make summary claims of the sort found in systematic reviews [see below].
Integrative Review Considered a form of research that reviews, critiques, and synthesizes representative literature on a topic in an integrated way such that new frameworks and perspectives on the topic are generated. The body of literature includes all studies that address related or identical hypotheses or research problems. A well-done integrative review meets the same standards as primary research in regard to clarity, rigor, and replication. This is the most common form of review in the social sciences.
Historical Review Few things rest in isolation from historical precedent. Historical literature reviews focus on examining research throughout a period of time, often starting with the first time an issue, concept, theory, phenomena emerged in the literature, then tracing its evolution within the scholarship of a discipline. The purpose is to place research in a historical context to show familiarity with state-of-the-art developments and to identify the likely directions for future research.
Methodological Review A review does not always focus on what someone said [findings], but how they came about saying what they say [method of analysis]. Reviewing methods of analysis provides a framework of understanding at different levels [i.e. those of theory, substantive fields, research approaches, and data collection and analysis techniques], how researchers draw upon a wide variety of knowledge ranging from the conceptual level to practical documents for use in fieldwork in the areas of ontological and epistemological consideration, quantitative and qualitative integration, sampling, interviewing, data collection, and data analysis. This approach helps highlight ethical issues which you should be aware of and consider as you go through your own study.
Systematic Review This form consists of an overview of existing evidence pertinent to a clearly formulated research question, which uses pre-specified and standardized methods to identify and critically appraise relevant research, and to collect, report, and analyze data from the studies that are included in the review. The goal is to deliberately document, critically evaluate, and summarize scientifically all of the research about a clearly defined research problem . Typically it focuses on a very specific empirical question, often posed in a cause-and-effect form, such as "To what extent does A contribute to B?" This type of literature review is primarily applied to examining prior research studies in clinical medicine and allied health fields, but it is increasingly being used in the social sciences.
Theoretical Review The purpose of this form is to examine the corpus of theory that has accumulated in regard to an issue, concept, theory, phenomena. The theoretical literature review helps to establish what theories already exist, the relationships between them, to what degree the existing theories have been investigated, and to develop new hypotheses to be tested. Often this form is used to help establish a lack of appropriate theories or reveal that current theories are inadequate for explaining new or emerging research problems. The unit of analysis can focus on a theoretical concept or a whole theory or framework.
NOTE : Most often the literature review will incorporate some combination of types. For example, a review that examines literature supporting or refuting an argument, assumption, or philosophical problem related to the research problem will also need to include writing supported by sources that establish the history of these arguments in the literature.
Baumeister, Roy F. and Mark R. Leary. "Writing Narrative Literature Reviews." Review of General Psychology 1 (September 1997): 311-320; Mark R. Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Kennedy, Mary M. "Defining a Literature." Educational Researcher 36 (April 2007): 139-147; Petticrew, Mark and Helen Roberts. Systematic Reviews in the Social Sciences: A Practical Guide . Malden, MA: Blackwell Publishers, 2006; Torracro, Richard. "Writing Integrative Literature Reviews: Guidelines and Examples." Human Resource Development Review 4 (September 2005): 356-367; Rocco, Tonette S. and Maria S. Plakhotnik. "Literature Reviews, Conceptual Frameworks, and Theoretical Frameworks: Terms, Functions, and Distinctions." Human Ressource Development Review 8 (March 2008): 120-130; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016.
Structure and Writing Style
I. Thinking About Your Literature Review
The structure of a literature review should include the following in support of understanding the research problem :
- An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review,
- Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches entirely],
- An explanation of how each work is similar to and how it varies from the others,
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research.
The critical evaluation of each work should consider :
- Provenance -- what are the author's credentials? Are the author's arguments supported by evidence [e.g. primary historical material, case studies, narratives, statistics, recent scientific findings]?
- Methodology -- were the techniques used to identify, gather, and analyze the data appropriate to addressing the research problem? Was the sample size appropriate? Were the results effectively interpreted and reported?
- Objectivity -- is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
- Persuasiveness -- which of the author's theses are most convincing or least convincing?
- Validity -- are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
II. Development of the Literature Review
Four Basic Stages of Writing 1. Problem formulation -- which topic or field is being examined and what are its component issues? 2. Literature search -- finding materials relevant to the subject being explored. 3. Data evaluation -- determining which literature makes a significant contribution to the understanding of the topic. 4. Analysis and interpretation -- discussing the findings and conclusions of pertinent literature.
Consider the following issues before writing the literature review: Clarify If your assignment is not specific about what form your literature review should take, seek clarification from your professor by asking these questions: 1. Roughly how many sources would be appropriate to include? 2. What types of sources should I review (books, journal articles, websites; scholarly versus popular sources)? 3. Should I summarize, synthesize, or critique sources by discussing a common theme or issue? 4. Should I evaluate the sources in any way beyond evaluating how they relate to understanding the research problem? 5. Should I provide subheadings and other background information, such as definitions and/or a history? Find Models Use the exercise of reviewing the literature to examine how authors in your discipline or area of interest have composed their literature review sections. Read them to get a sense of the types of themes you might want to look for in your own research or to identify ways to organize your final review. The bibliography or reference section of sources you've already read, such as required readings in the course syllabus, are also excellent entry points into your own research. Narrow the Topic The narrower your topic, the easier it will be to limit the number of sources you need to read in order to obtain a good survey of relevant resources. Your professor will probably not expect you to read everything that's available about the topic, but you'll make the act of reviewing easier if you first limit scope of the research problem. A good strategy is to begin by searching the USC Libraries Catalog for recent books about the topic and review the table of contents for chapters that focuses on specific issues. You can also review the indexes of books to find references to specific issues that can serve as the focus of your research. For example, a book surveying the history of the Israeli-Palestinian conflict may include a chapter on the role Egypt has played in mediating the conflict, or look in the index for the pages where Egypt is mentioned in the text. Consider Whether Your Sources are Current Some disciplines require that you use information that is as current as possible. This is particularly true in disciplines in medicine and the sciences where research conducted becomes obsolete very quickly as new discoveries are made. However, when writing a review in the social sciences, a survey of the history of the literature may be required. In other words, a complete understanding the research problem requires you to deliberately examine how knowledge and perspectives have changed over time. Sort through other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. You can also use this method to explore what is considered by scholars to be a "hot topic" and what is not.
III. Ways to Organize Your Literature Review
Chronology of Events If your review follows the chronological method, you could write about the materials according to when they were published. This approach should only be followed if a clear path of research building on previous research can be identified and that these trends follow a clear chronological order of development. For example, a literature review that focuses on continuing research about the emergence of German economic power after the fall of the Soviet Union. By Publication Order your sources by publication chronology, then, only if the order demonstrates a more important trend. For instance, you could order a review of literature on environmental studies of brown fields if the progression revealed, for example, a change in the soil collection practices of the researchers who wrote and/or conducted the studies. Thematic [“conceptual categories”] A thematic literature review is the most common approach to summarizing prior research in the social and behavioral sciences. Thematic reviews are organized around a topic or issue, rather than the progression of time, although the progression of time may still be incorporated into a thematic review. For example, a review of the Internet’s impact on American presidential politics could focus on the development of online political satire. While the study focuses on one topic, the Internet’s impact on American presidential politics, it would still be organized chronologically reflecting technological developments in media. The difference in this example between a "chronological" and a "thematic" approach is what is emphasized the most: themes related to the role of the Internet in presidential politics. Note that more authentic thematic reviews tend to break away from chronological order. A review organized in this manner would shift between time periods within each section according to the point being made. Methodological A methodological approach focuses on the methods utilized by the researcher. For the Internet in American presidential politics project, one methodological approach would be to look at cultural differences between the portrayal of American presidents on American, British, and French websites. Or the review might focus on the fundraising impact of the Internet on a particular political party. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed.
Other Sections of Your Literature Review Once you've decided on the organizational method for your literature review, the sections you need to include in the paper should be easy to figure out because they arise from your organizational strategy. In other words, a chronological review would have subsections for each vital time period; a thematic review would have subtopics based upon factors that relate to the theme or issue. However, sometimes you may need to add additional sections that are necessary for your study, but do not fit in the organizational strategy of the body. What other sections you include in the body is up to you. However, only include what is necessary for the reader to locate your study within the larger scholarship about the research problem.
Here are examples of other sections, usually in the form of a single paragraph, you may need to include depending on the type of review you write:
- Current Situation : Information necessary to understand the current topic or focus of the literature review.
- Sources Used : Describes the methods and resources [e.g., databases] you used to identify the literature you reviewed.
- History : The chronological progression of the field, the research literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Selection Methods : Criteria you used to select (and perhaps exclude) sources in your literature review. For instance, you might explain that your review includes only peer-reviewed [i.e., scholarly] sources.
- Standards : Description of the way in which you present your information.
- Questions for Further Research : What questions about the field has the review sparked? How will you further your research as a result of the review?
IV. Writing Your Literature Review
Once you've settled on how to organize your literature review, you're ready to write each section. When writing your review, keep in mind these issues.
Use Evidence A literature review section is, in this sense, just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence [citations] that demonstrates that what you are saying is valid. Be Selective Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the research problem, whether it is thematic, methodological, or chronological. Related items that provide additional information, but that are not key to understanding the research problem, can be included in a list of further readings . Use Quotes Sparingly Some short quotes are appropriate if you want to emphasize a point, or if what an author stated cannot be easily paraphrased. Sometimes you may need to quote certain terminology that was coined by the author, is not common knowledge, or taken directly from the study. Do not use extensive quotes as a substitute for using your own words in reviewing the literature. Summarize and Synthesize Remember to summarize and synthesize your sources within each thematic paragraph as well as throughout the review. Recapitulate important features of a research study, but then synthesize it by rephrasing the study's significance and relating it to your own work and the work of others. Keep Your Own Voice While the literature review presents others' ideas, your voice [the writer's] should remain front and center. For example, weave references to other sources into what you are writing but maintain your own voice by starting and ending the paragraph with your own ideas and wording. Use Caution When Paraphrasing When paraphrasing a source that is not your own, be sure to represent the author's information or opinions accurately and in your own words. Even when paraphrasing an author’s work, you still must provide a citation to that work.
V. Common Mistakes to Avoid
These are the most common mistakes made in reviewing social science research literature.
- Sources in your literature review do not clearly relate to the research problem;
- You do not take sufficient time to define and identify the most relevant sources to use in the literature review related to the research problem;
- Relies exclusively on secondary analytical sources rather than including relevant primary research studies or data;
- Uncritically accepts another researcher's findings and interpretations as valid, rather than examining critically all aspects of the research design and analysis;
- Does not describe the search procedures that were used in identifying the literature to review;
- Reports isolated statistical results rather than synthesizing them in chi-squared or meta-analytic methods; and,
- Only includes research that validates assumptions and does not consider contrary findings and alternative interpretations found in the literature.
Cook, Kathleen E. and Elise Murowchick. “Do Literature Review Skills Transfer from One Course to Another?” Psychology Learning and Teaching 13 (March 2014): 3-11; Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Jesson, Jill. Doing Your Literature Review: Traditional and Systematic Techniques . London: SAGE, 2011; Literature Review Handout. Online Writing Center. Liberty University; Literature Reviews. The Writing Center. University of North Carolina; Onwuegbuzie, Anthony J. and Rebecca Frels. Seven Steps to a Comprehensive Literature Review: A Multimodal and Cultural Approach . Los Angeles, CA: SAGE, 2016; Ridley, Diana. The Literature Review: A Step-by-Step Guide for Students . 2nd ed. Los Angeles, CA: SAGE, 2012; Randolph, Justus J. “A Guide to Writing the Dissertation Literature Review." Practical Assessment, Research, and Evaluation. vol. 14, June 2009; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016; Taylor, Dena. The Literature Review: A Few Tips On Conducting It. University College Writing Centre. University of Toronto; Writing a Literature Review. Academic Skills Centre. University of Canberra.
Writing Tip
Break Out of Your Disciplinary Box!
Thinking interdisciplinarily about a research problem can be a rewarding exercise in applying new ideas, theories, or concepts to an old problem. For example, what might cultural anthropologists say about the continuing conflict in the Middle East? In what ways might geographers view the need for better distribution of social service agencies in large cities than how social workers might study the issue? You don’t want to substitute a thorough review of core research literature in your discipline for studies conducted in other fields of study. However, particularly in the social sciences, thinking about research problems from multiple vectors is a key strategy for finding new solutions to a problem or gaining a new perspective. Consult with a librarian about identifying research databases in other disciplines; almost every field of study has at least one comprehensive database devoted to indexing its research literature.
Frodeman, Robert. The Oxford Handbook of Interdisciplinarity . New York: Oxford University Press, 2010.
Another Writing Tip
Don't Just Review for Content!
While conducting a review of the literature, maximize the time you devote to writing this part of your paper by thinking broadly about what you should be looking for and evaluating. Review not just what scholars are saying, but how are they saying it. Some questions to ask:
- How are they organizing their ideas?
- What methods have they used to study the problem?
- What theories have been used to explain, predict, or understand their research problem?
- What sources have they cited to support their conclusions?
- How have they used non-textual elements [e.g., charts, graphs, figures, etc.] to illustrate key points?
When you begin to write your literature review section, you'll be glad you dug deeper into how the research was designed and constructed because it establishes a means for developing more substantial analysis and interpretation of the research problem.
Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1 998.
Yet Another Writing Tip
When Do I Know I Can Stop Looking and Move On?
Here are several strategies you can utilize to assess whether you've thoroughly reviewed the literature:
- Look for repeating patterns in the research findings . If the same thing is being said, just by different people, then this likely demonstrates that the research problem has hit a conceptual dead end. At this point consider: Does your study extend current research? Does it forge a new path? Or, does is merely add more of the same thing being said?
- Look at sources the authors cite to in their work . If you begin to see the same researchers cited again and again, then this is often an indication that no new ideas have been generated to address the research problem.
- Search Google Scholar to identify who has subsequently cited leading scholars already identified in your literature review [see next sub-tab]. This is called citation tracking and there are a number of sources that can help you identify who has cited whom, particularly scholars from outside of your discipline. Here again, if the same authors are being cited again and again, this may indicate no new literature has been written on the topic.
Onwuegbuzie, Anthony J. and Rebecca Frels. Seven Steps to a Comprehensive Literature Review: A Multimodal and Cultural Approach . Los Angeles, CA: Sage, 2016; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016.
- << Previous: Theoretical Framework
- Next: Citation Tracking >>
- Last Updated: Apr 18, 2024 12:20 PM
- URL: https://libguides.usc.edu/writingguide

- University of Texas Libraries
Literature Reviews
- What is a literature review?
- Steps in the Literature Review Process
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Analyze Results
- Librarian Support
What is a Literature Review?
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important past and current research and practices. It provides background and context, and shows how your research will contribute to the field.
A literature review should:
- Provide a comprehensive and updated review of the literature;
- Explain why this review has taken place;
- Articulate a position or hypothesis;
- Acknowledge and account for conflicting and corroborating points of view
From S age Research Methods
Purpose of a Literature Review
A literature review can be written as an introduction to a study to:
- Demonstrate how a study fills a gap in research
- Compare a study with other research that's been done
Or it can be a separate work (a research article on its own) which:
- Organizes or describes a topic
- Describes variables within a particular issue/problem
Limitations of a Literature Review
Some of the limitations of a literature review are:
- It's a snapshot in time. Unlike other reviews, this one has beginning, a middle and an end. There may be future developments that could make your work less relevant.
- It may be too focused. Some niche studies may miss the bigger picture.
- It can be difficult to be comprehensive. There is no way to make sure all the literature on a topic was considered.
- It is easy to be biased if you stick to top tier journals. There may be other places where people are publishing exemplary research. Look to open access publications and conferences to reflect a more inclusive collection. Also, make sure to include opposing views (and not just supporting evidence).
Source: Grant, Maria J., and Andrew Booth. “A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies.” Health Information & Libraries Journal, vol. 26, no. 2, June 2009, pp. 91–108. Wiley Online Library, doi:10.1111/j.1471-1842.2009.00848.x.
Meryl Brodsky : Communication and Information Studies
Hannah Chapman Tripp : Biology, Neuroscience
Carolyn Cunningham : Human Development & Family Sciences, Psychology, Sociology
Larayne Dallas : Engineering
Janelle Hedstrom : Special Education, Curriculum & Instruction, Ed Leadership & Policy
Susan Macicak : Linguistics
Imelda Vetter : Dell Medical School
For help in other subject areas, please see the guide to library specialists by subject .
Periodically, UT Libraries runs a workshop covering the basics and library support for literature reviews. While we try to offer these once per academic year, we find providing the recording to be helpful to community members who have missed the session. Following is the most recent recording of the workshop, Conducting a Literature Review. To view the recording, a UT login is required.
- October 26, 2022 recording
- Last Updated: Oct 26, 2022 2:49 PM
- URL: https://guides.lib.utexas.edu/literaturereviews

- Open access
- Published: 14 August 2018
Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies
- Chris Cooper ORCID: orcid.org/0000-0003-0864-5607 1 ,
- Andrew Booth 2 ,
- Jo Varley-Campbell 1 ,
- Nicky Britten 3 &
- Ruth Garside 4
BMC Medical Research Methodology volume 18 , Article number: 85 ( 2018 ) Cite this article
200k Accesses
203 Citations
118 Altmetric
Metrics details
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before.
The purpose of this review is to determine if a shared model of the literature searching process can be detected across systematic review guidance documents and, if so, how this process is reported in the guidance and supported by published studies.
A literature review.
Two types of literature were reviewed: guidance and published studies. Nine guidance documents were identified, including: The Cochrane and Campbell Handbooks. Published studies were identified through ‘pearl growing’, citation chasing, a search of PubMed using the systematic review methods filter, and the authors’ topic knowledge.
The relevant sections within each guidance document were then read and re-read, with the aim of determining key methodological stages. Methodological stages were identified and defined. This data was reviewed to identify agreements and areas of unique guidance between guidance documents. Consensus across multiple guidance documents was used to inform selection of ‘key stages’ in the process of literature searching.
Eight key stages were determined relating specifically to literature searching in systematic reviews. They were: who should literature search, aims and purpose of literature searching, preparation, the search strategy, searching databases, supplementary searching, managing references and reporting the search process.
Conclusions
Eight key stages to the process of literature searching in systematic reviews were identified. These key stages are consistently reported in the nine guidance documents, suggesting consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews. Further research to determine the suitability of using the same process of literature searching for all types of systematic review is indicated.
Peer Review reports
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving review stakeholders clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before. This is in contrast to the information science literature, which has developed information processing models as an explicit basis for dialogue and empirical testing. Without an explicit model, research in the process of systematic literature searching will remain immature and potentially uneven, and the development of shared information models will be assumed but never articulated.
One way of developing such a conceptual model is by formally examining the implicit “programme theory” as embodied in key methodological texts. The aim of this review is therefore to determine if a shared model of the literature searching process in systematic reviews can be detected across guidance documents and, if so, how this process is reported and supported.
Identifying guidance
Key texts (henceforth referred to as “guidance”) were identified based upon their accessibility to, and prominence within, United Kingdom systematic reviewing practice. The United Kingdom occupies a prominent position in the science of health information retrieval, as quantified by such objective measures as the authorship of papers, the number of Cochrane groups based in the UK, membership and leadership of groups such as the Cochrane Information Retrieval Methods Group, the HTA-I Information Specialists’ Group and historic association with such centres as the UK Cochrane Centre, the NHS Centre for Reviews and Dissemination, the Centre for Evidence Based Medicine and the National Institute for Clinical Excellence (NICE). Coupled with the linguistic dominance of English within medical and health science and the science of systematic reviews more generally, this offers a justification for a purposive sample that favours UK, European and Australian guidance documents.
Nine guidance documents were identified. These documents provide guidance for different types of reviews, namely: reviews of interventions, reviews of health technologies, reviews of qualitative research studies, reviews of social science topics, and reviews to inform guidance.
Whilst these guidance documents occasionally offer additional guidance on other types of systematic reviews, we have focused on the core and stated aims of these documents as they relate to literature searching. Table 1 sets out: the guidance document, the version audited, their core stated focus, and a bibliographical pointer to the main guidance relating to literature searching.
Once a list of key guidance documents was determined, it was checked by six senior information professionals based in the UK for relevance to current literature searching in systematic reviews.
Identifying supporting studies
In addition to identifying guidance, the authors sought to populate an evidence base of supporting studies (henceforth referred to as “studies”) that contribute to existing search practice. Studies were first identified by the authors from their knowledge on this topic area and, subsequently, through systematic citation chasing key studies (‘pearls’ [ 1 ]) located within each key stage of the search process. These studies are identified in Additional file 1 : Appendix Table 1. Citation chasing was conducted by analysing the bibliography of references for each study (backwards citation chasing) and through Google Scholar (forward citation chasing). A search of PubMed using the systematic review methods filter was undertaken in August 2017 (see Additional file 1 ). The search terms used were: (literature search*[Title/Abstract]) AND sysrev_methods[sb] and 586 results were returned. These results were sifted for relevance to the key stages in Fig. 1 by CC.
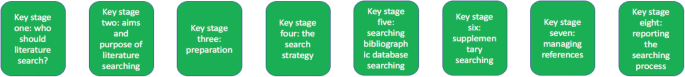
The key stages of literature search guidance as identified from nine key texts
Extracting the data
To reveal the implicit process of literature searching within each guidance document, the relevant sections (chapters) on literature searching were read and re-read, with the aim of determining key methodological stages. We defined a key methodological stage as a distinct step in the overall process for which specific guidance is reported, and action is taken, that collectively would result in a completed literature search.
The chapter or section sub-heading for each methodological stage was extracted into a table using the exact language as reported in each guidance document. The lead author (CC) then read and re-read these data, and the paragraphs of the document to which the headings referred, summarising section details. This table was then reviewed, using comparison and contrast to identify agreements and areas of unique guidance. Consensus across multiple guidelines was used to inform selection of ‘key stages’ in the process of literature searching.
Having determined the key stages to literature searching, we then read and re-read the sections relating to literature searching again, extracting specific detail relating to the methodological process of literature searching within each key stage. Again, the guidance was then read and re-read, first on a document-by-document-basis and, secondly, across all the documents above, to identify both commonalities and areas of unique guidance.
Results and discussion
Our findings.
We were able to identify consensus across the guidance on literature searching for systematic reviews suggesting a shared implicit model within the information retrieval community. Whilst the structure of the guidance varies between documents, the same key stages are reported, even where the core focus of each document is different. We were able to identify specific areas of unique guidance, where a document reported guidance not summarised in other documents, together with areas of consensus across guidance.
Unique guidance
Only one document provided guidance on the topic of when to stop searching [ 2 ]. This guidance from 2005 anticipates a topic of increasing importance with the current interest in time-limited (i.e. “rapid”) reviews. Quality assurance (or peer review) of literature searches was only covered in two guidance documents [ 3 , 4 ]. This topic has emerged as increasingly important as indicated by the development of the PRESS instrument [ 5 ]. Text mining was discussed in four guidance documents [ 4 , 6 , 7 , 8 ] where the automation of some manual review work may offer efficiencies in literature searching [ 8 ].
Agreement between guidance: Defining the key stages of literature searching
Where there was agreement on the process, we determined that this constituted a key stage in the process of literature searching to inform systematic reviews.
From the guidance, we determined eight key stages that relate specifically to literature searching in systematic reviews. These are summarised at Fig. 1 . The data extraction table to inform Fig. 1 is reported in Table 2 . Table 2 reports the areas of common agreement and it demonstrates that the language used to describe key stages and processes varies significantly between guidance documents.
For each key stage, we set out the specific guidance, followed by discussion on how this guidance is situated within the wider literature.
Key stage one: Deciding who should undertake the literature search
The guidance.
Eight documents provided guidance on who should undertake literature searching in systematic reviews [ 2 , 4 , 6 , 7 , 8 , 9 , 10 , 11 ]. The guidance affirms that people with relevant expertise of literature searching should ‘ideally’ be included within the review team [ 6 ]. Information specialists (or information scientists), librarians or trial search co-ordinators (TSCs) are indicated as appropriate researchers in six guidance documents [ 2 , 7 , 8 , 9 , 10 , 11 ].
How the guidance corresponds to the published studies
The guidance is consistent with studies that call for the involvement of information specialists and librarians in systematic reviews [ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ] and which demonstrate how their training as ‘expert searchers’ and ‘analysers and organisers of data’ can be put to good use [ 13 ] in a variety of roles [ 12 , 16 , 20 , 21 , 24 , 25 , 26 ]. These arguments make sense in the context of the aims and purposes of literature searching in systematic reviews, explored below. The need for ‘thorough’ and ‘replicable’ literature searches was fundamental to the guidance and recurs in key stage two. Studies have found poor reporting, and a lack of replicable literature searches, to be a weakness in systematic reviews [ 17 , 18 , 27 , 28 ] and they argue that involvement of information specialists/ librarians would be associated with better reporting and better quality literature searching. Indeed, Meert et al. [ 29 ] demonstrated that involving a librarian as a co-author to a systematic review correlated with a higher score in the literature searching component of a systematic review [ 29 ]. As ‘new styles’ of rapid and scoping reviews emerge, where decisions on how to search are more iterative and creative, a clear role is made here too [ 30 ].
Knowing where to search for studies was noted as important in the guidance, with no agreement as to the appropriate number of databases to be searched [ 2 , 6 ]. Database (and resource selection more broadly) is acknowledged as a relevant key skill of information specialists and librarians [ 12 , 15 , 16 , 31 ].
Whilst arguments for including information specialists and librarians in the process of systematic review might be considered self-evident, Koffel and Rethlefsen [ 31 ] have questioned if the necessary involvement is actually happening [ 31 ].
Key stage two: Determining the aim and purpose of a literature search
The aim: Five of the nine guidance documents use adjectives such as ‘thorough’, ‘comprehensive’, ‘transparent’ and ‘reproducible’ to define the aim of literature searching [ 6 , 7 , 8 , 9 , 10 ]. Analogous phrases were present in a further three guidance documents, namely: ‘to identify the best available evidence’ [ 4 ] or ‘the aim of the literature search is not to retrieve everything. It is to retrieve everything of relevance’ [ 2 ] or ‘A systematic literature search aims to identify all publications relevant to the particular research question’ [ 3 ]. The Joanna Briggs Institute reviewers’ manual was the only guidance document where a clear statement on the aim of literature searching could not be identified. The purpose of literature searching was defined in three guidance documents, namely to minimise bias in the resultant review [ 6 , 8 , 10 ]. Accordingly, eight of nine documents clearly asserted that thorough and comprehensive literature searches are required as a potential mechanism for minimising bias.
The need for thorough and comprehensive literature searches appears as uniform within the eight guidance documents that describe approaches to literature searching in systematic reviews of effectiveness. Reviews of effectiveness (of intervention or cost), accuracy and prognosis, require thorough and comprehensive literature searches to transparently produce a reliable estimate of intervention effect. The belief that all relevant studies have been ‘comprehensively’ identified, and that this process has been ‘transparently’ reported, increases confidence in the estimate of effect and the conclusions that can be drawn [ 32 ]. The supporting literature exploring the need for comprehensive literature searches focuses almost exclusively on reviews of intervention effectiveness and meta-analysis. Different ‘styles’ of review may have different standards however; the alternative, offered by purposive sampling, has been suggested in the specific context of qualitative evidence syntheses [ 33 ].
What is a comprehensive literature search?
Whilst the guidance calls for thorough and comprehensive literature searches, it lacks clarity on what constitutes a thorough and comprehensive literature search, beyond the implication that all of the literature search methods in Table 2 should be used to identify studies. Egger et al. [ 34 ], in an empirical study evaluating the importance of comprehensive literature searches for trials in systematic reviews, defined a comprehensive search for trials as:
a search not restricted to English language;
where Cochrane CENTRAL or at least two other electronic databases had been searched (such as MEDLINE or EMBASE); and
at least one of the following search methods has been used to identify unpublished trials: searches for (I) conference abstracts, (ii) theses, (iii) trials registers; and (iv) contacts with experts in the field [ 34 ].
Tricco et al. (2008) used a similar threshold of bibliographic database searching AND a supplementary search method in a review when examining the risk of bias in systematic reviews. Their criteria were: one database (limited using the Cochrane Highly Sensitive Search Strategy (HSSS)) and handsearching [ 35 ].
Together with the guidance, this would suggest that comprehensive literature searching requires the use of BOTH bibliographic database searching AND supplementary search methods.
Comprehensiveness in literature searching, in the sense of how much searching should be undertaken, remains unclear. Egger et al. recommend that ‘investigators should consider the type of literature search and degree of comprehension that is appropriate for the review in question, taking into account budget and time constraints’ [ 34 ]. This view tallies with the Cochrane Handbook, which stipulates clearly, that study identification should be undertaken ‘within resource limits’ [ 9 ]. This would suggest that the limitations to comprehension are recognised but it raises questions on how this is decided and reported [ 36 ].
What is the point of comprehensive literature searching?
The purpose of thorough and comprehensive literature searches is to avoid missing key studies and to minimize bias [ 6 , 8 , 10 , 34 , 37 , 38 , 39 ] since a systematic review based only on published (or easily accessible) studies may have an exaggerated effect size [ 35 ]. Felson (1992) sets out potential biases that could affect the estimate of effect in a meta-analysis [ 40 ] and Tricco et al. summarize the evidence concerning bias and confounding in systematic reviews [ 35 ]. Egger et al. point to non-publication of studies, publication bias, language bias and MEDLINE bias, as key biases [ 34 , 35 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. Comprehensive searches are not the sole factor to mitigate these biases but their contribution is thought to be significant [ 2 , 32 , 34 ]. Fehrmann (2011) suggests that ‘the search process being described in detail’ and that, where standard comprehensive search techniques have been applied, increases confidence in the search results [ 32 ].
Does comprehensive literature searching work?
Egger et al., and other study authors, have demonstrated a change in the estimate of intervention effectiveness where relevant studies were excluded from meta-analysis [ 34 , 47 ]. This would suggest that missing studies in literature searching alters the reliability of effectiveness estimates. This is an argument for comprehensive literature searching. Conversely, Egger et al. found that ‘comprehensive’ searches still missed studies and that comprehensive searches could, in fact, introduce bias into a review rather than preventing it, through the identification of low quality studies then being included in the meta-analysis [ 34 ]. Studies query if identifying and including low quality or grey literature studies changes the estimate of effect [ 43 , 48 ] and question if time is better invested updating systematic reviews rather than searching for unpublished studies [ 49 ], or mapping studies for review as opposed to aiming for high sensitivity in literature searching [ 50 ].
Aim and purpose beyond reviews of effectiveness
The need for comprehensive literature searches is less certain in reviews of qualitative studies, and for reviews where a comprehensive identification of studies is difficult to achieve (for example, in Public health) [ 33 , 51 , 52 , 53 , 54 , 55 ]. Literature searching for qualitative studies, and in public health topics, typically generates a greater number of studies to sift than in reviews of effectiveness [ 39 ] and demonstrating the ‘value’ of studies identified or missed is harder [ 56 ], since the study data do not typically support meta-analysis. Nussbaumer-Streit et al. (2016) have registered a review protocol to assess whether abbreviated literature searches (as opposed to comprehensive literature searches) has an impact on conclusions across multiple bodies of evidence, not only on effect estimates [ 57 ] which may develop this understanding. It may be that decision makers and users of systematic reviews are willing to trade the certainty from a comprehensive literature search and systematic review in exchange for different approaches to evidence synthesis [ 58 ], and that comprehensive literature searches are not necessarily a marker of literature search quality, as previously thought [ 36 ]. Different approaches to literature searching [ 37 , 38 , 59 , 60 , 61 , 62 ] and developing the concept of when to stop searching are important areas for further study [ 36 , 59 ].
The study by Nussbaumer-Streit et al. has been published since the submission of this literature review [ 63 ]. Nussbaumer-Streit et al. (2018) conclude that abbreviated literature searches are viable options for rapid evidence syntheses, if decision-makers are willing to trade the certainty from a comprehensive literature search and systematic review, but that decision-making which demands detailed scrutiny should still be based on comprehensive literature searches [ 63 ].
Key stage three: Preparing for the literature search
Six documents provided guidance on preparing for a literature search [ 2 , 3 , 6 , 7 , 9 , 10 ]. The Cochrane Handbook clearly stated that Cochrane authors (i.e. researchers) should seek advice from a trial search co-ordinator (i.e. a person with specific skills in literature searching) ‘before’ starting a literature search [ 9 ].
Two key tasks were perceptible in preparing for a literature searching [ 2 , 6 , 7 , 10 , 11 ]. First, to determine if there are any existing or on-going reviews, or if a new review is justified [ 6 , 11 ]; and, secondly, to develop an initial literature search strategy to estimate the volume of relevant literature (and quality of a small sample of relevant studies [ 10 ]) and indicate the resources required for literature searching and the review of the studies that follows [ 7 , 10 ].
Three documents summarised guidance on where to search to determine if a new review was justified [ 2 , 6 , 11 ]. These focused on searching databases of systematic reviews (The Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE)), institutional registries (including PROSPERO), and MEDLINE [ 6 , 11 ]. It is worth noting, however, that as of 2015, DARE (and NHS EEDs) are no longer being updated and so the relevance of this (these) resource(s) will diminish over-time [ 64 ]. One guidance document, ‘Systematic reviews in the Social Sciences’, noted, however, that databases are not the only source of information and unpublished reports, conference proceeding and grey literature may also be required, depending on the nature of the review question [ 2 ].
Two documents reported clearly that this preparation (or ‘scoping’) exercise should be undertaken before the actual search strategy is developed [ 7 , 10 ]).
The guidance offers the best available source on preparing the literature search with the published studies not typically reporting how their scoping informed the development of their search strategies nor how their search approaches were developed. Text mining has been proposed as a technique to develop search strategies in the scoping stages of a review although this work is still exploratory [ 65 ]. ‘Clustering documents’ and word frequency analysis have also been tested to identify search terms and studies for review [ 66 , 67 ]. Preparing for literature searches and scoping constitutes an area for future research.
Key stage four: Designing the search strategy
The Population, Intervention, Comparator, Outcome (PICO) structure was the commonly reported structure promoted to design a literature search strategy. Five documents suggested that the eligibility criteria or review question will determine which concepts of PICO will be populated to develop the search strategy [ 1 , 4 , 7 , 8 , 9 ]. The NICE handbook promoted multiple structures, namely PICO, SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) and multi-stranded approaches [ 4 ].
With the exclusion of The Joanna Briggs Institute reviewers’ manual, the guidance offered detail on selecting key search terms, synonyms, Boolean language, selecting database indexing terms and combining search terms. The CEE handbook suggested that ‘search terms may be compiled with the help of the commissioning organisation and stakeholders’ [ 10 ].
The use of limits, such as language or date limits, were discussed in all documents [ 2 , 3 , 4 , 6 , 7 , 8 , 9 , 10 , 11 ].
Search strategy structure
The guidance typically relates to reviews of intervention effectiveness so PICO – with its focus on intervention and comparator - is the dominant model used to structure literature search strategies [ 68 ]. PICOs – where the S denotes study design - is also commonly used in effectiveness reviews [ 6 , 68 ]. As the NICE handbook notes, alternative models to structure literature search strategies have been developed and tested. Booth provides an overview on formulating questions for evidence based practice [ 69 ] and has developed a number of alternatives to the PICO structure, namely: BeHEMoTh (Behaviour of interest; Health context; Exclusions; Models or Theories) for use when systematically identifying theory [ 55 ]; SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) for identification of social science and evaluation studies [ 69 ] and, working with Cooke and colleagues, SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) [ 70 ]. SPIDER has been compared to PICO and PICOs in a study by Methley et al. [ 68 ].
The NICE handbook also suggests the use of multi-stranded approaches to developing literature search strategies [ 4 ]. Glanville developed this idea in a study by Whitting et al. [ 71 ] and a worked example of this approach is included in the development of a search filter by Cooper et al. [ 72 ].
Writing search strategies: Conceptual and objective approaches
Hausner et al. [ 73 ] provide guidance on writing literature search strategies, delineating between conceptually and objectively derived approaches. The conceptual approach, advocated by and explained in the guidance documents, relies on the expertise of the literature searcher to identify key search terms and then develop key terms to include synonyms and controlled syntax. Hausner and colleagues set out the objective approach [ 73 ] and describe what may be done to validate it [ 74 ].
The use of limits
The guidance documents offer direction on the use of limits within a literature search. Limits can be used to focus literature searching to specific study designs or by other markers (such as by date) which limits the number of studies returned by a literature search. The use of limits should be described and the implications explored [ 34 ] since limiting literature searching can introduce bias (explored above). Craven et al. have suggested the use of a supporting narrative to explain decisions made in the process of developing literature searches and this advice would usefully capture decisions on the use of search limits [ 75 ].
Key stage five: Determining the process of literature searching and deciding where to search (bibliographic database searching)
Table 2 summarises the process of literature searching as reported in each guidance document. Searching bibliographic databases was consistently reported as the ‘first step’ to literature searching in all nine guidance documents.
Three documents reported specific guidance on where to search, in each case specific to the type of review their guidance informed, and as a minimum requirement [ 4 , 9 , 11 ]. Seven of the key guidance documents suggest that the selection of bibliographic databases depends on the topic of review [ 2 , 3 , 4 , 6 , 7 , 8 , 10 ], with two documents noting the absence of an agreed standard on what constitutes an acceptable number of databases searched [ 2 , 6 ].
The guidance documents summarise ‘how to’ search bibliographic databases in detail and this guidance is further contextualised above in terms of developing the search strategy. The documents provide guidance of selecting bibliographic databases, in some cases stating acceptable minima (i.e. The Cochrane Handbook states Cochrane CENTRAL, MEDLINE and EMBASE), and in other cases simply listing bibliographic database available to search. Studies have explored the value in searching specific bibliographic databases, with Wright et al. (2015) noting the contribution of CINAHL in identifying qualitative studies [ 76 ], Beckles et al. (2013) questioning the contribution of CINAHL to identifying clinical studies for guideline development [ 77 ], and Cooper et al. (2015) exploring the role of UK-focused bibliographic databases to identify UK-relevant studies [ 78 ]. The host of the database (e.g. OVID or ProQuest) has been shown to alter the search returns offered. Younger and Boddy [ 79 ] report differing search returns from the same database (AMED) but where the ‘host’ was different [ 79 ].
The average number of bibliographic database searched in systematic reviews has risen in the period 1994–2014 (from 1 to 4) [ 80 ] but there remains (as attested to by the guidance) no consensus on what constitutes an acceptable number of databases searched [ 48 ]. This is perhaps because thinking about the number of databases searched is the wrong question, researchers should be focused on which databases were searched and why, and which databases were not searched and why. The discussion should re-orientate to the differential value of sources but researchers need to think about how to report this in studies to allow findings to be generalised. Bethel (2017) has proposed ‘search summaries’, completed by the literature searcher, to record where included studies were identified, whether from database (and which databases specifically) or supplementary search methods [ 81 ]. Search summaries document both yield and accuracy of searches, which could prospectively inform resource use and decisions to search or not to search specific databases in topic areas. The prospective use of such data presupposes, however, that past searches are a potential predictor of future search performance (i.e. that each topic is to be considered representative and not unique). In offering a body of practice, this data would be of greater practicable use than current studies which are considered as little more than individual case studies [ 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 ].
When to database search is another question posed in the literature. Beyer et al. [ 91 ] report that databases can be prioritised for literature searching which, whilst not addressing the question of which databases to search, may at least bring clarity as to which databases to search first [ 91 ]. Paradoxically, this links to studies that suggest PubMed should be searched in addition to MEDLINE (OVID interface) since this improves the currency of systematic reviews [ 92 , 93 ]. Cooper et al. (2017) have tested the idea of database searching not as a primary search method (as suggested in the guidance) but as a supplementary search method in order to manage the volume of studies identified for an environmental effectiveness systematic review. Their case study compared the effectiveness of database searching versus a protocol using supplementary search methods and found that the latter identified more relevant studies for review than searching bibliographic databases [ 94 ].
Key stage six: Determining the process of literature searching and deciding where to search (supplementary search methods)
Table 2 also summaries the process of literature searching which follows bibliographic database searching. As Table 2 sets out, guidance that supplementary literature search methods should be used in systematic reviews recurs across documents, but the order in which these methods are used, and the extent to which they are used, varies. We noted inconsistency in the labelling of supplementary search methods between guidance documents.
Rather than focus on the guidance on how to use the methods (which has been summarised in a recent review [ 95 ]), we focus on the aim or purpose of supplementary search methods.
The Cochrane Handbook reported that ‘efforts’ to identify unpublished studies should be made [ 9 ]. Four guidance documents [ 2 , 3 , 6 , 9 ] acknowledged that searching beyond bibliographic databases was necessary since ‘databases are not the only source of literature’ [ 2 ]. Only one document reported any guidance on determining when to use supplementary methods. The IQWiG handbook reported that the use of handsearching (in their example) could be determined on a ‘case-by-case basis’ which implies that the use of these methods is optional rather than mandatory. This is in contrast to the guidance (above) on bibliographic database searching.
The issue for supplementary search methods is similar in many ways to the issue of searching bibliographic databases: demonstrating value. The purpose and contribution of supplementary search methods in systematic reviews is increasingly acknowledged [ 37 , 61 , 62 , 96 , 97 , 98 , 99 , 100 , 101 ] but understanding the value of the search methods to identify studies and data is unclear. In a recently published review, Cooper et al. (2017) reviewed the literature on supplementary search methods looking to determine the advantages, disadvantages and resource implications of using supplementary search methods [ 95 ]. This review also summarises the key guidance and empirical studies and seeks to address the question on when to use these search methods and when not to [ 95 ]. The guidance is limited in this regard and, as Table 2 demonstrates, offers conflicting advice on the order of searching, and the extent to which these search methods should be used in systematic reviews.
Key stage seven: Managing the references
Five of the documents provided guidance on managing references, for example downloading, de-duplicating and managing the output of literature searches [ 2 , 4 , 6 , 8 , 10 ]. This guidance typically itemised available bibliographic management tools rather than offering guidance on how to use them specifically [ 2 , 4 , 6 , 8 ]. The CEE handbook provided guidance on importing data where no direct export option is available (e.g. web-searching) [ 10 ].
The literature on using bibliographic management tools is not large relative to the number of ‘how to’ videos on platforms such as YouTube (see for example [ 102 ]). These YouTube videos confirm the overall lack of ‘how to’ guidance identified in this study and offer useful instruction on managing references. Bramer et al. set out methods for de-duplicating data and reviewing references in Endnote [ 103 , 104 ] and Gall tests the direct search function within Endnote to access databases such as PubMed, finding a number of limitations [ 105 ]. Coar et al. and Ahmed et al. consider the role of the free-source tool, Zotero [ 106 , 107 ]. Managing references is a key administrative function in the process of review particularly for documenting searches in PRISMA guidance.
Key stage eight: Documenting the search
The Cochrane Handbook was the only guidance document to recommend a specific reporting guideline: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 9 ]. Six documents provided guidance on reporting the process of literature searching with specific criteria to report [ 3 , 4 , 6 , 8 , 9 , 10 ]. There was consensus on reporting: the databases searched (and the host searched by), the search strategies used, and any use of limits (e.g. date, language, search filters (The CRD handbook called for these limits to be justified [ 6 ])). Three guidance documents reported that the number of studies identified should be recorded [ 3 , 6 , 10 ]. The number of duplicates identified [ 10 ], the screening decisions [ 3 ], a comprehensive list of grey literature sources searched (and full detail for other supplementary search methods) [ 8 ], and an annotation of search terms tested but not used [ 4 ] were identified as unique items in four documents.
The Cochrane Handbook was the only guidance document to note that the full search strategies for each database should be included in the Additional file 1 of the review [ 9 ].
All guidance documents should ultimately deliver completed systematic reviews that fulfil the requirements of the PRISMA reporting guidelines [ 108 ]. The guidance broadly requires the reporting of data that corresponds with the requirements of the PRISMA statement although documents typically ask for diverse and additional items [ 108 ]. In 2008, Sampson et al. observed a lack of consensus on reporting search methods in systematic reviews [ 109 ] and this remains the case as of 2017, as evidenced in the guidance documents, and in spite of the publication of the PRISMA guidelines in 2009 [ 110 ]. It is unclear why the collective guidance does not more explicitly endorse adherence to the PRISMA guidance.
Reporting of literature searching is a key area in systematic reviews since it sets out clearly what was done and how the conclusions of the review can be believed [ 52 , 109 ]. Despite strong endorsement in the guidance documents, specifically supported in PRISMA guidance, and other related reporting standards too (such as ENTREQ for qualitative evidence synthesis, STROBE for reviews of observational studies), authors still highlight the prevalence of poor standards of literature search reporting [ 31 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 ]. To explore issues experienced by authors in reporting literature searches, and look at uptake of PRISMA, Radar et al. [ 120 ] surveyed over 260 review authors to determine common problems and their work summaries the practical aspects of reporting literature searching [ 120 ]. Atkinson et al. [ 121 ] have also analysed reporting standards for literature searching, summarising recommendations and gaps for reporting search strategies [ 121 ].
One area that is less well covered by the guidance, but nevertheless appears in this literature, is the quality appraisal or peer review of literature search strategies. The PRESS checklist is the most prominent and it aims to develop evidence-based guidelines to peer review of electronic search strategies [ 5 , 122 , 123 ]. A corresponding guideline for documentation of supplementary search methods does not yet exist although this idea is currently being explored.
How the reporting of the literature searching process corresponds to critical appraisal tools is an area for further research. In the survey undertaken by Radar et al. (2014), 86% of survey respondents (153/178) identified a need for further guidance on what aspects of the literature search process to report [ 120 ]. The PRISMA statement offers a brief summary of what to report but little practical guidance on how to report it [ 108 ]. Critical appraisal tools for systematic reviews, such as AMSTAR 2 (Shea et al. [ 124 ]) and ROBIS (Whiting et al. [ 125 ]), can usefully be read alongside PRISMA guidance, since they offer greater detail on how the reporting of the literature search will be appraised and, therefore, they offer a proxy on what to report [ 124 , 125 ]. Further research in the form of a study which undertakes a comparison between PRISMA and quality appraisal checklists for systematic reviews would seem to begin addressing the call, identified by Radar et al., for further guidance on what to report [ 120 ].
Limitations
Other handbooks exist.
A potential limitation of this literature review is the focus on guidance produced in Europe (the UK specifically) and Australia. We justify the decision for our selection of the nine guidance documents reviewed in this literature review in section “ Identifying guidance ”. In brief, these nine guidance documents were selected as the most relevant health care guidance that inform UK systematic reviewing practice, given that the UK occupies a prominent position in the science of health information retrieval. We acknowledge the existence of other guidance documents, such as those from North America (e.g. the Agency for Healthcare Research and Quality (AHRQ) [ 126 ], The Institute of Medicine [ 127 ] and the guidance and resources produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 128 ]). We comment further on this directly below.
The handbooks are potentially linked to one another
What is not clear is the extent to which the guidance documents inter-relate or provide guidance uniquely. The Cochrane Handbook, first published in 1994, is notably a key source of reference in guidance and systematic reviews beyond Cochrane reviews. It is not clear to what extent broadening the sample of guidance handbooks to include North American handbooks, and guidance handbooks from other relevant countries too, would alter the findings of this literature review or develop further support for the process model. Since we cannot be clear, we raise this as a potential limitation of this literature review. On our initial review of a sample of North American, and other, guidance documents (before selecting the guidance documents considered in this review), however, we do not consider that the inclusion of these further handbooks would alter significantly the findings of this literature review.
This is a literature review
A further limitation of this review was that the review of published studies is not a systematic review of the evidence for each key stage. It is possible that other relevant studies could help contribute to the exploration and development of the key stages identified in this review.
This literature review would appear to demonstrate the existence of a shared model of the literature searching process in systematic reviews. We call this model ‘the conventional approach’, since it appears to be common convention in nine different guidance documents.
The findings reported above reveal eight key stages in the process of literature searching for systematic reviews. These key stages are consistently reported in the nine guidance documents which suggests consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews.
In Table 2 , we demonstrate consensus regarding the application of literature search methods. All guidance documents distinguish between primary and supplementary search methods. Bibliographic database searching is consistently the first method of literature searching referenced in each guidance document. Whilst the guidance uniformly supports the use of supplementary search methods, there is little evidence for a consistent process with diverse guidance across documents. This may reflect differences in the core focus across each document, linked to differences in identifying effectiveness studies or qualitative studies, for instance.
Eight of the nine guidance documents reported on the aims of literature searching. The shared understanding was that literature searching should be thorough and comprehensive in its aim and that this process should be reported transparently so that that it could be reproduced. Whilst only three documents explicitly link this understanding to minimising bias, it is clear that comprehensive literature searching is implicitly linked to ‘not missing relevant studies’ which is approximately the same point.
Defining the key stages in this review helps categorise the scholarship available, and it prioritises areas for development or further study. The supporting studies on preparing for literature searching (key stage three, ‘preparation’) were, for example, comparatively few, and yet this key stage represents a decisive moment in literature searching for systematic reviews. It is where search strategy structure is determined, search terms are chosen or discarded, and the resources to be searched are selected. Information specialists, librarians and researchers, are well placed to develop these and other areas within the key stages we identify.
This review calls for further research to determine the suitability of using the conventional approach. The publication dates of the guidance documents which underpin the conventional approach may raise questions as to whether the process which they each report remains valid for current systematic literature searching. In addition, it may be useful to test whether it is desirable to use the same process model of literature searching for qualitative evidence synthesis as that for reviews of intervention effectiveness, which this literature review demonstrates is presently recommended best practice.
Abbreviations
Behaviour of interest; Health context; Exclusions; Models or Theories
Cochrane Database of Systematic Reviews
The Cochrane Central Register of Controlled Trials
Database of Abstracts of Reviews of Effects
Enhancing transparency in reporting the synthesis of qualitative research
Institute for Quality and Efficiency in Healthcare
National Institute for Clinical Excellence
Population, Intervention, Comparator, Outcome
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Setting, Perspective, Intervention, Comparison, Evaluation
Sample, Phenomenon of Interest, Design, Evaluation, Research type
STrengthening the Reporting of OBservational studies in Epidemiology
Trial Search Co-ordinators
Booth A. Unpacking your literature search toolbox: on search styles and tactics. Health Information & Libraries Journal. 2008;25(4):313–7.
Article Google Scholar
Petticrew M, Roberts H. Systematic reviews in the social sciences: a practical guide. Oxford: Blackwell Publishing Ltd; 2006.
Book Google Scholar
Institute for Quality and Efficiency in Health Care (IQWiG). IQWiG Methods Resources. 7 Information retrieval 2014 [Available from: https://www.ncbi.nlm.nih.gov/books/NBK385787/ .
NICE: National Institute for Health and Care Excellence. Developing NICE guidelines: the manual 2014. Available from: https://www.nice.org.uk/media/default/about/what-we-do/our-programmes/developing-nice-guidelines-the-manual.pdf .
Sampson M. MJ, Lefebvre C, Moher D, Grimshaw J. Peer Review of Electronic Search Strategies: PRESS; 2008.
Google Scholar
Centre for Reviews & Dissemination. Systematic reviews – CRD’s guidance for undertaking reviews in healthcare. York: Centre for Reviews and Dissemination, University of York; 2009.
eunetha: European Network for Health Technology Assesment Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness 2016. Available from: http://www.eunethta.eu/sites/default/files/Guideline_Information_Retrieval_V1-1.pdf .
Kugley SWA, Thomas J, Mahood Q, Jørgensen AMK, Hammerstrøm K, Sathe N. Searching for studies: a guide to information retrieval for Campbell systematic reviews. Oslo: Campbell Collaboration. 2017; Available from: https://www.campbellcollaboration.org/library/searching-for-studies-information-retrieval-guide-campbell-reviews.html
Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. In: JPT H, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions; 2011.
Collaboration for Environmental Evidence. Guidelines for Systematic Review and Evidence Synthesis in Environmental Management.: Environmental Evidence:; 2013. Available from: http://www.environmentalevidence.org/wp-content/uploads/2017/01/Review-guidelines-version-4.2-final-update.pdf .
The Joanna Briggs Institute. Joanna Briggs institute reviewers’ manual. 2014th ed: the Joanna Briggs institute; 2014. Available from: https://joannabriggs.org/assets/docs/sumari/ReviewersManual-2014.pdf
Beverley CA, Booth A, Bath PA. The role of the information specialist in the systematic review process: a health information case study. Health Inf Libr J. 2003;20(2):65–74.
Article CAS Google Scholar
Harris MR. The librarian's roles in the systematic review process: a case study. Journal of the Medical Library Association. 2005;93(1):81–7.
PubMed PubMed Central Google Scholar
Egger JB. Use of recommended search strategies in systematic reviews and the impact of librarian involvement: a cross-sectional survey of recent authors. PLoS One. 2015;10(5):e0125931.
Li L, Tian J, Tian H, Moher D, Liang F, Jiang T, et al. Network meta-analyses could be improved by searching more sources and by involving a librarian. J Clin Epidemiol. 2014;67(9):1001–7.
Article PubMed Google Scholar
McGowan J, Sampson M. Systematic reviews need systematic searchers. J Med Libr Assoc. 2005;93(1):74–80.
Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ. Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol. 2015;68(6):617–26.
Weller AC. Mounting evidence that librarians are essential for comprehensive literature searches for meta-analyses and Cochrane reports. J Med Libr Assoc. 2004;92(2):163–4.
Swinkels A, Briddon J, Hall J. Two physiotherapists, one librarian and a systematic literature review: collaboration in action. Health Info Libr J. 2006;23(4):248–56.
Foster M. An overview of the role of librarians in systematic reviews: from expert search to project manager. EAHIL. 2015;11(3):3–7.
Lawson L. OPERATING OUTSIDE LIBRARY WALLS 2004.
Vassar M, Yerokhin V, Sinnett PM, Weiher M, Muckelrath H, Carr B, et al. Database selection in systematic reviews: an insight through clinical neurology. Health Inf Libr J. 2017;34(2):156–64.
Townsend WA, Anderson PF, Ginier EC, MacEachern MP, Saylor KM, Shipman BL, et al. A competency framework for librarians involved in systematic reviews. Journal of the Medical Library Association : JMLA. 2017;105(3):268–75.
Cooper ID, Crum JA. New activities and changing roles of health sciences librarians: a systematic review, 1990-2012. Journal of the Medical Library Association : JMLA. 2013;101(4):268–77.
Crum JA, Cooper ID. Emerging roles for biomedical librarians: a survey of current practice, challenges, and changes. Journal of the Medical Library Association : JMLA. 2013;101(4):278–86.
Dudden RF, Protzko SL. The systematic review team: contributions of the health sciences librarian. Med Ref Serv Q. 2011;30(3):301–15.
Golder S, Loke Y, McIntosh HM. Poor reporting and inadequate searches were apparent in systematic reviews of adverse effects. J Clin Epidemiol. 2008;61(5):440–8.
Maggio LA, Tannery NH, Kanter SL. Reproducibility of literature search reporting in medical education reviews. Academic medicine : journal of the Association of American Medical Colleges. 2011;86(8):1049–54.
Meert D, Torabi N, Costella J. Impact of librarians on reporting of the literature searching component of pediatric systematic reviews. Journal of the Medical Library Association : JMLA. 2016;104(4):267–77.
Morris M, Boruff JT, Gore GC. Scoping reviews: establishing the role of the librarian. Journal of the Medical Library Association : JMLA. 2016;104(4):346–54.
Koffel JB, Rethlefsen ML. Reproducibility of search strategies is poor in systematic reviews published in high-impact pediatrics, cardiology and surgery journals: a cross-sectional study. PLoS One. 2016;11(9):e0163309.
Article PubMed PubMed Central CAS Google Scholar
Fehrmann P, Thomas J. Comprehensive computer searches and reporting in systematic reviews. Research Synthesis Methods. 2011;2(1):15–32.
Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Systematic Reviews. 2016;5(1):74.
Article PubMed PubMed Central Google Scholar
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health technology assessment (Winchester, England). 2003;7(1):1–76.
Tricco AC, Tetzlaff J, Sampson M, Fergusson D, Cogo E, Horsley T, et al. Few systematic reviews exist documenting the extent of bias: a systematic review. J Clin Epidemiol. 2008;61(5):422–34.
Booth A. How much searching is enough? Comprehensive versus optimal retrieval for technology assessments. Int J Technol Assess Health Care. 2010;26(4):431–5.
Papaioannou D, Sutton A, Carroll C, Booth A, Wong R. Literature searching for social science systematic reviews: consideration of a range of search techniques. Health Inf Libr J. 2010;27(2):114–22.
Petticrew M. Time to rethink the systematic review catechism? Moving from ‘what works’ to ‘what happens’. Systematic Reviews. 2015;4(1):36.
Betrán AP, Say L, Gülmezoglu AM, Allen T, Hampson L. Effectiveness of different databases in identifying studies for systematic reviews: experience from the WHO systematic review of maternal morbidity and mortality. BMC Med Res Methodol. 2005;5
Felson DT. Bias in meta-analytic research. J Clin Epidemiol. 1992;45(8):885–92.
Article PubMed CAS Google Scholar
Franco A, Malhotra N, Simonovits G. Publication bias in the social sciences: unlocking the file drawer. Science. 2014;345(6203):1502–5.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med Res Methodol. 2017;17(1):64.
Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017;12(4):e0176210.
Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet (London, England). 1997;350(9074):326–9.
Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health technology assessment (Winchester, England). 2003;7(41):1–90.
Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol. 2005;58(8):769–76.
Mills EJ, Kanters S, Thorlund K, Chaimani A, Veroniki A-A, Ioannidis JPA. The effects of excluding treatments from network meta-analyses: survey. BMJ : British Medical Journal. 2013;347
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. The contribution of databases to the results of systematic reviews: a cross-sectional study. BMC Med Res Methodol. 2016;16(1):127.
van Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epidemiol. 2009;62(8):838–44.e3.
Buchberger B, Krabbe L, Lux B, Mattivi JT. Evidence mapping for decision making: feasibility versus accuracy - when to abandon high sensitivity in electronic searches. German medical science : GMS e-journal. 2016;14:Doc09.
Lorenc T, Pearson M, Jamal F, Cooper C, Garside R. The role of systematic reviews of qualitative evidence in evaluating interventions: a case study. Research Synthesis Methods. 2012;3(1):1–10.
Gough D. Weight of evidence: a framework for the appraisal of the quality and relevance of evidence. Res Pap Educ. 2007;22(2):213–28.
Barroso J, Gollop CJ, Sandelowski M, Meynell J, Pearce PF, Collins LJ. The challenges of searching for and retrieving qualitative studies. West J Nurs Res. 2003;25(2):153–78.
Britten N, Garside R, Pope C, Frost J, Cooper C. Asking more of qualitative synthesis: a response to Sally Thorne. Qual Health Res. 2017;27(9):1370–6.
Booth A, Carroll C. Systematic searching for theory to inform systematic reviews: is it feasible? Is it desirable? Health Info Libr J. 2015;32(3):220–35.
Kwon Y, Powelson SE, Wong H, Ghali WA, Conly JM. An assessment of the efficacy of searching in biomedical databases beyond MEDLINE in identifying studies for a systematic review on ward closures as an infection control intervention to control outbreaks. Syst Rev. 2014;3:135.
Nussbaumer-Streit B, Klerings I, Wagner G, Titscher V, Gartlehner G. Assessing the validity of abbreviated literature searches for rapid reviews: protocol of a non-inferiority and meta-epidemiologic study. Systematic Reviews. 2016;5:197.
Wagner G, Nussbaumer-Streit B, Greimel J, Ciapponi A, Gartlehner G. Trading certainty for speed - how much uncertainty are decisionmakers and guideline developers willing to accept when using rapid reviews: an international survey. BMC Med Res Methodol. 2017;17(1):121.
Ogilvie D, Hamilton V, Egan M, Petticrew M. Systematic reviews of health effects of social interventions: 1. Finding the evidence: how far should you go? J Epidemiol Community Health. 2005;59(9):804–8.
Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess Health Care. 2003;19(4):591–603.
Pearson M, Moxham T, Ashton K. Effectiveness of search strategies for qualitative research about barriers and facilitators of program delivery. Eval Health Prof. 2011;34(3):297–308.
Levay P, Raynor M, Tuvey D. The Contributions of MEDLINE, Other Bibliographic Databases and Various Search Techniques to NICE Public Health Guidance. 2015. 2015;10(1):19.
Nussbaumer-Streit B, Klerings I, Wagner G, Heise TL, Dobrescu AI, Armijo-Olivo S, et al. Abbreviated literature searches were viable alternatives to comprehensive searches: a meta-epidemiological study. J Clin Epidemiol. 2018;102:1–11.
Briscoe S, Cooper C, Glanville J, Lefebvre C. The loss of the NHS EED and DARE databases and the effect on evidence synthesis and evaluation. Res Synth Methods. 2017;8(3):256–7.
Stansfield C, O'Mara-Eves A, Thomas J. Text mining for search term development in systematic reviewing: A discussion of some methods and challenges. Research Synthesis Methods.n/a-n/a.
Petrova M, Sutcliffe P, Fulford KW, Dale J. Search terms and a validated brief search filter to retrieve publications on health-related values in Medline: a word frequency analysis study. Journal of the American Medical Informatics Association : JAMIA. 2012;19(3):479–88.
Stansfield C, Thomas J, Kavanagh J. 'Clustering' documents automatically to support scoping reviews of research: a case study. Res Synth Methods. 2013;4(3):230–41.
PubMed Google Scholar
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Andrew B. Clear and present questions: formulating questions for evidence based practice. Library Hi Tech. 2006;24(3):355–68.
Cooke A, Smith D, Booth A. Beyond PICO: the SPIDER tool for qualitative evidence synthesis. Qual Health Res. 2012;22(10):1435–43.
Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al. Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model. Health technology assessment (Winchester, England). 2006;10(36):iii-iv, xi-xiii, 1–154.
Cooper C, Levay P, Lorenc T, Craig GM. A population search filter for hard-to-reach populations increased search efficiency for a systematic review. J Clin Epidemiol. 2014;67(5):554–9.
Hausner E, Waffenschmidt S, Kaiser T, Simon M. Routine development of objectively derived search strategies. Systematic Reviews. 2012;1(1):19.
Hausner E, Guddat C, Hermanns T, Lampert U, Waffenschmidt S. Prospective comparison of search strategies for systematic reviews: an objective approach yielded higher sensitivity than a conceptual one. J Clin Epidemiol. 2016;77:118–24.
Craven J, Levay P. Recording database searches for systematic reviews - what is the value of adding a narrative to peer-review checklists? A case study of nice interventional procedures guidance. Evid Based Libr Inf Pract. 2011;6(4):72–87.
Wright K, Golder S, Lewis-Light K. What value is the CINAHL database when searching for systematic reviews of qualitative studies? Syst Rev. 2015;4:104.
Beckles Z, Glover S, Ashe J, Stockton S, Boynton J, Lai R, et al. Searching CINAHL did not add value to clinical questions posed in NICE guidelines. J Clin Epidemiol. 2013;66(9):1051–7.
Cooper C, Rogers M, Bethel A, Briscoe S, Lowe J. A mapping review of the literature on UK-focused health and social care databases. Health Inf Libr J. 2015;32(1):5–22.
Younger P, Boddy K. When is a search not a search? A comparison of searching the AMED complementary health database via EBSCOhost, OVID and DIALOG. Health Inf Libr J. 2009;26(2):126–35.
Lam MT, McDiarmid M. Increasing number of databases searched in systematic reviews and meta-analyses between 1994 and 2014. Journal of the Medical Library Association : JMLA. 2016;104(4):284–9.
Bethel A, editor Search summary tables for systematic reviews: results and findings. HLC Conference 2017a.
Aagaard T, Lund H, Juhl C. Optimizing literature search in systematic reviews - are MEDLINE, EMBASE and CENTRAL enough for identifying effect studies within the area of musculoskeletal disorders? BMC Med Res Methodol. 2016;16(1):161.
Adams CE, Frederick K. An investigation of the adequacy of MEDLINE searches for randomized controlled trials (RCTs) of the effects of mental health care. Psychol Med. 1994;24(3):741–8.
Kelly L, St Pierre-Hansen N. So many databases, such little clarity: searching the literature for the topic aboriginal. Canadian family physician Medecin de famille canadien. 2008;54(11):1572–3.
Lawrence DW. What is lost when searching only one literature database for articles relevant to injury prevention and safety promotion? Injury Prevention. 2008;14(6):401–4.
Lemeshow AR, Blum RE, Berlin JA, Stoto MA, Colditz GA. Searching one or two databases was insufficient for meta-analysis of observational studies. J Clin Epidemiol. 2005;58(9):867–73.
Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, et al. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol. 2003;56(10):943–55.
Stevinson C, Lawlor DA. Searching multiple databases for systematic reviews: added value or diminishing returns? Complementary Therapies in Medicine. 2004;12(4):228–32.
Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Control Clin Trials. 2000;21(5):476–87.
Taylor B, Wylie E, Dempster M, Donnelly M. Systematically retrieving research: a case study evaluating seven databases. Res Soc Work Pract. 2007;17(6):697–706.
Beyer FR, Wright K. Can we prioritise which databases to search? A case study using a systematic review of frozen shoulder management. Health Info Libr J. 2013;30(1):49–58.
Duffy S, de Kock S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE in-process searches via Ovid. Journal of the Medical Library Association : JMLA. 2016;104(4):309–12.
Katchamart W, Faulkner A, Feldman B, Tomlinson G, Bombardier C. PubMed had a higher sensitivity than Ovid-MEDLINE in the search for systematic reviews. J Clin Epidemiol. 2011;64(7):805–7.
Cooper C, Lovell R, Husk K, Booth A, Garside R. Supplementary search methods were more effective and offered better value than bibliographic database searching: a case study from public health and environmental enhancement (in Press). Research Synthesis Methods. 2017;
Cooper C, Booth, A., Britten, N., Garside, R. A comparison of results of empirical studies of supplementary search techniques and recommendations in review methodology handbooks: A methodological review. (In Press). BMC Systematic Reviews. 2017.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ (Clinical research ed). 2005;331(7524):1064–5.
Article PubMed Central Google Scholar
Hinde S, Spackman E. Bidirectional citation searching to completion: an exploration of literature searching methods. PharmacoEconomics. 2015;33(1):5–11.
Levay P, Ainsworth N, Kettle R, Morgan A. Identifying evidence for public health guidance: a comparison of citation searching with web of science and Google scholar. Res Synth Methods. 2016;7(1):34–45.
McManus RJ, Wilson S, Delaney BC, Fitzmaurice DA, Hyde CJ, Tobias RS, et al. Review of the usefulness of contacting other experts when conducting a literature search for systematic reviews. BMJ (Clinical research ed). 1998;317(7172):1562–3.
Westphal A, Kriston L, Holzel LP, Harter M, von Wolff A. Efficiency and contribution of strategies for finding randomized controlled trials: a case study from a systematic review on therapeutic interventions of chronic depression. Journal of public health research. 2014;3(2):177.
Matthews EJ, Edwards AG, Barker J, Bloor M, Covey J, Hood K, et al. Efficient literature searching in diffuse topics: lessons from a systematic review of research on communicating risk to patients in primary care. Health Libr Rev. 1999;16(2):112–20.
Bethel A. Endnote Training (YouTube Videos) 2017b [Available from: http://medicine.exeter.ac.uk/esmi/workstreams/informationscience/is_resources,_guidance_&_advice/ .
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association : JMLA. 2016;104(3):240–3.
Bramer WM, Milic J, Mast F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. Journal of the Medical Library Association : JMLA. 2017;105(1):84–7.
Gall C, Brahmi FA. Retrieval comparison of EndNote to search MEDLINE (Ovid and PubMed) versus searching them directly. Medical reference services quarterly. 2004;23(3):25–32.
Ahmed KK, Al Dhubaib BE. Zotero: a bibliographic assistant to researcher. J Pharmacol Pharmacother. 2011;2(4):303–5.
Coar JT, Sewell JP. Zotero: harnessing the power of a personal bibliographic manager. Nurse Educ. 2010;35(5):205–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Sampson M, McGowan J, Tetzlaff J, Cogo E, Moher D. No consensus exists on search reporting methods for systematic reviews. J Clin Epidemiol. 2008;61(8):748–54.
Toews LC. Compliance of systematic reviews in veterinary journals with preferred reporting items for systematic reviews and meta-analysis (PRISMA) literature search reporting guidelines. Journal of the Medical Library Association : JMLA. 2017;105(3):233–9.
Booth A. "brimful of STARLITE": toward standards for reporting literature searches. Journal of the Medical Library Association : JMLA. 2006;94(4):421–9. e205
Faggion CM Jr, Wu YC, Tu YK, Wasiak J. Quality of search strategies reported in systematic reviews published in stereotactic radiosurgery. Br J Radiol. 2016;89(1062):20150878.
Mullins MM, DeLuca JB, Crepaz N, Lyles CM. Reporting quality of search methods in systematic reviews of HIV behavioral interventions (2000–2010): are the searches clearly explained, systematic and reproducible? Research Synthesis Methods. 2014;5(2):116–30.
Yoshii A, Plaut DA, McGraw KA, Anderson MJ, Wellik KE. Analysis of the reporting of search strategies in Cochrane systematic reviews. Journal of the Medical Library Association : JMLA. 2009;97(1):21–9.
Bigna JJ, Um LN, Nansseu JR. A comparison of quality of abstracts of systematic reviews including meta-analysis of randomized controlled trials in high-impact general medicine journals before and after the publication of PRISMA extension for abstracts: a systematic review and meta-analysis. Syst Rev. 2016;5(1):174.
Akhigbe T, Zolnourian A, Bulters D. Compliance of systematic reviews articles in brain arteriovenous malformation with PRISMA statement guidelines: review of literature. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017;39:45–8.
Tao KM, Li XQ, Zhou QH, Moher D, Ling CQ, Yu WF. From QUOROM to PRISMA: a survey of high-impact medical journals' instructions to authors and a review of systematic reviews in anesthesia literature. PLoS One. 2011;6(11):e27611.
Wasiak J, Tyack Z, Ware R. Goodwin N. Jr. Poor methodological quality and reporting standards of systematic reviews in burn care management. International wound journal: Faggion CM; 2016.
Tam WW, Lo KK, Khalechelvam P. Endorsement of PRISMA statement and quality of systematic reviews and meta-analyses published in nursing journals: a cross-sectional study. BMJ Open. 2017;7(2):e013905.
Rader T, Mann M, Stansfield C, Cooper C, Sampson M. Methods for documenting systematic review searches: a discussion of common issues. Res Synth Methods. 2014;5(2):98–115.
Atkinson KM, Koenka AC, Sanchez CE, Moshontz H, Cooper H. Reporting standards for literature searches and report inclusion criteria: making research syntheses more transparent and easy to replicate. Res Synth Methods. 2015;6(1):87–95.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–52.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). 2017;358.
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Relevo R, Balshem H. Finding evidence for comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. 2011;64(11):1168–77.
Medicine Io. Standards for Systematic Reviews 2011 [Available from: http://www.nationalacademies.org/hmd/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews/Standards.aspx .
CADTH: Resources 2018.
Download references
Acknowledgements
CC acknowledges the supervision offered by Professor Chris Hyde.
This publication forms a part of CC’s PhD. CC’s PhD was funded through the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Project Number 16/54/11). The open access fee for this publication was paid for by Exeter Medical School.
RG and NB were partially supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and affiliations.
Institute of Health Research, University of Exeter Medical School, Exeter, UK
Chris Cooper & Jo Varley-Campbell
HEDS, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
Andrew Booth
Nicky Britten
European Centre for Environment and Human Health, University of Exeter Medical School, Truro, UK
Ruth Garside
You can also search for this author in PubMed Google Scholar
Contributions
CC conceived the idea for this study and wrote the first draft of the manuscript. CC discussed this publication in PhD supervision with AB and separately with JVC. CC revised the publication with input and comments from AB, JVC, RG and NB. All authors revised the manuscript prior to submission. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Chris Cooper .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Appendix tables and PubMed search strategy. Key studies used for pearl growing per key stage, working data extraction tables and the PubMed search strategy. (DOCX 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Cooper, C., Booth, A., Varley-Campbell, J. et al. Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies. BMC Med Res Methodol 18 , 85 (2018). https://doi.org/10.1186/s12874-018-0545-3
Download citation
Received : 20 September 2017
Accepted : 06 August 2018
Published : 14 August 2018
DOI : https://doi.org/10.1186/s12874-018-0545-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Literature Search Process
- Citation Chasing
- Tacit Models
- Unique Guidance
- Information Specialists
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
- Franklin University |
- Help & Support |
- Locations & Maps |

- | Research Guides
To access Safari eBooks,
- Select not listed in the Select Your Institution drop down menu.
- Enter your Franklin email address and click Go
- click "Already a user? Click here" link
- Enter your Franklin email and the password you used to create your Safari account.
Continue Close
Literature Review
- Getting Started
- Framing the Literature Review
Literature Review Process
- Mistakes to Avoid & Additional Help
The structure of a literature review should include the following :
- An overview of the subject, issue or theory under consideration, along with the objectives of the literature review,
- Division of works under review into themes or categories (e.g. works that support of a particular position, those against, and those offering alternative approaches entirely),
- An explanation of how each work is similar to and how it varies from the others,
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research
The critical evaluation of each work should consider :
- Provenance -- what are the author's credentials? Are the author's arguments supported by evidence (e.g. primary historical material, case studies, narratives, statistics, recent scientific findings)?
- Objectivity -- is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
- Persuasiveness -- which of the author's theses are most/least convincing?
- Value -- are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
Development of the Literature Review
Four stages:.
- Introduce the reader to the importance of the topic being studied . The reader is oriented to the significance of the study and the research questions or hypotheses to follow.
- Places the problem into a particular context that defines the parameters of what is to be investigated.
- Provides the framework for reporting the results and indicates what is probably necessary to conduct the study and explain how the findings will present this information.
- Literature search -- finding materials relevant to the subject being explored.
- Evaluation of resources -- determining which literature makes a significant contribution to the understanding of the topic.
- Analysis and interpretation -- discussing the findings and conclusions of pertinent literature.
Consider the following issues before writing the literature review:
Sources and expectations. if your assignment is not very specific about what form your literature review should take, seek clarification from your professor by asking these questions:.
- Roughly how many sources should I include?
- What types of sources should I review (books, journal articles, websites)?
- Should I summarize, synthesize, or critique your sources by discussing a common theme or issue?
- Should I evaluate the sources?
- Should I provide subheadings and other background information, such as definitions and/or a history?

Find Models. When reviewing the current literature, examine how authors in your discipline or area of interest have organized their literature reviews. Read not only for information, but also to get a sense of the types of themes you might want to look for in your own research review.
Narrow the topic. the narrower your topic, the easier it will be to limit the number of sources you need to read in order to obtain a good survey of relevant resources., consider whether your sources are current and applicable. s ome disciplines require that you use information that is as current as possible. this is very common in the sciences where research conducted only two years ago could be obsolete. however, when writing a review in the social sciences, a survey of the history of the literature may be what is needed because what is important is how perspectives have changed over the years or within a certain time period. try sorting through some other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. you can also use this method to consider what is consider by scholars to be a "hot topic" and what is not., follow the bread crumb trail. the bibliography or reference section of sources you read are excellent entry points for further exploration. you might find resourced listed in a bibliography that points you in the direction you wish to take your own research., ways to organize your literature review, chronologically: .
If your review follows the chronological method, you could write about the materials according to when they were published or the time period they cover.
By Publication:
Order your sources chronologically by publication date, only if the order demonstrates a more important trend. For instance, you could order a review of literature on environmental studies of brown fields if the progression revealed, for example, a change in the soil collection practices of the researchers who wrote and/or conducted the studies.
Conceptual Categories:
The literature review is organized around a topic or issue, rather than the progression of time. However, progression of time may still be an important factor in a thematic review. For example, a review of the Internet’s impact on American presidential politics could focus on the development of online political satire. While the study focuses on one topic, the Internet’s impact on American presidential politics, it will still be organized chronologically reflecting technological developments in media. The only difference here between a "chronological" and a "thematic" approach is what is emphasized the most.
Methodological:
A methodological approach focuses on the methods utilized by the researcher. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed.
Sections of Your Literature Review:
Once you've decided on the organizational method for your literature review, the sections you need to include should be easy to figure out because they arise from your organizational strategy.
Here are examples of other sections you may need to include depending on the type of review you write:
- Current Situation : information necessary to understand the topic or focus of the literature review.
- History : the chronological progression of the field, the literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Selection Methods : the criteria you used to select (and perhaps exclude) sources in your literature review. For instance, you might explain that your review includes only peer-reviewed articles and journals.
- Standards : the way in which you present your information.
- Questions for Further Research : What questions about the field has the review sparked? How will you further your research as a result of the review?
Writing Your Literature Review
Once you've settled on how to organize your literature review, you're ready to write each section. When writing your review, keep in mind these issues.
Use Evidence:
A literature review in this sense is just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence to show that what you are saying is valid.
Be Selective:
Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the research problem, whether it is thematic, methodological, or chronological.
Use Quotes Sparingly:
Some short quotes are okay if you want to emphasize a point, or if what the author said just cannot be rewritten in your own words. Sometimes you may need to quote certain terms that were coined by the author, not common knowledge, or taken directly from the study. Do not use extensive quotes as a substitute your own summary and interpretation of the literature.
Summarize and Synthesize:
Remember to summarize and synthesize your sources within each paragraph as well as throughout the review. Recapitulate important features of a research study, but then synthesize it by rephrasing the study's significance and relating it to their own work.
Keep Your Own Voice:
While the literature review presents others' ideas, your voice (the writer's) should remain front and center. For example, weave references to other sources into what you are writing but maintain your own voice by starting and ending the paragraph with your own ideas and wording.
Use Caution When Paraphrasing:
When paraphrasing a source that is not your own, be sure to represent the author's information or opinions accurately and in your own words. Even when paraphrasing an author’s work, you still must provide a citation to that work.
- << Previous: Getting Started
- Next: Mistakes to Avoid & Additional Help >>
- Last Updated: Oct 3, 2023 2:44 PM
- URL: https://guides.franklin.edu/LITREVIEW
- Skip to search box
- Skip to main content
- Princeton University Library
- Research Guides
- Writing Seminars
- Writing Seminar 163/164 And the Rest is Drag
- What is a Literature Review?
Writing Seminar 163/164 And the Rest is Drag: What is a Literature Review?
- Finding Books
- Finding Articles
- Specialized Databases
- Academic Integrity at Princeton This link opens in a new window
Finding Examples
It may be useful to look at other reviews to learn how researchers in the field "summarize and synthesize" the literature. Most research article or dissertation in the sciences will include a section which reviews the literature. Though the section may not be labeled as such, you will quickly recognize it by the number of citations and the discussion of the literature. Another option is to look for Review Articles, which are literature reviews as a stand alone article. Here are some resources where you can find Research Articles, Review Articles and Dissertations:
- Articles+ - Due to the interdisciplinary nature of gender & sexuality studies Articles+ can be a great place to start your research. Please make use of the filters on the left-hand side of the screen to help refine your searches.
- Gender Studies Database & LGBT Thought and Culture - Gender Studies Database & LGBT Though and Culture have a large corpus of reviews and research articles. As with Articles+ make sure to take advantage of the filters (type of publication, publication date) to help refine your searches.
- Google Scholar - Using the Cited By feature, hyperlinked below the search results, you can trace the scholarly conversation moving forward.
- Dissertations @ Princeton - Provides access to many Princeton dissertations, full text is available for most published after 1996.
- Purdue OWL - The Purdue OWL site provides tips and examples of literature reviews and is a great source for reviewing citation styles
*** Note about using Review Articles in your research - while they are useful in helping you to locate articles on your topic, remember that you must go to and use the original source if you intend to include a study mentioned in the review. The only time you would cite a review article is if they have made an original insight in their work that you talk about in your paper. Going to the original research paper allows you to verify the information about that study and determine whether the points made in the review are valid and accurate.
What is a literature review?
A literature review surveys scholarly articles, books and other sources relevant to a particular issue, area of research, or theory. The purpose is to offer an overview of significant literature published on a topic.
A literature review may constitute an essential chapter of a thesis or dissertation, or may be a self-contained review of writings on a subject. In either case, its purpose is to:
- Place each work in the context of its contribution to the understanding of the subject under review
- Describe the relationship of each work to the others under consideration
- Identify new ways to interpret, and shed light on any gaps in, previous research
- Resolve conflicts amongst seemingly contradictory previous studies
- Identify areas of prior scholarship to prevent duplication of effort
- Point the way forward for further research
- Place one's original work (in the case of theses or dissertations) in the context of existing literature
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information. It might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. And depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant.
Similar to primary research, development of the literature review requires four stages:
- Problem formulation—which topic or field is being examined and what are its component issues?
- Literature search—finding materials relevant to the subject being explored
- Data evaluation—determining which literature makes a significant contribution to the understanding of the topic
- Analysis and interpretation—discussing the findings and conclusions of pertinent literature
Remember, this is a process and not necessarily a linear one. As you search and evaluate the literature, you may refine your topic or head in a different direction which will take you back to the search stage. In fact, it is useful to evaluate as you go along so you don't spend hours researching one aspect of your topic only to find yourself more interested in another.
The main focus of an academic research paper is to develop a new argument, and a research paper will contain a literature review as one of its parts. In a research paper, you use the literature as a foundation and as support for a new insight that you contribute. The focus of a literature review, however, is to summarize and synthesize the arguments and ideas of others without adding new contributions.

- << Previous: Specialized Databases
- Next: Academic Integrity at Princeton >>
- Subjects: Writing Program
- Last Updated: Jan 30, 2024 2:56 PM
- URL: https://libguides.princeton.edu/andtherestisdrag
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Anaesth
- v.63(8); 2019 Aug
Formulating a good research question: Pearls and pitfalls
Wilson fandino.
Guys' and St Thomas' Hospital National Health Service Foundation Trust, London, United Kingdom
The process of formulating a good research question can be challenging and frustrating. While a comprehensive literature review is compulsory, the researcher usually encounters methodological difficulties in the conduct of the study, particularly if the primary study question has not been adequately selected in accordance with the clinical dilemma that needs to be addressed. Therefore, optimising time and resources before embarking in the design of a clinical protocol can make an impact on the final results of the research project. Researchers have developed effective ways to convey the message of how to build a good research question that can be easily recalled under the acronyms of PICOT (population, intervention, comparator, outcome, and time frame) and FINER (feasible, interesting, novel, ethical, and relevant). In line with these concepts, this article highlights the main issues faced by clinicians, when developing a research question.
INTRODUCTION
What is your research question? This is very often one of the first queries made by statisticians, when researchers come up with an interesting idea. In fact, the findings of a study may only acquire relevance if they provide an accurate and unbiased answer to a specific question,[ 1 , 2 ] and it has been suggested that up to one-third of the time spent in the whole process—from the conception of an idea to the publication of the manuscript—could be invested in finding the right primary study question.[ 3 ] Furthermore, selecting a good research question can be a time-consuming and challenging task: in one retrospective study, Mayo et al . reported that 3 out of 10 articles published would have needed a major rewording of the question.[ 1 ] This paper explores some recommendations to consider before starting any research project, and outlines the main difficulties faced by young and experienced clinicians, when it comes time to turn an exciting idea into a valuable and feasible research question.
OPTIMISATION OF TIME AND RESOURCES
Focusing on the primary research question.
The process of developing a new idea usually stems from a dilemma inherent to the clinical practice.[ 2 , 3 , 4 ] However, once the problem has been identified, it is tempting to formulate multiple research questions. Conducting a clinical trial with more than one primary study question would not be feasible. First, because each question may require a different research design, and second, because the necessary statistical power of the study would demand unaffordable sample sizes. It is the duty of editors and reviewers to make sure that authors clearly identify the primary research question, and as a consequence, studies approaching more than one primary research question may not be suitable for publication.
Working in the right environment
Teamwork is essential to find the appropriate research question. Working in the right environment will enable the investigator to interact with colleagues with different backgrounds, and create opportunities to exchange experiences in a collaborative way between clinicians and researchers. Likewise, it is of paramount importance to get involved colleagues with expertise in the field (lead clinicians, education supervisors, research mentors, department chairs, epidemiologists, biostatisticians, and ethical consultants, among others), and ask for their guidance.[ 5 , 6 , 7 , 8 ]
Evaluating the pertinence of the study
The researcher should wonder if, on the basis of the research question formulated, there is a need for a study to address the problem, as clinical research usually entails a large investment of resources and workforce involvement. Thus, if the answer to the posed clinical question seems to be evident before starting the study, investing in research to address the problem would become superfluous. For example, in a clinical trial, Herzog-Niescery et al . compared laryngeal masks with cuffed and uncuffed tracheal tubes, in the context of surgeons' exposure to sevoflurane, in infants undergoing adenoidectomy. However, it appears obvious that cuffed tracheal tubes are preferred to minimise surgeons' exposure to volatile gases, as authors concluded after recruiting 60 patients.[ 9 ]
Conducting a thorough literature review
Any research project requires the identification of at least one of three problems: the evidence is scarce, the existing literature yields conflicting results, or the results could be improved. Hence, a comprehensive review of the topic is imperative, as it allows the researcher to identify this gap in the literature, formulate a hypothesis and develop a research question.[ 2 ] To this end, it is crucial to be attentive to new ideas, keep the imagination roaming with reflective attitude, and remain sceptical to the new-gained information.[ 4 , 7 ]
Narrowing the research question
A broad research question may encompass an unaffordable extensive topic. For instance, do supraglottic devices provide similar conditions for the visualization of the glottis aperture in a German hospital? Such a general research question usually needs to be narrowed, not only by cutting away unnecessary components (a German hospital is irrelevant in this context), but also by defining a target population, a specific intervention, an alternative treatment or procedure to be compared with the intervention, a measurable primary outcome, and a time frame of the study. In contrast, an example of a good research question would be: among children younger than 1 year of age undergoing elective minor procedures, to what extent the insertion times are different, comparing the Supreme™ laryngeal mask airway (LMA) to Proseal™ LMA, when placed after reaching a BIS index <60?[ 10 ] In this example, the core ingredients of the research question can be easily identified as: children <1 year of age undergoing minor elective procedures, Supreme™ LMA, Proseal™ LMA and insertion times at anaesthetic induction when reaching a BIS index <60. These components are usually gathered in the literature under the acronym of PICOT (population, intervention, comparator, outcome and time frame, respectively).[ 1 , 3 , 5 ]
PICOT FRAMEWORK
Table 1 summarises the foremost questions likely to be addressed when working on PICOT frame.[ 1 , 6 , 8 ] These components are also applicable to observational studies, where the exposure takes place of the intervention.[ 1 , 11 ] Remarkably, if after browsing the title and the abstract of a paper, the reader is not able to clearly identify the PICOT parameters, and elucidate the question posed by the authors, there should be reasonable scepticism regarding the scientific rigor of the work.[ 12 , 13 ] All these elements are crucial in the design and methodology of a clinical trial, as they can affect the feasibility and reliability of results. Having formulated the primary study question in the context of the PICOT framework [ Table 1 ],[ 1 , 6 , 8 ] the researcher should be able to elucidate which design is most suitable for their work, determine what type of data needs to be collected, and write a structured introduction tailored to what they want to know, explicitly mentioning the primary study hypothesis, which should lead to formulate the main research question.[ 1 , 2 , 6 , 8 ]
Key questions to be answered when working with the PICOT framework (population, intervention, comparator, outcome, and time frame) in a clinical research design
Occasionally, the intended population of the study needs to be modified, in order to overcome any potential ethical issues, and/or for the sake of convenience and feasibility of the project. Yet, the researcher must be aware that the external validity of the results may be compromised. As an illustration, in a randomised clinical trial, authors compared the ease of tracheal tube insertion between C-MAC video laryngoscope and direct laryngoscopy, in patients presenting to the emergency department with an indication of rapid sequence intubation. However, owing to the existence of ethical concerns, a substantial amount of patients requiring emergency tracheal intubation, including patients with major maxillofacial trauma and ongoing cardiopulmonary resuscitation, had to be excluded from the trial.[ 14 ] In fact, the design of prospective studies to explore this subset of patients can be challenging, not only because of ethical considerations, but because of the low incidence of these cases. In another study, Metterlein et al . compared the glottis visualisation among five different supraglottic airway devices, using fibreroptic-guided tracheal intubation in an adult population. Despite that the study was aimed to explore the ease of intubation in patients with anticipated difficult airway (thus requiring fibreoptic tracheal intubation), authors decided to enrol patients undergoing elective laser treatment for genital condylomas, as a strategy to hasten the recruitment process and optimise resources.[ 15 ]
Intervention
Anaesthetic interventions can be classified into pharmacological (experimental treatment) and nonpharmacological. Among nonpharmacological interventions, the most common include anaesthetic techniques, monitoring instruments and airway devices. For example, it would be appropriate to examine the ease of insertion of Supreme™ LMA, when compared with ProSeal™ LMA. Notwithstanding, a common mistake is the tendency to be focused on the data aimed to be collected (the “stated” objective), rather than the question that needs to be answered (the “latent” objective).[ 1 , 4 ] In one clinical trial, authors stated: “we compared the Supreme™ and ProSeal™ LMAs in infants by measuring their performance characteristics, including insertion features, ventilation parameters, induced changes in haemodynamics, and rates of postoperative complications”.[ 10 ] Here, the research question has been centered on the measurements (insertion characteristics, haemodynamic variables, LMA insertion characteristics, ventilation parameters) rather than the clinical problem that needs to be addressed (is Supreme™ LMA easier to insert than ProSeal™ LMA?).
Comparators in clinical research can also be pharmacological (e.g., gold standard or placebo) or nonpharmacological. Typically, not more than two comparator groups are included in a clinical trial. Multiple comparisons should be generally avoided, unless there is enough statistical power to address the end points of interest, and statistical analyses have been adjusted for multiple testing. For instance, in the aforementioned study of Metterlein et al .,[ 15 ] authors compared five supraglottic airway devices by recruiting only 10--12 participants per group. In spite of the authors' recommendation of using two supraglottic devices based on the results of the study, there was no mention of statistical adjustments for multiple comparisons, and given the small sample size, larger clinical trials will undoubtedly be needed to confirm or refute these findings.[ 15 ]
A clear formulation of the primary outcome results of vital importance in clinical research, as the primary statistical analyses, including the sample size calculation (and therefore, the estimation of the effect size and statistical power), will be derived from the main outcome of interest. While it is clear that using more than one primary outcome would not be appropriate, it would be equally inadequate to include multiple point measurements of the same variable as the primary outcome (e.g., visual analogue scale for pain at 1, 2, 6, and 12 h postoperatively).
Composite outcomes, in which multiple primary endpoints are combined, may make it difficult to draw any conclusions based on the study findings. For example, in a clinical trial, 200 children undergoing ophthalmic surgery were recruited to explore the incidence of respiratory adverse events, when comparing desflurane with sevoflurane, following the removal of flexible LMA during the emergence of the anaesthesia. The primary outcome was the number of respiratory events, including breath holding, coughing, secretions requiring suction, laryngospasm, bronchospasm, and mild desaturation.[ 16 ] Should authors had claimed a significant difference between these anaesthetic volatiles, it would have been important to elucidate whether those differences were due to serious adverse events, like laryngospasm or bronchospasm, or the results were explained by any of the other events (e.g., secretions requiring suction). While it is true that clinical trials evaluating the occurrence of adverse events like laryngospasm/bronchospasm,[ 16 , 17 ] or life-threating complications following a tracheal intubation (e.g., inadvertent oesophageal placement, dental damage or injury of the larynx/pharynx)[ 14 ] are almost invariably underpowered, because the incidence of such events is expected to be low, subjective outcomes like coughing or secretions requiring suction should be avoided, as they are highly dependent on the examiner's criteria.[ 16 ]
Secondary outcomes are useful to document potential side effects (e.g., gastric insufflation after placing a supraglottic device), and evaluate the adherence (say, airway leak pressure) and safety of the intervention (for instance, occurrence, or laryngospasm/bronchospasm).[ 17 ] Nevertheless, the problem of addressing multiple secondary outcomes without the adequate statistical power is habitual in medical literature. A good illustration of this issue can be found in a study evaluating the performance of two supraglottic devices in 50 anaesthetised infants and neonates, whereby authors could not draw any conclusions in regard to potential differences in the occurrence of complications, because the sample size calculated made the study underpowered to explore those differences.[ 17 ]
Among PICOT components, the time frame is the most likely to be omitted or inappropriate.[ 1 , 12 ] There are two key aspects of the time component that need to be clearly specified in the research question: the time of measuring the outcome variables (e.g. visual analogue scale for pain at 1, 2, 6, and 12 h postoperatively), and the duration of each measurement (when indicated). The omission of these details in the study protocol might lead to substantial differences in the methodology used. For instance, if a study is designed to compare the insertion times of three different supraglottic devices, and researchers do not specify the exact moment of LMA insertion in the clinical trial protocol (i.e., at the anaesthetic induction after reaching a BIS index < 60), placing an LMA with insufficient depth of anaesthesia would have compromised the internal validity of the results, because inserting a supraglottic device in those patients would have resulted in failed attempts and longer insertion times.[ 10 ]
FINER CRITERIA
A well-elaborated research question may not necessarily be a good question. The proposed study also requires being achievable from both ethical and realistic perspectives, interesting and useful to the clinical practice, and capable to formulate new hypotheses, that may contribute to the generation of knowledge. Researchers have developed an effective way to convey the message of how to build a good research question, that is usually recalled under the acronym of FINER (feasible, interesting, novel, ethical and relevant).[ 5 , 6 , 7 ] Table 2 highlights the main characteristics of FINER criteria.[ 7 ]
Main features of FINER criteria (Feasibility, interest, novelty, ethics, and relevance) to formulate a good research question. Adapted from Cummings et al .[ 7 ]
Novelty and relevance
Although it is clear that any research project should commence with an accurate literature interpretation, in many instances it represents the start and the end of the research: the reader will soon realise that the answer to several questions can be easily found in the published literature.[ 5 ] When the question overcomes the test of a thorough literature review, the project may become novel (there is a gap in the knowledge, and therefore, there is a need for new evidence on the topic) and relevant (the paper may contribute to change the clinical practice). In this context, it is important to distinguish the difference between statistical significance and clinical relevance: in the aforementioned study of Oba et al .,[ 10 ] despite the means of insertion times were reported as significant for the Supreme™ LMA, as compared with ProSeal™ LMA, the difference found in the insertion times (528 vs. 486 sec, respectively), although reported as significant, had little or no clinical relevance.[ 10 ] Conversely, a statistically significant difference of 12 sec might be of clinical relevance in neonates weighing <5 kg.[ 17 ] Thus, statistical tests must be interpreted in the context of a clinically meaningful effect size, which should be previously defined by the researcher.
Feasibility and ethical aspects
Among FINER criteria, there are two potential barriers that may prevent the successful conduct of the project and publication of the manuscript: feasibility and ethical aspects. These obstacles are usually related to the target population, as discussed above. Feasibility refers not only to the budget but also to the complexity of the design, recruitment strategy, blinding, adequacy of the sample size, measurement of the outcome, time of follow-up of participants, and commitment of clinicians, among others.[ 3 , 7 ] Funding, as a component of feasibility, may also be implicated in the ethical principles of clinical research, because the choice of the primary study question may be markedly influenced by the specific criteria demanded in the interest of potential funders.
Discussing ethical issues with local committees is compulsory, as rules applied might vary among countries.[ 18 ] Potential risks and benefits need to be carefully weighed, based upon the four principles of respect for autonomy, beneficence, non-maleficence, and justice.[ 19 ] Although many of these issues may be related to the population target (e.g., conducting a clinical trial in patients with ongoing cardiopulmonary resuscitation would be inappropriate, as would be anaesthetising patients undergoing elective LASER treatment for condylomas, to examine the performance of supraglottic airway devices),[ 14 , 15 ] ethical conflicts may also arise from the intervention (particularly those involving the occurrence of side effects or complications, and their potential for reversibility), comparison (e.g., use of placebo or sham procedures),[ 19 ] outcome (surrogate outcomes should be considered in lieu of long term outcomes), or time frame (e.g., unnecessary longer exposition to an intervention). Thus, FINER criteria should not be conceived without a concomitant examination of the PICOT checklist, and consequently, PICOT framework and FINER criteria should not be seen as separated components, but rather complementary ingredients of a good research question.
Undoubtedly, no research project can be conducted if it is deemed unfeasible, and most institutional review boards would not be in a position to approve a work with major ethical problems. Nonetheless, whether or not the findings are interesting, is a subjective matter. Engaging the attention of readers also depends upon a number of factors, including the manner of presenting the problem, the background of the topic, the intended audience, and the reader's expectations. Furthermore, the interest is usually linked to the novelty and relevance of the topic, and it is worth nothing that editors and peer reviewers of high-impact medical journals are usually reluctant to accept any publication, if there is no novelty inherent to the research hypothesis, or there is a lack of relevance in the results.[ 11 ] Nevertheless, a considerable number of papers have been published without any novelty or relevance in the topic addressed. This is probably reflected in a recent survey, according to which only a third of respondents declared to have read thoroughly the most recent papers downloaded, and at least half of those manuscripts remained unread.[ 20 ] The same study reported that up to one-third of papers examined remained uncited after 5 years of publication, and only 20% of papers accounted for 80% of the citations.[ 20 ]
Formulating a good research question can be fascinating, albeit challenging, even for experienced investigators. While it is clear that clinical experience in combination with the accurate interpretation of literature and teamwork are essential to develop new ideas, the formulation of a clinical problem usually requires the compliance with PICOT framework in conjunction with FINER criteria, in order to translate a clinical dilemma into a researchable question. Working in the right environment with the adequate support of experienced researchers, will certainly make a difference in the generation of knowledge. By doing this, a lot of time will be saved in the search of the primary study question, and undoubtedly, there will be more chances to become a successful researcher.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Open access
- Published: 08 June 2023
Guidance to best tools and practices for systematic reviews
- Kat Kolaski 1 ,
- Lynne Romeiser Logan 2 &
- John P. A. Ioannidis 3
Systematic Reviews volume 12 , Article number: 96 ( 2023 ) Cite this article
18k Accesses
14 Citations
76 Altmetric
Metrics details
Data continue to accumulate indicating that many systematic reviews are methodologically flawed, biased, redundant, or uninformative. Some improvements have occurred in recent years based on empirical methods research and standardization of appraisal tools; however, many authors do not routinely or consistently apply these updated methods. In addition, guideline developers, peer reviewers, and journal editors often disregard current methodological standards. Although extensively acknowledged and explored in the methodological literature, most clinicians seem unaware of these issues and may automatically accept evidence syntheses (and clinical practice guidelines based on their conclusions) as trustworthy.
A plethora of methods and tools are recommended for the development and evaluation of evidence syntheses. It is important to understand what these are intended to do (and cannot do) and how they can be utilized. Our objective is to distill this sprawling information into a format that is understandable and readily accessible to authors, peer reviewers, and editors. In doing so, we aim to promote appreciation and understanding of the demanding science of evidence synthesis among stakeholders. We focus on well-documented deficiencies in key components of evidence syntheses to elucidate the rationale for current standards. The constructs underlying the tools developed to assess reporting, risk of bias, and methodological quality of evidence syntheses are distinguished from those involved in determining overall certainty of a body of evidence. Another important distinction is made between those tools used by authors to develop their syntheses as opposed to those used to ultimately judge their work.
Exemplar methods and research practices are described, complemented by novel pragmatic strategies to improve evidence syntheses. The latter include preferred terminology and a scheme to characterize types of research evidence. We organize best practice resources in a Concise Guide that can be widely adopted and adapted for routine implementation by authors and journals. Appropriate, informed use of these is encouraged, but we caution against their superficial application and emphasize their endorsement does not substitute for in-depth methodological training. By highlighting best practices with their rationale, we hope this guidance will inspire further evolution of methods and tools that can advance the field.
Part 1. The state of evidence synthesis
Evidence syntheses are commonly regarded as the foundation of evidence-based medicine (EBM). They are widely accredited for providing reliable evidence and, as such, they have significantly influenced medical research and clinical practice. Despite their uptake throughout health care and ubiquity in contemporary medical literature, some important aspects of evidence syntheses are generally overlooked or not well recognized. Evidence syntheses are mostly retrospective exercises, they often depend on weak or irreparably flawed data, and they may use tools that have acknowledged or yet unrecognized limitations. They are complicated and time-consuming undertakings prone to bias and errors. Production of a good evidence synthesis requires careful preparation and high levels of organization in order to limit potential pitfalls [ 1 ]. Many authors do not recognize the complexity of such an endeavor and the many methodological challenges they may encounter. Failure to do so is likely to result in research and resource waste.
Given their potential impact on people’s lives, it is crucial for evidence syntheses to correctly report on the current knowledge base. In order to be perceived as trustworthy, reliable demonstration of the accuracy of evidence syntheses is equally imperative [ 2 ]. Concerns about the trustworthiness of evidence syntheses are not recent developments. From the early years when EBM first began to gain traction until recent times when thousands of systematic reviews are published monthly [ 3 ] the rigor of evidence syntheses has always varied. Many systematic reviews and meta-analyses had obvious deficiencies because original methods and processes had gaps, lacked precision, and/or were not widely known. The situation has improved with empirical research concerning which methods to use and standardization of appraisal tools. However, given the geometrical increase in the number of evidence syntheses being published, a relatively larger pool of unreliable evidence syntheses is being published today.
Publication of methodological studies that critically appraise the methods used in evidence syntheses is increasing at a fast pace. This reflects the availability of tools specifically developed for this purpose [ 4 , 5 , 6 ]. Yet many clinical specialties report that alarming numbers of evidence syntheses fail on these assessments. The syntheses identified report on a broad range of common conditions including, but not limited to, cancer, [ 7 ] chronic obstructive pulmonary disease, [ 8 ] osteoporosis, [ 9 ] stroke, [ 10 ] cerebral palsy, [ 11 ] chronic low back pain, [ 12 ] refractive error, [ 13 ] major depression, [ 14 ] pain, [ 15 ] and obesity [ 16 , 17 ]. The situation is even more concerning with regard to evidence syntheses included in clinical practice guidelines (CPGs) [ 18 , 19 , 20 ]. Astonishingly, in a sample of CPGs published in 2017–18, more than half did not apply even basic systematic methods in the evidence syntheses used to inform their recommendations [ 21 ].
These reports, while not widely acknowledged, suggest there are pervasive problems not limited to evidence syntheses that evaluate specific kinds of interventions or include primary research of a particular study design (eg, randomized versus non-randomized) [ 22 ]. Similar concerns about the reliability of evidence syntheses have been expressed by proponents of EBM in highly circulated medical journals [ 23 , 24 , 25 , 26 ]. These publications have also raised awareness about redundancy, inadequate input of statistical expertise, and deficient reporting. These issues plague primary research as well; however, there is heightened concern for the impact of these deficiencies given the critical role of evidence syntheses in policy and clinical decision-making.
Methods and guidance to produce a reliable evidence synthesis
Several international consortiums of EBM experts and national health care organizations currently provide detailed guidance (Table 1 ). They draw criteria from the reporting and methodological standards of currently recommended appraisal tools, and regularly review and update their methods to reflect new information and changing needs. In addition, they endorse the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for rating the overall quality of a body of evidence [ 27 ]. These groups typically certify or commission systematic reviews that are published in exclusive databases (eg, Cochrane, JBI) or are used to develop government or agency sponsored guidelines or health technology assessments (eg, National Institute for Health and Care Excellence [NICE], Scottish Intercollegiate Guidelines Network [SIGN], Agency for Healthcare Research and Quality [AHRQ]). They offer developers of evidence syntheses various levels of methodological advice, technical and administrative support, and editorial assistance. Use of specific protocols and checklists are required for development teams within these groups, but their online methodological resources are accessible to any potential author.
Notably, Cochrane is the largest single producer of evidence syntheses in biomedical research; however, these only account for 15% of the total [ 28 ]. The World Health Organization requires Cochrane standards be used to develop evidence syntheses that inform their CPGs [ 29 ]. Authors investigating questions of intervention effectiveness in syntheses developed for Cochrane follow the Methodological Expectations of Cochrane Intervention Reviews [ 30 ] and undergo multi-tiered peer review [ 31 , 32 ]. Several empirical evaluations have shown that Cochrane systematic reviews are of higher methodological quality compared with non-Cochrane reviews [ 4 , 7 , 9 , 11 , 14 , 32 , 33 , 34 , 35 ]. However, some of these assessments have biases: they may be conducted by Cochrane-affiliated authors, and they sometimes use scales and tools developed and used in the Cochrane environment and by its partners. In addition, evidence syntheses published in the Cochrane database are not subject to space or word restrictions, while non-Cochrane syntheses are often limited. As a result, information that may be relevant to the critical appraisal of non-Cochrane reviews is often removed or is relegated to online-only supplements that may not be readily or fully accessible [ 28 ].
Influences on the state of evidence synthesis
Many authors are familiar with the evidence syntheses produced by the leading EBM organizations but can be intimidated by the time and effort necessary to apply their standards. Instead of following their guidance, authors may employ methods that are discouraged or outdated 28]. Suboptimal methods described in in the literature may then be taken up by others. For example, the Newcastle–Ottawa Scale (NOS) is a commonly used tool for appraising non-randomized studies [ 36 ]. Many authors justify their selection of this tool with reference to a publication that describes the unreliability of the NOS and recommends against its use [ 37 ]. Obviously, the authors who cite this report for that purpose have not read it. Authors and peer reviewers have a responsibility to use reliable and accurate methods and not copycat previous citations or substandard work [ 38 , 39 ]. Similar cautions may potentially extend to automation tools. These have concentrated on evidence searching [ 40 ] and selection given how demanding it is for humans to maintain truly up-to-date evidence [ 2 , 41 ]. Cochrane has deployed machine learning to identify randomized controlled trials (RCTs) and studies related to COVID-19, [ 2 , 42 ] but such tools are not yet commonly used [ 43 ]. The routine integration of automation tools in the development of future evidence syntheses should not displace the interpretive part of the process.
Editorials about unreliable or misleading systematic reviews highlight several of the intertwining factors that may contribute to continued publication of unreliable evidence syntheses: shortcomings and inconsistencies of the peer review process, lack of endorsement of current standards on the part of journal editors, the incentive structure of academia, industry influences, publication bias, and the lure of “predatory” journals [ 44 , 45 , 46 , 47 , 48 ]. At this juncture, clarification of the extent to which each of these factors contribute remains speculative, but their impact is likely to be synergistic.
Over time, the generalized acceptance of the conclusions of systematic reviews as incontrovertible has affected trends in the dissemination and uptake of evidence. Reporting of the results of evidence syntheses and recommendations of CPGs has shifted beyond medical journals to press releases and news headlines and, more recently, to the realm of social media and influencers. The lay public and policy makers may depend on these outlets for interpreting evidence syntheses and CPGs. Unfortunately, communication to the general public often reflects intentional or non-intentional misrepresentation or “spin” of the research findings [ 49 , 50 , 51 , 52 ] News and social media outlets also tend to reduce conclusions on a body of evidence and recommendations for treatment to binary choices (eg, “do it” versus “don’t do it”) that may be assigned an actionable symbol (eg, red/green traffic lights, smiley/frowning face emoji).
Strategies for improvement
Many authors and peer reviewers are volunteer health care professionals or trainees who lack formal training in evidence synthesis [ 46 , 53 ]. Informing them about research methodology could increase the likelihood they will apply rigorous methods [ 25 , 33 , 45 ]. We tackle this challenge, from both a theoretical and a practical perspective, by offering guidance applicable to any specialty. It is based on recent methodological research that is extensively referenced to promote self-study. However, the information presented is not intended to be substitute for committed training in evidence synthesis methodology; instead, we hope to inspire our target audience to seek such training. We also hope to inform a broader audience of clinicians and guideline developers influenced by evidence syntheses. Notably, these communities often include the same members who serve in different capacities.
In the following sections, we highlight methodological concepts and practices that may be unfamiliar, problematic, confusing, or controversial. In Part 2, we consider various types of evidence syntheses and the types of research evidence summarized by them. In Part 3, we examine some widely used (and misused) tools for the critical appraisal of systematic reviews and reporting guidelines for evidence syntheses. In Part 4, we discuss how to meet methodological conduct standards applicable to key components of systematic reviews. In Part 5, we describe the merits and caveats of rating the overall certainty of a body of evidence. Finally, in Part 6, we summarize suggested terminology, methods, and tools for development and evaluation of evidence syntheses that reflect current best practices.
Part 2. Types of syntheses and research evidence
A good foundation for the development of evidence syntheses requires an appreciation of their various methodologies and the ability to correctly identify the types of research potentially available for inclusion in the synthesis.
Types of evidence syntheses
Systematic reviews have historically focused on the benefits and harms of interventions; over time, various types of systematic reviews have emerged to address the diverse information needs of clinicians, patients, and policy makers [ 54 ] Systematic reviews with traditional components have become defined by the different topics they assess (Table 2.1 ). In addition, other distinctive types of evidence syntheses have evolved, including overviews or umbrella reviews, scoping reviews, rapid reviews, and living reviews. The popularity of these has been increasing in recent years [ 55 , 56 , 57 , 58 ]. A summary of the development, methods, available guidance, and indications for these unique types of evidence syntheses is available in Additional File 2 A.
Both Cochrane [ 30 , 59 ] and JBI [ 60 ] provide methodologies for many types of evidence syntheses; they describe these with different terminology, but there is obvious overlap (Table 2.2 ). The majority of evidence syntheses published by Cochrane (96%) and JBI (62%) are categorized as intervention reviews. This reflects the earlier development and dissemination of their intervention review methodologies; these remain well-established [ 30 , 59 , 61 ] as both organizations continue to focus on topics related to treatment efficacy and harms. In contrast, intervention reviews represent only about half of the total published in the general medical literature, and several non-intervention review types contribute to a significant proportion of the other half.
Types of research evidence
There is consensus on the importance of using multiple study designs in evidence syntheses; at the same time, there is a lack of agreement on methods to identify included study designs. Authors of evidence syntheses may use various taxonomies and associated algorithms to guide selection and/or classification of study designs. These tools differentiate categories of research and apply labels to individual study designs (eg, RCT, cross-sectional). A familiar example is the Design Tree endorsed by the Centre for Evidence-Based Medicine [ 70 ]. Such tools may not be helpful to authors of evidence syntheses for multiple reasons.
Suboptimal levels of agreement and accuracy even among trained methodologists reflect challenges with the application of such tools [ 71 , 72 ]. Problematic distinctions or decision points (eg, experimental or observational, controlled or uncontrolled, prospective or retrospective) and design labels (eg, cohort, case control, uncontrolled trial) have been reported [ 71 ]. The variable application of ambiguous study design labels to non-randomized studies is common, making them especially prone to misclassification [ 73 ]. In addition, study labels do not denote the unique design features that make different types of non-randomized studies susceptible to different biases, including those related to how the data are obtained (eg, clinical trials, disease registries, wearable devices). Given this limitation, it is important to be aware that design labels preclude the accurate assignment of non-randomized studies to a “level of evidence” in traditional hierarchies [ 74 ].
These concerns suggest that available tools and nomenclature used to distinguish types of research evidence may not uniformly apply to biomedical research and non-health fields that utilize evidence syntheses (eg, education, economics) [ 75 , 76 ]. Moreover, primary research reports often do not describe study design or do so incompletely or inaccurately; thus, indexing in PubMed and other databases does not address the potential for misclassification [ 77 ]. Yet proper identification of research evidence has implications for several key components of evidence syntheses. For example, search strategies limited by index terms using design labels or study selection based on labels applied by the authors of primary studies may cause inconsistent or unjustified study inclusions and/or exclusions [ 77 ]. In addition, because risk of bias (RoB) tools consider attributes specific to certain types of studies and study design features, results of these assessments may be invalidated if an inappropriate tool is used. Appropriate classification of studies is also relevant for the selection of a suitable method of synthesis and interpretation of those results.
An alternative to these tools and nomenclature involves application of a few fundamental distinctions that encompass a wide range of research designs and contexts. While these distinctions are not novel, we integrate them into a practical scheme (see Fig. 1 ) designed to guide authors of evidence syntheses in the basic identification of research evidence. The initial distinction is between primary and secondary studies. Primary studies are then further distinguished by: 1) the type of data reported (qualitative or quantitative); and 2) two defining design features (group or single-case and randomized or non-randomized). The different types of studies and study designs represented in the scheme are described in detail in Additional File 2 B. It is important to conceptualize their methods as complementary as opposed to contrasting or hierarchical [ 78 ]; each offers advantages and disadvantages that determine their appropriateness for answering different kinds of research questions in an evidence synthesis.
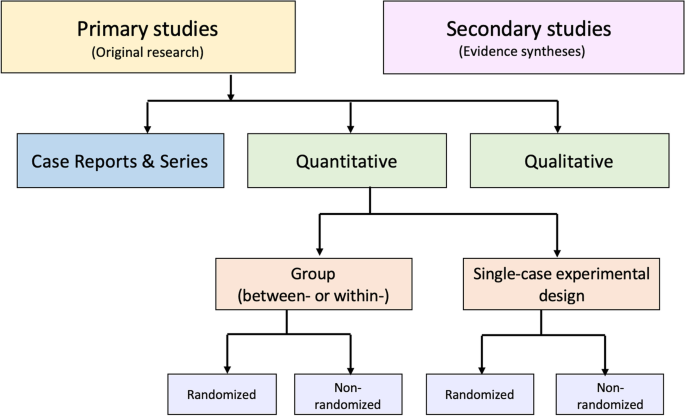
Distinguishing types of research evidence
Application of these basic distinctions may avoid some of the potential difficulties associated with study design labels and taxonomies. Nevertheless, debatable methodological issues are raised when certain types of research identified in this scheme are included in an evidence synthesis. We briefly highlight those associated with inclusion of non-randomized studies, case reports and series, and a combination of primary and secondary studies.
Non-randomized studies
When investigating an intervention’s effectiveness, it is important for authors to recognize the uncertainty of observed effects reported by studies with high RoB. Results of statistical analyses that include such studies need to be interpreted with caution in order to avoid misleading conclusions [ 74 ]. Review authors may consider excluding randomized studies with high RoB from meta-analyses. Non-randomized studies of intervention (NRSI) are affected by a greater potential range of biases and thus vary more than RCTs in their ability to estimate a causal effect [ 79 ]. If data from NRSI are synthesized in meta-analyses, it is helpful to separately report their summary estimates [ 6 , 74 ].
Nonetheless, certain design features of NRSI (eg, which parts of the study were prospectively designed) may help to distinguish stronger from weaker ones. Cochrane recommends that authors of a review including NRSI focus on relevant study design features when determining eligibility criteria instead of relying on non-informative study design labels [ 79 , 80 ] This process is facilitated by a study design feature checklist; guidance on using the checklist is included with developers’ description of the tool [ 73 , 74 ]. Authors collect information about these design features during data extraction and then consider it when making final study selection decisions and when performing RoB assessments of the included NRSI.
Case reports and case series
Correctly identified case reports and case series can contribute evidence not well captured by other designs [ 81 ]; in addition, some topics may be limited to a body of evidence that consists primarily of uncontrolled clinical observations. Murad and colleagues offer a framework for how to include case reports and series in an evidence synthesis [ 82 ]. Distinguishing between cohort studies and case series in these syntheses is important, especially for those that rely on evidence from NRSI. Additional data obtained from studies misclassified as case series can potentially increase the confidence in effect estimates. Mathes and Pieper provide authors of evidence syntheses with specific guidance on distinguishing between cohort studies and case series, but emphasize the increased workload involved [ 77 ].
Primary and secondary studies
Synthesis of combined evidence from primary and secondary studies may provide a broad perspective on the entirety of available literature on a topic. This is, in fact, the recommended strategy for scoping reviews that may include a variety of sources of evidence (eg, CPGs, popular media). However, except for scoping reviews, the synthesis of data from primary and secondary studies is discouraged unless there are strong reasons to justify doing so.
Combining primary and secondary sources of evidence is challenging for authors of other types of evidence syntheses for several reasons [ 83 ]. Assessments of RoB for primary and secondary studies are derived from conceptually different tools, thus obfuscating the ability to make an overall RoB assessment of a combination of these study types. In addition, authors who include primary and secondary studies must devise non-standardized methods for synthesis. Note this contrasts with well-established methods available for updating existing evidence syntheses with additional data from new primary studies [ 84 , 85 , 86 ]. However, a new review that synthesizes data from primary and secondary studies raises questions of validity and may unintentionally support a biased conclusion because no existing methodological guidance is currently available [ 87 ].
Recommendations
We suggest that journal editors require authors to identify which type of evidence synthesis they are submitting and reference the specific methodology used for its development. This will clarify the research question and methods for peer reviewers and potentially simplify the editorial process. Editors should announce this practice and include it in the instructions to authors. To decrease bias and apply correct methods, authors must also accurately identify the types of research evidence included in their syntheses.
Part 3. Conduct and reporting
The need to develop criteria to assess the rigor of systematic reviews was recognized soon after the EBM movement began to gain international traction [ 88 , 89 ]. Systematic reviews rapidly became popular, but many were very poorly conceived, conducted, and reported. These problems remain highly prevalent [ 23 ] despite development of guidelines and tools to standardize and improve the performance and reporting of evidence syntheses [ 22 , 28 ]. Table 3.1 provides some historical perspective on the evolution of tools developed specifically for the evaluation of systematic reviews, with or without meta-analysis.
These tools are often interchangeably invoked when referring to the “quality” of an evidence synthesis. However, quality is a vague term that is frequently misused and misunderstood; more precisely, these tools specify different standards for evidence syntheses. Methodological standards address how well a systematic review was designed and performed [ 5 ]. RoB assessments refer to systematic flaws or limitations in the design, conduct, or analysis of research that distort the findings of the review [ 4 ]. Reporting standards help systematic review authors describe the methodology they used and the results of their synthesis in sufficient detail [ 92 ]. It is essential to distinguish between these evaluations: a systematic review may be biased, it may fail to report sufficient information on essential features, or it may exhibit both problems; a thoroughly reported systematic evidence synthesis review may still be biased and flawed while an otherwise unbiased one may suffer from deficient documentation.
We direct attention to the currently recommended tools listed in Table 3.1 but concentrate on AMSTAR-2 (update of AMSTAR [A Measurement Tool to Assess Systematic Reviews]) and ROBIS (Risk of Bias in Systematic Reviews), which evaluate methodological quality and RoB, respectively. For comparison and completeness, we include PRISMA 2020 (update of the 2009 Preferred Reporting Items for Systematic Reviews of Meta-Analyses statement), which offers guidance on reporting standards. The exclusive focus on these three tools is by design; it addresses concerns related to the considerable variability in tools used for the evaluation of systematic reviews [ 28 , 88 , 96 , 97 ]. We highlight the underlying constructs these tools were designed to assess, then describe their components and applications. Their known (or potential) uptake and impact and limitations are also discussed.
Evaluation of conduct
Development.
AMSTAR [ 5 ] was in use for a decade prior to the 2017 publication of AMSTAR-2; both provide a broad evaluation of methodological quality of intervention systematic reviews, including flaws arising through poor conduct of the review [ 6 ]. ROBIS, published in 2016, was developed to specifically assess RoB introduced by the conduct of the review; it is applicable to systematic reviews of interventions and several other types of reviews [ 4 ]. Both tools reflect a shift to a domain-based approach as opposed to generic quality checklists. There are a few items unique to each tool; however, similarities between items have been demonstrated [ 98 , 99 ]. AMSTAR-2 and ROBIS are recommended for use by: 1) authors of overviews or umbrella reviews and CPGs to evaluate systematic reviews considered as evidence; 2) authors of methodological research studies to appraise included systematic reviews; and 3) peer reviewers for appraisal of submitted systematic review manuscripts. For authors, these tools may function as teaching aids and inform conduct of their review during its development.
Description
Systematic reviews that include randomized and/or non-randomized studies as evidence can be appraised with AMSTAR-2 and ROBIS. Other characteristics of AMSTAR-2 and ROBIS are summarized in Table 3.2 . Both tools define categories for an overall rating; however, neither tool is intended to generate a total score by simply calculating the number of responses satisfying criteria for individual items [ 4 , 6 ]. AMSTAR-2 focuses on the rigor of a review’s methods irrespective of the specific subject matter. ROBIS places emphasis on a review’s results section— this suggests it may be optimally applied by appraisers with some knowledge of the review’s topic as they may be better equipped to determine if certain procedures (or lack thereof) would impact the validity of a review’s findings [ 98 , 100 ]. Reliability studies show AMSTAR-2 overall confidence ratings strongly correlate with the overall RoB ratings in ROBIS [ 100 , 101 ].
Interrater reliability has been shown to be acceptable for AMSTAR-2 [ 6 , 11 , 102 ] and ROBIS [ 4 , 98 , 103 ] but neither tool has been shown to be superior in this regard [ 100 , 101 , 104 , 105 ]. Overall, variability in reliability for both tools has been reported across items, between pairs of raters, and between centers [ 6 , 100 , 101 , 104 ]. The effects of appraiser experience on the results of AMSTAR-2 and ROBIS require further evaluation [ 101 , 105 ]. Updates to both tools should address items shown to be prone to individual appraisers’ subjective biases and opinions [ 11 , 100 ]; this may involve modifications of the current domains and signaling questions as well as incorporation of methods to make an appraiser’s judgments more explicit. Future revisions of these tools may also consider the addition of standards for aspects of systematic review development currently lacking (eg, rating overall certainty of evidence, [ 99 ] methods for synthesis without meta-analysis [ 105 ]) and removal of items that assess aspects of reporting that are thoroughly evaluated by PRISMA 2020.
Application
A good understanding of what is required to satisfy the standards of AMSTAR-2 and ROBIS involves study of the accompanying guidance documents written by the tools’ developers; these contain detailed descriptions of each item’s standards. In addition, accurate appraisal of a systematic review with either tool requires training. Most experts recommend independent assessment by at least two appraisers with a process for resolving discrepancies as well as procedures to establish interrater reliability, such as pilot testing, a calibration phase or exercise, and development of predefined decision rules [ 35 , 99 , 100 , 101 , 103 , 104 , 106 ]. These methods may, to some extent, address the challenges associated with the diversity in methodological training, subject matter expertise, and experience using the tools that are likely to exist among appraisers.
The standards of AMSTAR, AMSTAR-2, and ROBIS have been used in many methodological studies and epidemiological investigations. However, the increased publication of overviews or umbrella reviews and CPGs has likely been a greater influence on the widening acceptance of these tools. Critical appraisal of the secondary studies considered evidence is essential to the trustworthiness of both the recommendations of CPGs and the conclusions of overviews. Currently both Cochrane [ 55 ] and JBI [ 107 ] recommend AMSTAR-2 and ROBIS in their guidance for authors of overviews or umbrella reviews. However, ROBIS and AMSTAR-2 were released in 2016 and 2017, respectively; thus, to date, limited data have been reported about the uptake of these tools or which of the two may be preferred [ 21 , 106 ]. Currently, in relation to CPGs, AMSTAR-2 appears to be overwhelmingly popular compared to ROBIS. A Google Scholar search of this topic (search terms “AMSTAR 2 AND clinical practice guidelines,” “ROBIS AND clinical practice guidelines” 13 May 2022) found 12,700 hits for AMSTAR-2 and 1,280 for ROBIS. The apparent greater appeal of AMSTAR-2 may relate to its longer track record given the original version of the tool was in use for 10 years prior to its update in 2017.
Barriers to the uptake of AMSTAR-2 and ROBIS include the real or perceived time and resources necessary to complete the items they include and appraisers’ confidence in their own ratings [ 104 ]. Reports from comparative studies available to date indicate that appraisers find AMSTAR-2 questions, responses, and guidance to be clearer and simpler compared with ROBIS [ 11 , 101 , 104 , 105 ]. This suggests that for appraisal of intervention systematic reviews, AMSTAR-2 may be a more practical tool than ROBIS, especially for novice appraisers [ 101 , 103 , 104 , 105 ]. The unique characteristics of each tool, as well as their potential advantages and disadvantages, should be taken into consideration when deciding which tool should be used for an appraisal of a systematic review. In addition, the choice of one or the other may depend on how the results of an appraisal will be used; for example, a peer reviewer’s appraisal of a single manuscript versus an appraisal of multiple systematic reviews in an overview or umbrella review, CPG, or systematic methodological study.
Authors of overviews and CPGs report results of AMSTAR-2 and ROBIS appraisals for each of the systematic reviews they include as evidence. Ideally, an independent judgment of their appraisals can be made by the end users of overviews and CPGs; however, most stakeholders, including clinicians, are unlikely to have a sophisticated understanding of these tools. Nevertheless, they should at least be aware that AMSTAR-2 and ROBIS ratings reported in overviews and CPGs may be inaccurate because the tools are not applied as intended by their developers. This can result from inadequate training of the overview or CPG authors who perform the appraisals, or to modifications of the appraisal tools imposed by them. The potential variability in overall confidence and RoB ratings highlights why appraisers applying these tools need to support their judgments with explicit documentation; this allows readers to judge for themselves whether they agree with the criteria used by appraisers [ 4 , 108 ]. When these judgments are explicit, the underlying rationale used when applying these tools can be assessed [ 109 ].
Theoretically, we would expect an association of AMSTAR-2 with improved methodological rigor and an association of ROBIS with lower RoB in recent systematic reviews compared to those published before 2017. To our knowledge, this has not yet been demonstrated; however, like reports about the actual uptake of these tools, time will tell. Additional data on user experience is also needed to further elucidate the practical challenges and methodological nuances encountered with the application of these tools. This information could potentially inform the creation of unifying criteria to guide and standardize the appraisal of evidence syntheses [ 109 ].
Evaluation of reporting
Complete reporting is essential for users to establish the trustworthiness and applicability of a systematic review’s findings. Efforts to standardize and improve the reporting of systematic reviews resulted in the 2009 publication of the PRISMA statement [ 92 ] with its accompanying explanation and elaboration document [ 110 ]. This guideline was designed to help authors prepare a complete and transparent report of their systematic review. In addition, adherence to PRISMA is often used to evaluate the thoroughness of reporting of published systematic reviews [ 111 ]. The updated version, PRISMA 2020 [ 93 ], and its guidance document [ 112 ] were published in 2021. Items on the original and updated versions of PRISMA are organized by the six basic review components they address (title, abstract, introduction, methods, results, discussion). The PRISMA 2020 update is a considerably expanded version of the original; it includes standards and examples for the 27 original and 13 additional reporting items that capture methodological advances and may enhance the replicability of reviews [ 113 ].
The original PRISMA statement fostered the development of various PRISMA extensions (Table 3.3 ). These include reporting guidance for scoping reviews and reviews of diagnostic test accuracy and for intervention reviews that report on the following: harms outcomes, equity issues, the effects of acupuncture, the results of network meta-analyses and analyses of individual participant data. Detailed reporting guidance for specific systematic review components (abstracts, protocols, literature searches) is also available.
Uptake and impact
The 2009 PRISMA standards [ 92 ] for reporting have been widely endorsed by authors, journals, and EBM-related organizations. We anticipate the same for PRISMA 2020 [ 93 ] given its co-publication in multiple high-impact journals. However, to date, there is a lack of strong evidence for an association between improved systematic review reporting and endorsement of PRISMA 2009 standards [ 43 , 111 ]. Most journals require a PRISMA checklist accompany submissions of systematic review manuscripts. However, the accuracy of information presented on these self-reported checklists is not necessarily verified. It remains unclear which strategies (eg, authors’ self-report of checklists, peer reviewer checks) might improve adherence to the PRISMA reporting standards; in addition, the feasibility of any potentially effective strategies must be taken into consideration given the structure and limitations of current research and publication practices [ 124 ].
Pitfalls and limitations of PRISMA, AMSTAR-2, and ROBIS
Misunderstanding of the roles of these tools and their misapplication may be widespread problems. PRISMA 2020 is a reporting guideline that is most beneficial if consulted when developing a review as opposed to merely completing a checklist when submitting to a journal; at that point, the review is finished, with good or bad methodological choices. However, PRISMA checklists evaluate how completely an element of review conduct was reported, but do not evaluate the caliber of conduct or performance of a review. Thus, review authors and readers should not think that a rigorous systematic review can be produced by simply following the PRISMA 2020 guidelines. Similarly, it is important to recognize that AMSTAR-2 and ROBIS are tools to evaluate the conduct of a review but do not substitute for conceptual methodological guidance. In addition, they are not intended to be simple checklists. In fact, they have the potential for misuse or abuse if applied as such; for example, by calculating a total score to make a judgment about a review’s overall confidence or RoB. Proper selection of a response for the individual items on AMSTAR-2 and ROBIS requires training or at least reference to their accompanying guidance documents.
Not surprisingly, it has been shown that compliance with the PRISMA checklist is not necessarily associated with satisfying the standards of ROBIS [ 125 ]. AMSTAR-2 and ROBIS were not available when PRISMA 2009 was developed; however, they were considered in the development of PRISMA 2020 [ 113 ]. Therefore, future studies may show a positive relationship between fulfillment of PRISMA 2020 standards for reporting and meeting the standards of tools evaluating methodological quality and RoB.
Choice of an appropriate tool for the evaluation of a systematic review first involves identification of the underlying construct to be assessed. For systematic reviews of interventions, recommended tools include AMSTAR-2 and ROBIS for appraisal of conduct and PRISMA 2020 for completeness of reporting. All three tools were developed rigorously and provide easily accessible and detailed user guidance, which is necessary for their proper application and interpretation. When considering a manuscript for publication, training in these tools can sensitize peer reviewers and editors to major issues that may affect the review’s trustworthiness and completeness of reporting. Judgment of the overall certainty of a body of evidence and formulation of recommendations rely, in part, on AMSTAR-2 or ROBIS appraisals of systematic reviews. Therefore, training on the application of these tools is essential for authors of overviews and developers of CPGs. Peer reviewers and editors considering an overview or CPG for publication must hold their authors to a high standard of transparency regarding both the conduct and reporting of these appraisals.
Part 4. Meeting conduct standards
Many authors, peer reviewers, and editors erroneously equate fulfillment of the items on the PRISMA checklist with superior methodological rigor. For direction on methodology, we refer them to available resources that provide comprehensive conceptual guidance [ 59 , 60 ] as well as primers with basic step-by-step instructions [ 1 , 126 , 127 ]. This section is intended to complement study of such resources by facilitating use of AMSTAR-2 and ROBIS, tools specifically developed to evaluate methodological rigor of systematic reviews. These tools are widely accepted by methodologists; however, in the general medical literature, they are not uniformly selected for the critical appraisal of systematic reviews [ 88 , 96 ].
To enable their uptake, Table 4.1 links review components to the corresponding appraisal tool items. Expectations of AMSTAR-2 and ROBIS are concisely stated, and reasoning provided.
Issues involved in meeting the standards for seven review components (identified in bold in Table 4.1 ) are addressed in detail. These were chosen for elaboration for one (or both) of two reasons: 1) the component has been identified as potentially problematic for systematic review authors based on consistent reports of their frequent AMSTAR-2 or ROBIS deficiencies [ 9 , 11 , 15 , 88 , 128 , 129 ]; and/or 2) the review component is judged by standards of an AMSTAR-2 “critical” domain. These have the greatest implications for how a systematic review will be appraised: if standards for any one of these critical domains are not met, the review is rated as having “critically low confidence.”
Research question
Specific and unambiguous research questions may have more value for reviews that deal with hypothesis testing. Mnemonics for the various elements of research questions are suggested by JBI and Cochrane (Table 2.1 ). These prompt authors to consider the specialized methods involved for developing different types of systematic reviews; however, while inclusion of the suggested elements makes a review compliant with a particular review’s methods, it does not necessarily make a research question appropriate. Table 4.2 lists acronyms that may aid in developing the research question. They include overlapping concepts of importance in this time of proliferating reviews of uncertain value [ 130 ]. If these issues are not prospectively contemplated, systematic review authors may establish an overly broad scope, or develop runaway scope allowing them to stray from predefined choices relating to key comparisons and outcomes.
Once a research question is established, searching on registry sites and databases for existing systematic reviews addressing the same or a similar topic is necessary in order to avoid contributing to research waste [ 131 ]. Repeating an existing systematic review must be justified, for example, if previous reviews are out of date or methodologically flawed. A full discussion on replication of intervention systematic reviews, including a consensus checklist, can be found in the work of Tugwell and colleagues [ 84 ].
Protocol development is considered a core component of systematic reviews [ 125 , 126 , 132 ]. Review protocols may allow researchers to plan and anticipate potential issues, assess validity of methods, prevent arbitrary decision-making, and minimize bias that can be introduced by the conduct of the review. Registration of a protocol that allows public access promotes transparency of the systematic review’s methods and processes and reduces the potential for duplication [ 132 ]. Thinking early and carefully about all the steps of a systematic review is pragmatic and logical and may mitigate the influence of the authors’ prior knowledge of the evidence [ 133 ]. In addition, the protocol stage is when the scope of the review can be carefully considered by authors, reviewers, and editors; this may help to avoid production of overly ambitious reviews that include excessive numbers of comparisons and outcomes or are undisciplined in their study selection.
An association with attainment of AMSTAR standards in systematic reviews with published prospective protocols has been reported [ 134 ]. However, completeness of reporting does not seem to be different in reviews with a protocol compared to those without one [ 135 ]. PRISMA-P [ 116 ] and its accompanying elaboration and explanation document [ 136 ] can be used to guide and assess the reporting of protocols. A final version of the review should fully describe any protocol deviations. Peer reviewers may compare the submitted manuscript with any available pre-registered protocol; this is required if AMSTAR-2 or ROBIS are used for critical appraisal.
There are multiple options for the recording of protocols (Table 4.3 ). Some journals will peer review and publish protocols. In addition, many online sites offer date-stamped and publicly accessible protocol registration. Some of these are exclusively for protocols of evidence syntheses; others are less restrictive and offer researchers the capacity for data storage, sharing, and other workflow features. These sites document protocol details to varying extents and have different requirements [ 137 ]. The most popular site for systematic reviews, the International Prospective Register of Systematic Reviews (PROSPERO), for example, only registers reviews that report on an outcome with direct relevance to human health. The PROSPERO record documents protocols for all types of reviews except literature and scoping reviews. Of note, PROSPERO requires authors register their review protocols prior to any data extraction [ 133 , 138 ]. The electronic records of most of these registry sites allow authors to update their protocols and facilitate transparent tracking of protocol changes, which are not unexpected during the progress of the review [ 139 ].
Study design inclusion
For most systematic reviews, broad inclusion of study designs is recommended [ 126 ]. This may allow comparison of results between contrasting study design types [ 126 ]. Certain study designs may be considered preferable depending on the type of review and nature of the research question. However, prevailing stereotypes about what each study design does best may not be accurate. For example, in systematic reviews of interventions, randomized designs are typically thought to answer highly specific questions while non-randomized designs often are expected to reveal greater information about harms or real-word evidence [ 126 , 140 , 141 ]. This may be a false distinction; randomized trials may be pragmatic [ 142 ], they may offer important (and more unbiased) information on harms [ 143 ], and data from non-randomized trials may not necessarily be more real-world-oriented [ 144 ].
Moreover, there may not be any available evidence reported by RCTs for certain research questions; in some cases, there may not be any RCTs or NRSI. When the available evidence is limited to case reports and case series, it is not possible to test hypotheses nor provide descriptive estimates or associations; however, a systematic review of these studies can still offer important insights [ 81 , 145 ]. When authors anticipate that limited evidence of any kind may be available to inform their research questions, a scoping review can be considered. Alternatively, decisions regarding inclusion of indirect as opposed to direct evidence can be addressed during protocol development [ 146 ]. Including indirect evidence at an early stage of intervention systematic review development allows authors to decide if such studies offer any additional and/or different understanding of treatment effects for their population or comparison of interest. Issues of indirectness of included studies are accounted for later in the process, during determination of the overall certainty of evidence (see Part 5 for details).
Evidence search
Both AMSTAR-2 and ROBIS require systematic and comprehensive searches for evidence. This is essential for any systematic review. Both tools discourage search restrictions based on language and publication source. Given increasing globalism in health care, the practice of including English-only literature should be avoided [ 126 ]. There are many examples in which language bias (different results in studies published in different languages) has been documented [ 147 , 148 ]. This does not mean that all literature, in all languages, is equally trustworthy [ 148 ]; however, the only way to formally probe for the potential of such biases is to consider all languages in the initial search. The gray literature and a search of trials may also reveal important details about topics that would otherwise be missed [ 149 , 150 , 151 ]. Again, inclusiveness will allow review authors to investigate whether results differ in gray literature and trials [ 41 , 151 , 152 , 153 ].
Authors should make every attempt to complete their review within one year as that is the likely viable life of a search. (1) If that is not possible, the search should be updated close to the time of completion [ 154 ]. Different research topics may warrant less of a delay, for example, in rapidly changing fields (as in the case of the COVID-19 pandemic), even one month may radically change the available evidence.
Excluded studies
AMSTAR-2 requires authors to provide references for any studies excluded at the full text phase of study selection along with reasons for exclusion; this allows readers to feel confident that all relevant literature has been considered for inclusion and that exclusions are defensible.
Risk of bias assessment of included studies
The design of the studies included in a systematic review (eg, RCT, cohort, case series) should not be equated with appraisal of its RoB. To meet AMSTAR-2 and ROBIS standards, systematic review authors must examine RoB issues specific to the design of each primary study they include as evidence. It is unlikely that a single RoB appraisal tool will be suitable for all research designs. In addition to tools for randomized and non-randomized studies, specific tools are available for evaluation of RoB in case reports and case series [ 82 ] and single-case experimental designs [ 155 , 156 ]. Note the RoB tools selected must meet the standards of the appraisal tool used to judge the conduct of the review. For example, AMSTAR-2 identifies four sources of bias specific to RCTs and NRSI that must be addressed by the RoB tool(s) chosen by the review authors. The Cochrane RoB-2 [ 157 ] tool for RCTs and ROBINS-I [ 158 ] for NRSI for RoB assessment meet the AMSTAR-2 standards. Appraisers on the review team should not modify any RoB tool without complete transparency and acknowledgment that they have invalidated the interpretation of the tool as intended by its developers [ 159 ]. Conduct of RoB assessments is not addressed AMSTAR-2; to meet ROBIS standards, two independent reviewers should complete RoB assessments of included primary studies.
Implications of the RoB assessments must be explicitly discussed and considered in the conclusions of the review. Discussion of the overall RoB of included studies may consider the weight of the studies at high RoB, the importance of the sources of bias in the studies being summarized, and if their importance differs in relationship to the outcomes reported. If a meta-analysis is performed, serious concerns for RoB of individual studies should be accounted for in these results as well. If the results of the meta-analysis for a specific outcome change when studies at high RoB are excluded, readers will have a more accurate understanding of this body of evidence. However, while investigating the potential impact of specific biases is a useful exercise, it is important to avoid over-interpretation, especially when there are sparse data.
Synthesis methods for quantitative data
Syntheses of quantitative data reported by primary studies are broadly categorized as one of two types: meta-analysis, and synthesis without meta-analysis (Table 4.4 ). Before deciding on one of these methods, authors should seek methodological advice about whether reported data can be transformed or used in other ways to provide a consistent effect measure across studies [ 160 , 161 ].
Meta-analysis
Systematic reviews that employ meta-analysis should not be referred to simply as “meta-analyses.” The term meta-analysis strictly refers to a specific statistical technique used when study effect estimates and their variances are available, yielding a quantitative summary of results. In general, methods for meta-analysis involve use of a weighted average of effect estimates from two or more studies. If considered carefully, meta-analysis increases the precision of the estimated magnitude of effect and can offer useful insights about heterogeneity and estimates of effects. We refer to standard references for a thorough introduction and formal training [ 165 , 166 , 167 ].
There are three common approaches to meta-analysis in current health care–related systematic reviews (Table 4.4 ). Aggregate meta-analyses is the most familiar to authors of evidence syntheses and their end users. This standard meta-analysis combines data on effect estimates reported by studies that investigate similar research questions involving direct comparisons of an intervention and comparator. Results of these analyses provide a single summary intervention effect estimate. If the included studies in a systematic review measure an outcome differently, their reported results may be transformed to make them comparable [ 161 ]. Forest plots visually present essential information about the individual studies and the overall pooled analysis (see Additional File 4 for details).
Less familiar and more challenging meta-analytical approaches used in secondary research include individual participant data (IPD) and network meta-analyses (NMA); PRISMA extensions provide reporting guidelines for both [ 117 , 118 ]. In IPD, the raw data on each participant from each eligible study are re-analyzed as opposed to the study-level data analyzed in aggregate data meta-analyses [ 168 ]. This may offer advantages, including the potential for limiting concerns about bias and allowing more robust analyses [ 163 ]. As suggested by the description in Table 4.4 , NMA is a complex statistical approach. It combines aggregate data [ 169 ] or IPD [ 170 ] for effect estimates from direct and indirect comparisons reported in two or more studies of three or more interventions. This makes it a potentially powerful statistical tool; while multiple interventions are typically available to treat a condition, few have been evaluated in head-to-head trials [ 171 ]. Both IPD and NMA facilitate a broader scope, and potentially provide more reliable and/or detailed results; however, compared with standard aggregate data meta-analyses, their methods are more complicated, time-consuming, and resource-intensive, and they have their own biases, so one needs sufficient funding, technical expertise, and preparation to employ them successfully [ 41 , 172 , 173 ].
Several items in AMSTAR-2 and ROBIS address meta-analysis; thus, understanding the strengths, weaknesses, assumptions, and limitations of methods for meta-analyses is important. According to the standards of both tools, plans for a meta-analysis must be addressed in the review protocol, including reasoning, description of the type of quantitative data to be synthesized, and the methods planned for combining the data. This should not consist of stock statements describing conventional meta-analysis techniques; rather, authors are expected to anticipate issues specific to their research questions. Concern for the lack of training in meta-analysis methods among systematic review authors cannot be overstated. For those with training, the use of popular software (eg, RevMan [ 174 ], MetaXL [ 175 ], JBI SUMARI [ 176 ]) may facilitate exploration of these methods; however, such programs cannot substitute for the accurate interpretation of the results of meta-analyses, especially for more complex meta-analytical approaches.
Synthesis without meta-analysis
There are varied reasons a meta-analysis may not be appropriate or desirable [ 160 , 161 ]. Syntheses that informally use statistical methods other than meta-analysis are variably referred to as descriptive, narrative, or qualitative syntheses or summaries; these terms are also applied to syntheses that make no attempt to statistically combine data from individual studies. However, use of such imprecise terminology is discouraged; in order to fully explore the results of any type of synthesis, some narration or description is needed to supplement the data visually presented in tabular or graphic forms [ 63 , 177 ]. In addition, the term “qualitative synthesis” is easily confused with a synthesis of qualitative data in a qualitative or mixed methods review. “Synthesis without meta-analysis” is currently the preferred description of other ways to combine quantitative data from two or more studies. Use of this specific terminology when referring to these types of syntheses also implies the application of formal methods (Table 4.4 ).
Methods for syntheses without meta-analysis involve structured presentations of the data in any tables and plots. In comparison to narrative descriptions of each study, these are designed to more effectively and transparently show patterns and convey detailed information about the data; they also allow informal exploration of heterogeneity [ 178 ]. In addition, acceptable quantitative statistical methods (Table 4.4 ) are formally applied; however, it is important to recognize these methods have significant limitations for the interpretation of the effectiveness of an intervention [ 160 ]. Nevertheless, when meta-analysis is not possible, the application of these methods is less prone to bias compared with an unstructured narrative description of included studies [ 178 , 179 ].
Vote counting is commonly used in systematic reviews and involves a tally of studies reporting results that meet some threshold of importance applied by review authors. Until recently, it has not typically been identified as a method for synthesis without meta-analysis. Guidance on an acceptable vote counting method based on direction of effect is currently available [ 160 ] and should be used instead of narrative descriptions of such results (eg, “more than half the studies showed improvement”; “only a few studies reported adverse effects”; “7 out of 10 studies favored the intervention”). Unacceptable methods include vote counting by statistical significance or magnitude of effect or some subjective rule applied by the authors.
AMSTAR-2 and ROBIS standards do not explicitly address conduct of syntheses without meta-analysis, although AMSTAR-2 items 13 and 14 might be considered relevant. Guidance for the complete reporting of syntheses without meta-analysis for systematic reviews of interventions is available in the Synthesis without Meta-analysis (SWiM) guideline [ 180 ] and methodological guidance is available in the Cochrane Handbook [ 160 , 181 ].
Familiarity with AMSTAR-2 and ROBIS makes sense for authors of systematic reviews as these appraisal tools will be used to judge their work; however, training is necessary for authors to truly appreciate and apply methodological rigor. Moreover, judgment of the potential contribution of a systematic review to the current knowledge base goes beyond meeting the standards of AMSTAR-2 and ROBIS. These tools do not explicitly address some crucial concepts involved in the development of a systematic review; this further emphasizes the need for author training.
We recommend that systematic review authors incorporate specific practices or exercises when formulating a research question at the protocol stage, These should be designed to raise the review team’s awareness of how to prevent research and resource waste [ 84 , 130 ] and to stimulate careful contemplation of the scope of the review [ 30 ]. Authors’ training should also focus on justifiably choosing a formal method for the synthesis of quantitative and/or qualitative data from primary research; both types of data require specific expertise. For typical reviews that involve syntheses of quantitative data, statistical expertise is necessary, initially for decisions about appropriate methods, [ 160 , 161 ] and then to inform any meta-analyses [ 167 ] or other statistical methods applied [ 160 ].
Part 5. Rating overall certainty of evidence
Report of an overall certainty of evidence assessment in a systematic review is an important new reporting standard of the updated PRISMA 2020 guidelines [ 93 ]. Systematic review authors are well acquainted with assessing RoB in individual primary studies, but much less familiar with assessment of overall certainty across an entire body of evidence. Yet a reliable way to evaluate this broader concept is now recognized as a vital part of interpreting the evidence.
Historical systems for rating evidence are based on study design and usually involve hierarchical levels or classes of evidence that use numbers and/or letters to designate the level/class. These systems were endorsed by various EBM-related organizations. Professional societies and regulatory groups then widely adopted them, often with modifications for application to the available primary research base in specific clinical areas. In 2002, a report issued by the AHRQ identified 40 systems to rate quality of a body of evidence [ 182 ]. A critical appraisal of systems used by prominent health care organizations published in 2004 revealed limitations in sensibility, reproducibility, applicability to different questions, and usability to different end users [ 183 ]. Persistent use of hierarchical rating schemes to describe overall quality continues to complicate the interpretation of evidence. This is indicated by recent reports of poor interpretability of systematic review results by readers [ 184 , 185 , 186 ] and misleading interpretations of the evidence related to the “spin” systematic review authors may put on their conclusions [ 50 , 187 ].
Recognition of the shortcomings of hierarchical rating systems raised concerns that misleading clinical recommendations could result even if based on a rigorous systematic review. In addition, the number and variability of these systems were considered obstacles to quick and accurate interpretations of the evidence by clinicians, patients, and policymakers [ 183 ]. These issues contributed to the development of the GRADE approach. An international working group, that continues to actively evaluate and refine it, first introduced GRADE in 2004 [ 188 ]. Currently more than 110 organizations from 19 countries around the world have endorsed or are using GRADE [ 189 ].
GRADE approach to rating overall certainty
GRADE offers a consistent and sensible approach for two separate processes: rating the overall certainty of a body of evidence and the strength of recommendations. The former is the expected conclusion of a systematic review, while the latter is pertinent to the development of CPGs. As such, GRADE provides a mechanism to bridge the gap from evidence synthesis to application of the evidence for informed clinical decision-making [ 27 , 190 ]. We briefly examine the GRADE approach but only as it applies to rating overall certainty of evidence in systematic reviews.
In GRADE, use of “certainty” of a body of evidence is preferred over the term “quality.” [ 191 ] Certainty refers to the level of confidence systematic review authors have that, for each outcome, an effect estimate represents the true effect. The GRADE approach to rating confidence in estimates begins with identifying the study type (RCT or NRSI) and then systematically considers criteria to rate the certainty of evidence up or down (Table 5.1 ).
This process results in assignment of one of the four GRADE certainty ratings to each outcome; these are clearly conveyed with the use of basic interpretation symbols (Table 5.2 ) [ 192 ]. Notably, when multiple outcomes are reported in a systematic review, each outcome is assigned a unique certainty rating; thus different levels of certainty may exist in the body of evidence being examined.
GRADE’s developers acknowledge some subjectivity is involved in this process [ 193 ]. In addition, they emphasize that both the criteria for rating evidence up and down (Table 5.1 ) as well as the four overall certainty ratings (Table 5.2 ) reflect a continuum as opposed to discrete categories [ 194 ]. Consequently, deciding whether a study falls above or below the threshold for rating up or down may not be straightforward, and preliminary overall certainty ratings may be intermediate (eg, between low and moderate). Thus, the proper application of GRADE requires systematic review authors to take an overall view of the body of evidence and explicitly describe the rationale for their final ratings.
Advantages of GRADE
Outcomes important to the individuals who experience the problem of interest maintain a prominent role throughout the GRADE process [ 191 ]. These outcomes must inform the research questions (eg, PICO [population, intervention, comparator, outcome]) that are specified a priori in a systematic review protocol. Evidence for these outcomes is then investigated and each critical or important outcome is ultimately assigned a certainty of evidence as the end point of the review. Notably, limitations of the included studies have an impact at the outcome level. Ultimately, the certainty ratings for each outcome reported in a systematic review are considered by guideline panels. They use a different process to formulate recommendations that involves assessment of the evidence across outcomes [ 201 ]. It is beyond our scope to describe the GRADE process for formulating recommendations; however, it is critical to understand how these two outcome-centric concepts of certainty of evidence in the GRADE framework are related and distinguished. An in-depth illustration using examples from recently published evidence syntheses and CPGs is provided in Additional File 5 A (Table AF5A-1).
The GRADE approach is applicable irrespective of whether the certainty of the primary research evidence is high or very low; in some circumstances, indirect evidence of higher certainty may be considered if direct evidence is unavailable or of low certainty [ 27 ]. In fact, most interventions and outcomes in medicine have low or very low certainty of evidence based on GRADE and there seems to be no major improvement over time [ 202 , 203 ]. This is still a very important (even if sobering) realization for calibrating our understanding of medical evidence. A major appeal of the GRADE approach is that it offers a common framework that enables authors of evidence syntheses to make complex judgments about evidence certainty and to convey these with unambiguous terminology. This prevents some common mistakes made by review authors, including overstating results (or under-reporting harms) [ 187 ] and making recommendations for treatment. This is illustrated in Table AF5A-2 (Additional File 5 A), which compares the concluding statements made about overall certainty in a systematic review with and without application of the GRADE approach.
Theoretically, application of GRADE should improve consistency of judgments about certainty of evidence, both between authors and across systematic reviews. In one empirical evaluation conducted by the GRADE Working Group, interrater reliability of two individual raters assessing certainty of the evidence for a specific outcome increased from ~ 0.3 without using GRADE to ~ 0.7 by using GRADE [ 204 ]. However, others report variable agreement among those experienced in GRADE assessments of evidence certainty [ 190 ]. Like any other tool, GRADE requires training in order to be properly applied. The intricacies of the GRADE approach and the necessary subjectivity involved suggest that improving agreement may require strict rules for its application; alternatively, use of general guidance and consensus among review authors may result in less consistency but provide important information for the end user [ 190 ].
GRADE caveats
Simply invoking “the GRADE approach” does not automatically ensure GRADE methods were employed by authors of a systematic review (or developers of a CPG). Table 5.3 lists the criteria the GRADE working group has established for this purpose. These criteria highlight the specific terminology and methods that apply to rating the certainty of evidence for outcomes reported in a systematic review [ 191 ], which is different from rating overall certainty across outcomes considered in the formulation of recommendations [ 205 ]. Modifications of standard GRADE methods and terminology are discouraged as these may detract from GRADE’s objectives to minimize conceptual confusion and maximize clear communication [ 206 ].
Nevertheless, GRADE is prone to misapplications [ 207 , 208 ], which can distort a systematic review’s conclusions about the certainty of evidence. Systematic review authors without proper GRADE training are likely to misinterpret the terms “quality” and “grade” and to misunderstand the constructs assessed by GRADE versus other appraisal tools. For example, review authors may reference the standard GRADE certainty ratings (Table 5.2 ) to describe evidence for their outcome(s) of interest. However, these ratings are invalidated if authors omit or inadequately perform RoB evaluations of each included primary study. Such deficiencies in RoB assessments are unacceptable but not uncommon, as reported in methodological studies of systematic reviews and overviews [ 104 , 186 , 209 , 210 ]. GRADE ratings are also invalidated if review authors do not formally address and report on the other criteria (Table 5.1 ) necessary for a GRADE certainty rating.
Other caveats pertain to application of a GRADE certainty of evidence rating in various types of evidence syntheses. Current adaptations of GRADE are described in Additional File 5 B and included on Table 6.3 , which is introduced in the next section.
The expected culmination of a systematic review should be a rating of overall certainty of a body of evidence for each outcome reported. The GRADE approach is recommended for making these judgments for outcomes reported in systematic reviews of interventions and can be adapted for other types of reviews. This represents the initial step in the process of making recommendations based on evidence syntheses. Peer reviewers should ensure authors meet the minimal criteria for supporting the GRADE approach when reviewing any evidence synthesis that reports certainty ratings derived using GRADE. Authors and peer reviewers of evidence syntheses unfamiliar with GRADE are encouraged to seek formal training and take advantage of the resources available on the GRADE website [ 211 , 212 ].
Part 6. Concise Guide to best practices
Accumulating data in recent years suggest that many evidence syntheses (with or without meta-analysis) are not reliable. This relates in part to the fact that their authors, who are often clinicians, can be overwhelmed by the plethora of ways to evaluate evidence. They tend to resort to familiar but often inadequate, inappropriate, or obsolete methods and tools and, as a result, produce unreliable reviews. These manuscripts may not be recognized as such by peer reviewers and journal editors who may disregard current standards. When such a systematic review is published or included in a CPG, clinicians and stakeholders tend to believe that it is trustworthy. A vicious cycle in which inadequate methodology is rewarded and potentially misleading conclusions are accepted is thus supported. There is no quick or easy way to break this cycle; however, increasing awareness of best practices among all these stakeholder groups, who often have minimal (if any) training in methodology, may begin to mitigate it. This is the rationale for inclusion of Parts 2 through 5 in this guidance document. These sections present core concepts and important methodological developments that inform current standards and recommendations. We conclude by taking a direct and practical approach.
Inconsistent and imprecise terminology used in the context of development and evaluation of evidence syntheses is problematic for authors, peer reviewers and editors, and may lead to the application of inappropriate methods and tools. In response, we endorse use of the basic terms (Table 6.1 ) defined in the PRISMA 2020 statement [ 93 ]. In addition, we have identified several problematic expressions and nomenclature. In Table 6.2 , we compile suggestions for preferred terms less likely to be misinterpreted.
We also propose a Concise Guide (Table 6.3 ) that summarizes the methods and tools recommended for the development and evaluation of nine types of evidence syntheses. Suggestions for specific tools are based on the rigor of their development as well as the availability of detailed guidance from their developers to ensure their proper application. The formatting of the Concise Guide addresses a well-known source of confusion by clearly distinguishing the underlying methodological constructs that these tools were designed to assess. Important clarifications and explanations follow in the guide’s footnotes; associated websites, if available, are listed in Additional File 6 .
To encourage uptake of best practices, journal editors may consider adopting or adapting the Concise Guide in their instructions to authors and peer reviewers of evidence syntheses. Given the evolving nature of evidence synthesis methodology, the suggested methods and tools are likely to require regular updates. Authors of evidence syntheses should monitor the literature to ensure they are employing current methods and tools. Some types of evidence syntheses (eg, rapid, economic, methodological) are not included in the Concise Guide; for these, authors are advised to obtain recommendations for acceptable methods by consulting with their target journal.
We encourage the appropriate and informed use of the methods and tools discussed throughout this commentary and summarized in the Concise Guide (Table 6.3 ). However, we caution against their application in a perfunctory or superficial fashion. This is a common pitfall among authors of evidence syntheses, especially as the standards of such tools become associated with acceptance of a manuscript by a journal. Consequently, published evidence syntheses may show improved adherence to the requirements of these tools without necessarily making genuine improvements in their performance.
In line with our main objective, the suggested tools in the Concise Guide address the reliability of evidence syntheses; however, we recognize that the utility of systematic reviews is an equally important concern. An unbiased and thoroughly reported evidence synthesis may still not be highly informative if the evidence itself that is summarized is sparse, weak and/or biased [ 24 ]. Many intervention systematic reviews, including those developed by Cochrane [ 203 ] and those applying GRADE [ 202 ], ultimately find no evidence, or find the evidence to be inconclusive (eg, “weak,” “mixed,” or of “low certainty”). This often reflects the primary research base; however, it is important to know what is known (or not known) about a topic when considering an intervention for patients and discussing treatment options with them.
Alternatively, the frequency of “empty” and inconclusive reviews published in the medical literature may relate to limitations of conventional methods that focus on hypothesis testing; these have emphasized the importance of statistical significance in primary research and effect sizes from aggregate meta-analyses [ 183 ]. It is becoming increasingly apparent that this approach may not be appropriate for all topics [ 130 ]. Development of the GRADE approach has facilitated a better understanding of significant factors (beyond effect size) that contribute to the overall certainty of evidence. Other notable responses include the development of integrative synthesis methods for the evaluation of complex interventions [ 230 , 231 ], the incorporation of crowdsourcing and machine learning into systematic review workflows (eg the Cochrane Evidence Pipeline) [ 2 ], the shift in paradigm to living systemic review and NMA platforms [ 232 , 233 ] and the proposal of a new evidence ecosystem that fosters bidirectional collaborations and interactions among a global network of evidence synthesis stakeholders [ 234 ]. These evolutions in data sources and methods may ultimately make evidence syntheses more streamlined, less duplicative, and more importantly, they may be more useful for timely policy and clinical decision-making; however, that will only be the case if they are rigorously reported and conducted.
We look forward to others’ ideas and proposals for the advancement of methods for evidence syntheses. For now, we encourage dissemination and uptake of the currently accepted best tools and practices for their development and evaluation; at the same time, we stress that uptake of appraisal tools, checklists, and software programs cannot substitute for proper education in the methodology of evidence syntheses and meta-analysis. Authors, peer reviewers, and editors must strive to make accurate and reliable contributions to the present evidence knowledge base; online alerts, upcoming technology, and accessible education may make this more feasible than ever before. Our intention is to improve the trustworthiness of evidence syntheses across disciplines, topics, and types of evidence syntheses. All of us must continue to study, teach, and act cooperatively for that to happen.
Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60.
Article PubMed Google Scholar
Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for cochrane reviews. J Clin Epidemiol. 2021;133:140–51.
Article PubMed PubMed Central Google Scholar
Fontelo P, Liu F. A review of recent publication trends from top publishing countries. Syst Rev. 2018;7(1):147.
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:1–7.
Article Google Scholar
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Goldkuhle M, Narayan VM, Weigl A, Dahm P, Skoetz N. A systematic assessment of Cochrane reviews and systematic reviews published in high-impact medical journals related to cancer. BMJ Open. 2018;8(3): e020869.
Ho RS, Wu X, Yuan J, Liu S, Lai X, Wong SY, et al. Methodological quality of meta-analyses on treatments for chronic obstructive pulmonary disease: a cross-sectional study using the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) tool. NPJ Prim Care Respir Med. 2015;25:14102.
Tsoi AKN, Ho LTF, Wu IXY, Wong CHL, Ho RST, Lim JYY, et al. Methodological quality of systematic reviews on treatments for osteoporosis: a cross-sectional study. Bone. 2020;139(June): 115541.
Arienti C, Lazzarini SG, Pollock A, Negrini S. Rehabilitation interventions for improving balance following stroke: an overview of systematic reviews. PLoS ONE. 2019;14(7):1–23.
Kolaski K, Romeiser Logan L, Goss KD, Butler C. Quality appraisal of systematic reviews of interventions for children with cerebral palsy reveals critically low confidence. Dev Med Child Neurol. 2021;63(11):1316–26.
Almeida MO, Yamato TP, Parreira PCS, do Costa LOP, Kamper S, Saragiotto BT. Overall confidence in the results of systematic reviews on exercise therapy for chronic low back pain: a cross-sectional analysis using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 tool. Braz J Phys Ther. 2020;24(2):103–17.
Mayo-Wilson E, Ng SM, Chuck RS, Li T. The quality of systematic reviews about interventions for refractive error can be improved: a review of systematic reviews. BMC Ophthalmol. 2017;17(1):1–10.
Matthias K, Rissling O, Pieper D, Morche J, Nocon M, Jacobs A, et al. The methodological quality of systematic reviews on the treatment of adult major depression needs improvement according to AMSTAR 2: a cross-sectional study. Heliyon. 2020;6(9): e04776.
Article CAS PubMed PubMed Central Google Scholar
Riado Minguez D, Kowalski M, Vallve Odena M, Longin Pontzen D, Jelicic Kadic A, Jeric M, et al. Methodological and reporting quality of systematic reviews published in the highest ranking journals in the field of pain. Anesth Analg. 2017;125(4):1348–54.
Churuangsuk C, Kherouf M, Combet E, Lean M. Low-carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews. Obes Rev. 2018;19(12):1700–18.
Article CAS PubMed Google Scholar
Storman M, Storman D, Jasinska KW, Swierz MJ, Bala MM. The quality of systematic reviews/meta-analyses published in the field of bariatrics: a cross-sectional systematic survey using AMSTAR 2 and ROBIS. Obes Rev. 2020;21(5):1–11.
Franco JVA, Arancibia M, Meza N, Madrid E, Kopitowski K. [Clinical practice guidelines: concepts, limitations and challenges]. Medwave. 2020;20(3):e7887 ([Spanish]).
Brito JP, Tsapas A, Griebeler ML, Wang Z, Prutsky GJ, Domecq JP, et al. Systematic reviews supporting practice guideline recommendations lack protection against bias. J Clin Epidemiol. 2013;66(6):633–8.
Zhou Q, Wang Z, Shi Q, Zhao S, Xun Y, Liu H, et al. Clinical epidemiology in China series. Paper 4: the reporting and methodological quality of Chinese clinical practice guidelines published between 2014 and 2018: a systematic review. J Clin Epidemiol. 2021;140:189–99.
Lunny C, Ramasubbu C, Puil L, Liu T, Gerrish S, Salzwedel DM, et al. Over half of clinical practice guidelines use non-systematic methods to inform recommendations: a methods study. PLoS ONE. 2021;16(4):1–21.
Faber T, Ravaud P, Riveros C, Perrodeau E, Dechartres A. Meta-analyses including non-randomized studies of therapeutic interventions: a methodological review. BMC Med Res Methodol. 2016;16(1):1–26.
Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94(3):485–514.
Møller MH, Ioannidis JPA, Darmon M. Are systematic reviews and meta-analyses still useful research? We are not sure. Intensive Care Med. 2018;44(4):518–20.
Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PMM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: who’s listening? Lancet. 2016;387(10027):1573–86.
Barnard ND, Willet WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA. 2017;318(15):1435–6.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. 2016;13(5):1–31.
World Health Organization. WHO handbook for guideline development, 2nd edn. WHO; 2014. Available from: https://www.who.int/publications/i/item/9789241548960 . Cited 2022 Jan 20
Higgins J, Lasserson T, Chandler J, Tovey D, Thomas J, Flemying E, et al. Methodological expectations of Cochrane intervention reviews. Cochrane; 2022. Available from: https://community.cochrane.org/mecir-manual/key-points-and-introduction . Cited 2022 Jul 19
Cumpston M, Chandler J. Chapter II: Planning a Cochrane review. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook . Cited 2022 Jan 30
Henderson LK, Craig JC, Willis NS, Tovey D, Webster AC. How to write a cochrane systematic review. Nephrology. 2010;15(6):617–24.
Page MJ, Altman DG, Shamseer L, McKenzie JE, Ahmadzai N, Wolfe D, et al. Reproducible research practices are underused in systematic reviews of biomedical interventions. J Clin Epidemiol. 2018;94:8–18.
Lorenz RC, Matthias K, Pieper D, Wegewitz U, Morche J, Nocon M, et al. AMSTAR 2 overall confidence rating: lacking discriminating capacity or requirement of high methodological quality? J Clin Epidemiol. 2020;119:142–4.
Posadzki P, Pieper D, Bajpai R, Makaruk H, Könsgen N, Neuhaus AL, et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health. 2020;20(1):1–12.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastile-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital; 2009. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Cited 2022 Jul 19
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Stang A, Jonas S, Poole C. Case study in major quotation errors: a critical commentary on the Newcastle-Ottawa scale. Eur J Epidemiol. 2018;33(11):1025–31.
Ioannidis JPA. Massive citations to misleading methods and research tools: Matthew effect, quotation error and citation copying. Eur J Epidemiol. 2018;33(11):1021–3.
Khalil H, Ameen D, Zarnegar A. Tools to support the automation of systematic reviews: a scoping review. J Clin Epidemiol. 2022;144:22–42.
Crequit P, Boutron I, Meerpohl J, Williams H, Craig J, Ravaud P. Future of evidence ecosystem series: 2. Current opportunities and need for better tools and methods. J Clin Epidemiol. 2020;123:143–52.
Shemilt I, Noel-Storr A, Thomas J, Featherstone R, Mavergames C. Machine learning reduced workload for the cochrane COVID-19 study register: development and evaluation of the cochrane COVID-19 study classifier. Syst Rev. 2022;11(1):15.
Nguyen P-Y, Kanukula R, McKensie J, Alqaidoom Z, Brennan SE, Haddaway N, et al. Changing patterns in reporting and sharing of review data in systematic reviews with meta-analysis of the effects of interventions: a meta-research study. medRxiv; 2022 Available from: https://doi.org/10.1101/2022.04.11.22273688v3 . Cited 2022 Nov 18
Afshari A, Møller MH. Broken science and the failure of academics—resignation or reaction? Acta Anaesthesiol Scand. 2018;62(8):1038–40.
Butler E, Granholm A, Aneman A. Trustworthy systematic reviews–can journals do more? Acta Anaesthesiol Scand. 2019;63(4):558–9.
Negrini S, Côté P, Kiekens C. Methodological quality of systematic reviews on interventions for children with cerebral palsy: the evidence pyramid paradox. Dev Med Child Neurol. 2021;63(11):1244–5.
Page MJ, Moher D. Mass production of systematic reviews and meta-analyses: an exercise in mega-silliness? Milbank Q. 2016;94(3):515–9.
Clarke M, Chalmers I. Reflections on the history of systematic reviews. BMJ Evid Based Med. 2018;23(4):121–2.
Alnemer A, Khalid M, Alhuzaim W, Alnemer A, Ahmed B, Alharbi B, et al. Are health-related tweets evidence based? Review and analysis of health-related tweets on twitter. J Med Internet Res. 2015;17(10): e246.
PubMed PubMed Central Google Scholar
Haber N, Smith ER, Moscoe E, Andrews K, Audy R, Bell W, et al. Causal language and strength of inference in academic and media articles shared in social media (CLAIMS): a systematic review. PLoS ONE. 2018;13(5): e196346.
Swetland SB, Rothrock AN, Andris H, Davis B, Nguyen L, Davis P, et al. Accuracy of health-related information regarding COVID-19 on Twitter during a global pandemic. World Med Heal Policy. 2021;13(3):503–17.
Nascimento DP, Almeida MO, Scola LFC, Vanin AA, Oliveira LA, Costa LCM, et al. Letter to the editor – not even the top general medical journals are free of spin: a wake-up call based on an overview of reviews. J Clin Epidemiol. 2021;139:232–4.
Ioannidis JPA, Fanelli D, Dunne DD, Goodman SN. Meta-research: evaluation and improvement of research methods and practices. PLoS Biol. 2015;13(10):1–7.
Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):1–9.
Pollock M, Fernandez R, Becker LA, Pieper D, Hartling L. Chapter V: overviews of reviews. Cochrane handbook for systematic reviews of interventions. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-v . Cited 2022 Mar 7
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16(1):1–10.
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22.
Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction—the why, what, when, and how. J Clin Epidemiol. 2017;91:23–30.
Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook . Cited 2022 Jan 25
Aromataris E, Munn Z. JBI Manual for Evidence Synthesis [internet]. JBI; 2020 [cited 2022 Jan 15]. Available from: https://synthesismanual.jbi.global .
Tufanaru C, Munn Z, Aromartaris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. JBI; 2020 [cited 2022 Jan 25]. Available from: https://synthesismanual.jbi.global .
Leeflang MMG, Davenport C, Bossuyt PM. Defining the review question. In: Deeks JJ, Bossuyt PM, Leeflang MMG, Takwoingi Y, editors. Cochrane handbook for systematic reviews of diagnostic test accuracy [internet]. Cochrane; 2022 [cited 2022 Mar 30]. Available from: https://training.cochrane.org/6-defining-review-question .
Noyes J, Booth A, Cargo M, Flemming K, Harden A, Harris J, et al.Qualitative evidence. In: Higgins J, Tomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions [internet]. Cochrane; 2022 [cited 2022 Mar 30]. Available from: https://training.cochrane.org/handbook/current/chapter-21#section-21-5 .
Lockwood C, Porritt K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, et al. Chapter 2: Systematic reviews of qualitative evidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. JBI; 2020 [cited 2022 Jul 11]. Available from: https://synthesismanual.jbi.global .
Debray TPA, Damen JAAG, Snell KIE, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ. 2017;356:i6460.
Moola S, Munn Z, Tufanaru C, Aromartaris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. JBI; 2020 [cited 2022 Mar 30]. Available from: https://synthesismanual.jbi.global/ .
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–49.
Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–57.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: Systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. JBI; 2020 [cited 2022 Mar 30]. Available from: https://synthesismanual.jbi.global/ .
Centre for Evidence-Based Medicine. Study designs. CEBM; 2016. Available from: https://www.cebm.ox.ac.uk/resources/ebm-tools/study-designs . Cited 2022 Aug 30
Hartling L, Bond K, Santaguida PL, Viswanathan M, Dryden DM. Testing a tool for the classification of study designs in systematic reviews of interventions and exposures showed moderate reliability and low accuracy. J Clin Epidemiol. 2011;64(8):861–71.
Crowe M, Sheppard L, Campbell A. Reliability analysis for a proposed critical appraisal tool demonstrated value for diverse research designs. J Clin Epidemiol. 2012;65(4):375–83.
Reeves BC, Wells GA, Waddington H. Quasi-experimental study designs series—paper 5: a checklist for classifying studies evaluating the effects on health interventions—a taxonomy without labels. J Clin Epidemiol. 2017;89:30–42.
Reeves BC, Deeks JJ, Higgins JPT, Shea B, Tugwell P, Wells GA. Chapter 24: including non-randomized studies on intervention effects. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-24 . Cited 2022 Mar 1
Reeves B. A framework for classifying study designs to evaluate health care interventions. Forsch Komplementarmed Kl Naturheilkd. 2004;11(Suppl 1):13–7.
Google Scholar
Rockers PC, Røttingen J, Shemilt I. Inclusion of quasi-experimental studies in systematic reviews of health systems research. Health Policy. 2015;119(4):511–21.
Mathes T, Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med Res Methodol. 2017;17(1):8–13.
Jhangiani R, Cuttler C, Leighton D. Single subject research. In: Jhangiani R, Cuttler C, Leighton D, editors. Research methods in psychology, 4th edn. Pressbooks KPU; 2019. Available from: https://kpu.pressbooks.pub/psychmethods4e/part/single-subject-research/ . Cited 2022 Aug 15
Higgins JP, Ramsay C, Reeves BC, Deeks JJ, Shea B, Valentine JC, et al. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4(1):12–25.
Cumpston M, Lasserson T, Chandler J, Page M. 3.4.1 Criteria for considering studies for this review, Chapter III: Reporting the review. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-iii#section-iii-3-4-1 . Cited 2022 Oct 12
Kooistra B, Dijkman B, Einhorn TA, Bhandari M. How to design a good case series. J Bone Jt Surg. 2009;91(Suppl 3):21–6.
Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. Evid Based Med. 2018;23(2):60–3.
Robinson K, Chou R, Berkman N, Newberry S, FU R, Hartling L, et al. Methods guide for comparative effectiveness reviews integrating bodies of evidence: existing systematic reviews and primary studies. AHRQ; 2015. Available from: https://archive.org/details/integrating-evidence-report-150226 . Cited 2022 Aug 7
Tugwell P, Welch VA, Karunananthan S, Maxwell LJ, Akl EA, Avey MT, et al. When to replicate systematic reviews of interventions: consensus checklist. BMJ. 2020;370: m2864.
Tsertsvadze A, Maglione M, Chou R, Garritty C, Coleman C, Lux L, et al. Updating comparative effectiveness reviews:current efforts in AHRQ’s effective health care program. J Clin Epidemiol. 2011;64(11):1208–15.
Cumpston M, Chandler J. Chapter IV: Updating a review. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook . Cited 2022 Aug 2
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):1–8.
Pussegoda K, Turner L, Garritty C, Mayhew A, Skidmore B, Stevens A, et al. Identifying approaches for assessing methodological and reporting quality of systematic reviews: a descriptive study. Syst Rev. 2017;6(1):1–12.
Bhaumik S. Use of evidence for clinical practice guideline development. Trop Parasitol. 2017;7(2):65–71.
Moher D, Eastwood S, Olkin I, Drummond R, Stroup D. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–900.
Stroup D, Berlin J, Morton S, Olkin I, Williamson G, Rennie D, et al. Meta-analysis of observational studies in epidemiology A proposal for reporting. JAMA. 2000;238(15):2008–12.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44(11):1271–8.
Centre for Evidence-Based Medicine. Critical appraisal tools. CEBM; 2015. Available from: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools . Cited 2022 Apr 10
Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8(3):1–16.
Article CAS Google Scholar
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):1–11.
Banzi R, Cinquini M, Gonzalez-Lorenzo M, Pecoraro V, Capobussi M, Minozzi S. Quality assessment versus risk of bias in systematic reviews: AMSTAR and ROBIS had similar reliability but differed in their construct and applicability. J Clin Epidemiol. 2018;99:24–32.
Swierz MJ, Storman D, Zajac J, Koperny M, Weglarz P, Staskiewicz W, et al. Similarities, reliability and gaps in assessing the quality of conduct of systematic reviews using AMSTAR-2 and ROBIS: systematic survey of nutrition reviews. BMC Med Res Methodol. 2021;21(1):1–10.
Pieper D, Puljak L, González-Lorenzo M, Minozzi S. Minor differences were found between AMSTAR 2 and ROBIS in the assessment of systematic reviews including both randomized and nonrandomized studies. J Clin Epidemiol. 2019;108:26–33.
Lorenz RC, Matthias K, Pieper D, Wegewitz U, Morche J, Nocon M, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133–40.
Leclercq V, Hiligsmann M, Parisi G, Beaudart C, Tirelli E, Bruyère O. Best-worst scaling identified adequate statistical methods and literature search as the most important items of AMSTAR2 (A measurement tool to assess systematic reviews). J Clin Epidemiol. 2020;128:74–82.
Bühn S, Mathes T, Prengel P, Wegewitz U, Ostermann T, Robens S, et al. The risk of bias in systematic reviews tool showed fair reliability and good construct validity. J Clin Epidemiol. 2017;91:121–8.
Gates M, Gates A, Duarte G, Cary M, Becker M, Prediger B, et al. Quality and risk of bias appraisals of systematic reviews are inconsistent across reviewers and centers. J Clin Epidemiol. 2020;125:9–15.
Perry R, Whitmarsh A, Leach V, Davies P. A comparison of two assessment tools used in overviews of systematic reviews: ROBIS versus AMSTAR-2. Syst Rev. 2021;10(1):273.
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):1–19.
Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Chapter 10: umbrella reviews. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. Available from: https://synthesismanual.jbi.global . Cited 2022 Jul 11
Pieper D, Lorenz RC, Rombey T, Jacobs A, Rissling O, Freitag S, et al. Authors should clearly report how they derived the overall rating when applying AMSTAR 2—a cross-sectional study. J Clin Epidemiol. 2021;129:97–103.
Franco JVA, Meza N. Authors should also report the support for judgment when applying AMSTAR 2. J Clin Epidemiol. 2021;138:240.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7): e1000100.
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and extensions: a scoping review. Syst Rev. 2017;6(1):263.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2016;70:68–89.
Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10(4): e1001419.
Moher D, Shamseer L, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA. 2015;313(16):1657–65.
Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al. PRISMA harms checklist: Improving harms reporting in systematic reviews. BMJ. 2016;352: i157.
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy studies The PRISMA-DTA statement. JAMA. 2018;319(4):388–96.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Wang X, Chen Y, Liu Y, Yao L, Estill J, Bian Z, et al. Reporting items for systematic reviews and meta-analyses of acupuncture: the PRISMA for acupuncture checklist. BMC Complement Altern Med. 2019;19(1):1–10.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. J Med Libr Assoc. 2021;109(2):174–200.
Blanco D, Altman D, Moher D, Boutron I, Kirkham JJ, Cobo E. Scoping review on interventions to improve adherence to reporting guidelines in health research. BMJ Open. 2019;9(5): e26589.
Koster TM, Wetterslev J, Gluud C, Keus F, van der Horst ICC. Systematic overview and critical appraisal of meta-analyses of interventions in intensive care medicine. Acta Anaesthesiol Scand. 2018;62(8):1041–9.
Johnson BT, Hennessy EA. Systematic reviews and meta-analyses in the health sciences: best practice methods for research syntheses. Soc Sci Med. 2019;233:237–51.
Pollock A, Berge E. How to do a systematic review. Int J Stroke. 2018;13(2):138–56.
Gagnier JJ, Kellam PJ. Reporting and methodological quality of systematic reviews in the orthopaedic literature. J Bone Jt Surg. 2013;95(11):1–7.
Martinez-Monedero R, Danielian A, Angajala V, Dinalo JE, Kezirian EJ. Methodological quality of systematic reviews and meta-analyses published in high-impact otolaryngology journals. Otolaryngol Head Neck Surg. 2020;163(5):892–905.
Boutron I, Crequit P, Williams H, Meerpohl J, Craig J, Ravaud P. Future of evidence ecosystem series 1. Introduction-evidence synthesis ecosystem needs dramatic change. J Clin Epidemiol. 2020;123:135–42.
Ioannidis JPA, Bhattacharya S, Evers JLH, Der Veen F, Van SE, Barratt CLR, et al. Protect us from poor-quality medical research. Hum Reprod. 2018;33(5):770–6.
Lasserson T, Thomas J, Higgins J. Section 1.5 Protocol development, Chapter 1: Starting a review. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/archive/v6/chapter-01#section-1-5 . Cited 2022 Mar 20
Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1(1):7–10.
Allers K, Hoffmann F, Mathes T, Pieper D. Systematic reviews with published protocols compared to those without: more effort, older search. J Clin Epidemiol. 2018;95:102–10.
Ge L, Tian J, Li Y, Pan J, Li G, Wei D, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol. 2018;93:45–55.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647.
Pieper D, Rombey T. Where to prospectively register a systematic review. Syst Rev. 2022;11(1):8.
PROSPERO. PROSPERO will require earlier registration. NIHR; 2022. Available from: https://www.crd.york.ac.uk/prospero/ . Cited 2022 Mar 20
Kirkham JJ, Altman DG, Williamson PR. Bias due to changes in specified outcomes during the systematic review process. PLoS ONE. 2010;5(3):3–7.
Victora CG, Habicht JP, Bryce J. Evidence-based public health: moving beyond randomized trials. Am J Public Health. 2004;94(3):400–5.
Peinemann F, Kleijnen J. Development of an algorithm to provide awareness in choosing study designs for inclusion in systematic reviews of healthcare interventions: a method study. BMJ Open. 2015;5(8): e007540.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350: h2147.
Junqueira DR, Phillips R, Zorzela L, Golder S, Loke Y, Moher D, et al. Time to improve the reporting of harms in randomized controlled trials. J Clin Epidemiol. 2021;136:216–20.
Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JPA. Routinely collected data and comparative effectiveness evidence: promises and limitations. CMAJ. 2016;188(8):E158–64.
Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92(3):423–33.
Abdelhamid AS, Loke YK, Parekh-Bhurke S, Chen Y-F, Sutton A, Eastwood A, et al. Use of indirect comparison methods in systematic reviews: a survey of cochrane review authors. Res Synth Methods. 2012;3(2):71–9.
Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115–23.
Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19(2):159–66.
Jones CW, Keil LG, Weaver MA, Platts-Mills TF. Clinical trials registries are under-utilized in the conduct of systematic reviews: a cross-sectional analysis. Syst Rev. 2014;3(1):1–7.
Baudard M, Yavchitz A, Ravaud P, Perrodeau E, Boutron I. Impact of searching clinical trial registries in systematic reviews of pharmaceutical treatments: methodological systematic review and reanalysis of meta-analyses. BMJ. 2017;356: j448.
Fanelli D, Costas R, Ioannidis JPA. Meta-assessment of bias in science. Proc Natl Acad Sci USA. 2017;114(14):3714–9.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med Res Methodol. 2017;17(1):64.
Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;2:MR000010.
Shojania K, Sampson M, Ansari MT, Ji J, Garritty C, Radar T, et al. Updating systematic reviews. AHRQ Technical Reviews. 2007: Report 07–0087.
Tate RL, Perdices M, Rosenkoetter U, Wakim D, Godbee K, Togher L, et al. Revision of a method quality rating scale for single-case experimental designs and n-of-1 trials: The 15-item Risk of Bias in N-of-1 Trials (RoBiNT) Scale. Neuropsychol Rehabil. 2013;23(5):619–38.
Tate RL, Perdices M, McDonald S, Togher L, Rosenkoetter U. The design, conduct and report of single-case research: Resources to improve the quality of the neurorehabilitation literature. Neuropsychol Rehabil. 2014;24(3–4):315–31.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4894.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Igelström E, Campbell M, Craig P, Katikireddi SV. Cochrane’s risk of bias tool for non-randomized studies (ROBINS-I) is frequently misapplied: a methodological systematic review. J Clin Epidemiol. 2021;140:22–32.
McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-12 . Cited 2022 Apr 10
Ioannidis J, Patsopoulos N, Rothstein H. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–5.
Stewart LA, Tierney JF. To IPD or not to IPD? Eval Health Prof. 2002;25(1):76–97.
Tierney JF, Stewart LA, Clarke M. Chapter 26: Individual participant data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-26 . Cited 2022 Oct 12
Chaimani A, Caldwell D, Li T, Higgins J, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook . Cited 2022 Oct 12.
Cooper H, Hedges L, Valentine J. The handbook of research synthesis and meta-analysis. 3rd ed. Russell Sage Foundation; 2019.
Sutton AJ, Abrams KR, Jones DR, Sheldon T, Song F. Methods for meta-analysis in medical research. Methods for meta-analysis in medical research; 2000.
Deeks J, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic review of interventions. Cochrane; 2022. Available from: http://www.training.cochrane.org/handbook . Cited 2022 Mar 20.
Clarke MJ. Individual patient data meta-analyses. Best Pract Res Clin Obstet Gynaecol. 2005;19(1):47–55.
Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34(11):1489–96.
Debray T, Schuit E, Efthimiou O, Reitsma J, Ioannidis J, Salanti G, et al. An overview of methods for network meta-analysis using individual participant data: when do benefits arise? Stat Methods Med Res. 2016;27(5):1351–64.
Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis : a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017;15(1):943.
Tierney JF, Vale C, Riley R, Smith CT, Stewart L, Clarke M, et al. Individual participant data (IPD) metaanalyses of randomised controlled trials: guidance on their use. PLoS Med. 2015;12(7): e1001855.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11.
Cochrane Training. Review Manager RevMan Web. Cochrane; 2022. Available from: https://training.cochrane.org/online-learning/core-software/revman . Cited 2022 Jun 24
MetaXL. MetalXL. Epi Gear; 2016. Available from: http://epigear.com/index_files/metaxl.html . Cited 2022 Jun 24.
JBI. JBI SUMARI. JBI; 2019. Available from: https://sumari.jbi.global/ . Cited 2022 Jun 24.
Ryan R. Cochrane Consumers and Communication Review Group: data synthesis and analysis. Cochrane Consumers and Communication Review Group; 2013. Available from: http://cccrg.cochrane.org . Cited 2022 Jun 24
McKenzie JE, Beller EM, Forbes AB. Introduction to systematic reviews and meta-analysis. Respirology. 2016;21(4):626–37.
Campbell M, Katikireddi SV, Sowden A, Thomson H. Lack of transparency in reporting narrative synthesis of quantitative data: a methodological assessment of systematic reviews. J Clin Epidemiol. 2019;105:1–9.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368: l6890.
McKenzie JE, Brennan S, Ryan R. Summarizing study characteristics and preparing for synthesis. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook . Cited 2022 Oct 12
AHRQ. Systems to rate the strength of scientific evidence. Evidence report/technology assessment no. 47. AHRQ; 2002. Available from: https://archive.ahrq.gov/clinic/epcsums/strengthsum.htm . Cited 2022 Apr 10.
Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches. BMC Health Serv Res. 2004;4(1):38.
Ioannidis JPA. Meta-research: the art of getting it wrong. Res Synth Methods. 2010;1(3–4):169–84.
Lai NM, Teng CL, Lee ML. Interpreting systematic reviews: are we ready to make our own conclusions? A cross sectional study. BMC Med. 2011;9(1):30.
Glenton C, Santesso N, Rosenbaum S, Nilsen ES, Rader T, Ciapponi A, et al. Presenting the results of Cochrane systematic reviews to a consumer audience: a qualitative study. Med Decis Making. 2010;30(5):566–77.
Yavchitz A, Ravaud P, Altman DG, Moher D, HrobjartssonA, Lasserson T, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56–65.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:7454.
GRADE Working Group. Organizations. GRADE; 2022 [cited 2023 May 2]. Available from: www.gradeworkinggroup.org .
Hartling L, Fernandes RM, Seida J, Vandermeer B, Dryden DM. From the trenches: a cross-sectional study applying the grade tool in systematic reviews of healthcare interventions. PLoS One. 2012;7(4):e34697.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13.
Schünemann H, Brozek J, Guyatt G, Oxman AD, Editors. Section 6.3.2. Symbolic representation. GRADE Handbook [internet]. GRADE; 2013 [cited 2022 Jan 27]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html#h.lr8e9vq954 .
Siemieniuk R, Guyatt G What is GRADE? [internet] BMJ Best Practice; 2017 [cited 2022 Jul 20]. Available from: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/ .
Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66(2):151–7.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6.
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - Study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - Imprecision. J Clin Epidemiol. 2011;64(12):1283–93.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - Inconsistency. J Clin Epidemiol. 2011;64(12):1294–302.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - Indirectness. J Clin Epidemiol. 2011;64(12):1303–10.
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence - Publication bias. J Clin Epidemiol. 2011;64(12):1277–82.
Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation - Determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–35.
Fleming PS, Koletsi D, Ioannidis JPA, Pandis N. High quality of the evidence for medical and other health-related interventions was uncommon in Cochrane systematic reviews. J Clin Epidemiol. 2016;78:34–42.
Howick J, Koletsi D, Pandis N, Fleming PS, Loef M, Walach H, et al. The quality of evidence for medical interventions does not improve or worsen: a metaepidemiological study of Cochrane reviews. J Clin Epidemiol. 2020;126:154–9.
Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J Clin Epidemiol. 2013;66(7):736-742.e5.
Schünemann H, Brozek J, Guyatt G, Oxman A, editors. Section 5.4: Overall quality of evidence. GRADE Handbook. GRADE; 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html#h.lr8e9vq954a . Cited 2022 Mar 25.
GRADE Working Group. Criteria for using GRADE. GRADE; 2016. Available from: https://www.gradeworkinggroup.org/docs/Criteria_for_using_GRADE_2016-04-05.pdf . Cited 2022 Jan 26
Werner SS, Binder N, Toews I, Schünemann HJ, Meerpohl JJ, Schwingshackl L. Use of GRADE in evidence syntheses published in high-impact-factor nutrition journals: a methodological survey. J Clin Epidemiol. 2021;135:54–69.
Zhang S, Wu QJ, Liu SX. A methodologic survey on use of the GRADE approach in evidence syntheses published in high-impact factor urology and nephrology journals. BMC Med Res Methodol. 2022;22(1):220.
Li L, Tian J, Tian H, Sun R, Liu Y, Yang K. Quality and transparency of overviews of systematic reviews. J Evid Based Med. 2012;5(3):166–73.
Pieper D, Buechter R, Jerinic P, Eikermann M. Overviews of reviews often have limited rigor: a systematic review. J Clin Epidemiol. 2012;65(12):1267–73.
Cochrane Editorial Unit. Appendix 1: Checklist for auditing GRADE and SoF tables in protocols of intervention reviews. Cochrane Training; 2022. Available from: https://training.cochrane.org/gomo/modules/522/resources/8307/Checklist for GRADE and SoF methods in Protocols for Gomo.pdf. Cited 2022 Mar 12
Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. Cochrane; 2016. Available from: https://cccrg.cochrane.org/author-resources .
Cunningham M, France EF, Ring N, Uny I, Duncan EA, Roberts RJ, et al. Developing a reporting guideline to improve meta-ethnography in health research: the eMERGe mixed-methods study. Heal Serv Deliv Res. 2019;7(4):1–116.
Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
Gates M, Gates G, Pieper D, Fernandes R, Tricco A, Moher D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378:e070849.
Whiting PF, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM, Rutjes AWSS, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(4):529–36.
Hayden JA, van der Windt DA, Cartwright JL, Co P. Research and reporting methods assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6.
Critical Appraisal Skills Programme. CASP qualitative checklist. CASP; 2018. Available from: https://casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018_fillable_form.pdf . Cited 2022 Apr 26
Hannes K, Lockwood C, Pearson A. A comparative analysis of three online appraisal instruments’ ability to assess validity in qualitative research. Qual Health Res. 2010;20(12):1736–43.
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Heal Policy Manag. 2014;3(3):123–8.
Lewin S, Bohren M, Rashidian A, Munthe-Kaas H, Glenton C, Colvin CJ, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 2: how to make an overall CERQual assessment of confidence and create a Summary of Qualitative Findings table. Implement Sci. 2018;13(suppl 1):10.
Munn Z, Porritt K, Lockwood C, Aromataris E, Pearson A. Establishing confidence in the output of qualitative research synthesis: the ConQual approach. BMC Med Res Methodol. 2014;14(1):108.
Flemming K, Booth A, Hannes K, Cargo M, Noyes J. Cochrane Qualitative and Implementation Methods Group guidance series—paper 6: reporting guidelines for qualitative, implementation, and process evaluation evidence syntheses. J Clin Epidemiol. 2018;97:79–85.
Lockwood C, Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int J Evid Based Health. 2015;13(3):179–87.
Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J Clin Epidemiol. 2020;122:129–41.
Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol. 2020;122:142–52.
Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE Guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020;121:62–70.
Janiaud P, Agarwal A, Belbasis L, Tzoulaki I. An umbrella review of umbrella reviews for non-randomized observational evidence on putative risk and protective factors [internet]. OSF protocol; 2021 [cited 2022 May 28]. Available from: https://osf.io/xj5cf/ .
Mokkink LB, Prinsen CA, Patrick DL, Alonso J, Bouter LM, et al. COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs) - user manual. COSMIN; 2018 [cited 2022 Feb 15]. Available from: http://www.cosmin.nl/ .
Thomas J, M P, Noyes J, Chandler J, Rehfuess E, Tugwell P, et al. Chapter 17: Intervention complexity. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2022. Available from: https://training.cochrane.org/handbook/current/chapter-17 . Cited 2022 Oct 12
Guise JM, Chang C, Butler M, Viswanathan M, Tugwell P. AHRQ series on complex intervention systematic reviews—paper 1: an introduction to a series of articles that provide guidance and tools for reviews of complex interventions. J Clin Epidemiol. 2017;90:6–10.
Riaz IB, He H, Ryu AJ, Siddiqi R, Naqvi SAA, Yao Y, et al. A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma [formula presented]. Eur Urol. 2021;80(6):712–23.
Créquit P, Trinquart L, Ravaud P. Live cumulative network meta-analysis: protocol for second-line treatments in advanced non-small-cell lung cancer with wild-type or unknown status for epidermal growth factor receptor. BMJ Open. 2016;6(8):e011841.
Ravaud P, Créquit P, Williams HC, Meerpohl J, Craig JC, Boutron I. Future of evidence ecosystem series: 3. From an evidence synthesis ecosystem to an evidence ecosystem. J Clin Epidemiol. 2020;123:153–61.
Download references
Acknowledgements
Michelle Oakman Hayes for her assistance with the graphics, Mike Clarke for his willingness to answer our seemingly arbitrary questions, and Bernard Dan for his encouragement of this project.
The work of John Ioannidis has been supported by an unrestricted gift from Sue and Bob O’Donnell to Stanford University.
Author information
Authors and affiliations.
Departments of Orthopaedic Surgery, Pediatrics, and Neurology, Wake Forest School of Medicine, Winston-Salem, NC, USA
Kat Kolaski
Department of Physical Medicine and Rehabilitation, SUNY Upstate Medical University, Syracuse, NY, USA
Lynne Romeiser Logan
Departments of Medicine, of Epidemiology and Population Health, of Biomedical Data Science, and of Statistics, and Meta-Research Innovation Center at Stanford (METRICS), Stanford University School of Medicine, Stanford, CA, USA
John P. A. Ioannidis
You can also search for this author in PubMed Google Scholar
Contributions
All authors participated in the development of the ideas, writing, and review of this manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Kat Kolaski .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’ s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been published simultaneously in BMC Systematic Reviews, Acta Anaesthesiologica Scandinavica, BMC Infectious Diseases, British Journal of Pharmacology, JBI Evidence Synthesis, the Journal of Bone and Joint Surgery Reviews , and the Journal of Pediatric Rehabilitation Medicine .
Supplementary Information
Additional file 2a..
Overviews, scoping reviews, rapid reviews and living reviews.
Additional file 2B.
Practical scheme for distinguishing types of research evidence.
Additional file 4.
Presentation of forest plots.
Additional file 5A.
Illustrations of the GRADE approach.
Additional file 5B.
Adaptations of GRADE for evidence syntheses.
Additional file 6.
Links to Concise Guide online resources.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kolaski, K., Logan, L.R. & Ioannidis, J.P.A. Guidance to best tools and practices for systematic reviews. Syst Rev 12 , 96 (2023). https://doi.org/10.1186/s13643-023-02255-9
Download citation
Received : 03 October 2022
Accepted : 19 February 2023
Published : 08 June 2023
DOI : https://doi.org/10.1186/s13643-023-02255-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Certainty of evidence
- Critical appraisal
- Methodological quality
- Risk of bias
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
This section addresses such questions broadly while providing general guidance for writing a narrative literature review that evaluates the most pertinent studies. The literature review process should begin before the research is conducted. As Boote and Beile (2005, p. 3) suggested, researchers should be "scholars before researchers."
When writing a literature review it is important to start with a brief introduction, followed by the text broken up into subsections and conclude with a summary to bring everything together. A summary table including title, author, publication date and key findings is a useful feature to present in your review (see Table 1 for an example).
a description of the publication. a summary of the publication's main points. an evaluation of the publication's contribution to the topic. identification of critical gaps, points of disagreement, or potentially flawed methodology or theoretical approaches. indicates potential directions for future research.
The literature review process is further broken down into subtopics on formulating the research problem, developing and validating the review protocol, searching the literature, screening for inclusion, assessing quality, extracting data, analyzing and synthesizing data, and reporting the findings.
Abstract. Performing a literature review is a critical first step in research to understanding the state-of-the-art and identifying gaps and challenges in the field. A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in ...
Step 3: Search the Literature. The quality of literature review is highly dependent on the literature collected for the review—"Garbage-in, garbage-out.". The literature search finds materials for the review; therefore, a systematic review depends on a systematic search of literature. Channels for literature search.
The objective of a Literature Review is to find previous published scholarly works relevant to an specific topic. A literature review is important because it: Explains the background of research on a topic. Demonstrates why a topic is significant to a subject area. Discovers relationships between research studies/ideas.
• Important authors who are working on your topic ... inconsistencies in theory and findings, and areas or issues pertinent to future study 3. Conclude by providing some insight into the relationship between the central topic of the literature review and a larger area of study such as a discipline, a scientific endeavor, or a profession.
The literature review can serve various functions in the contexts of education and research. It aids in identifying knowledge gaps, informing research methodology, and developing a theoretical framework during the planning stages of a research study or project, as well as reporting of review findings in the context of the existing literature.
A literature review may consist of simply a summary of key sources, but in the social sciences, a literature review usually has an organizational pattern and combines both summary and synthesis, often within specific conceptual categories.A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information in a way that ...
In composing a literature review, it is important to note that it is often this third layer of knowledge that ... Development of the Literature Review Four Stages 1. Problem formulation -- which topic or field is being examined and what are its component issues? ... discussing the findings and conclusions of pertinent literature. Consider the ...
9.3. Types of Review Articles and Brief Illustrations. EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic.
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important ...
Determining which literature makes a significant contribution to the understanding of the topic. Analysis and interpretation Discussing the findings and conclusions of pertinent literature. Write and Publish Produce a formatted document that you can get published in an appropriate journal to inform everyone of your findings.
When writing a literature review it is important to start with a brief introduction, followed by the text broken up into subsections and conclude with a summary to bring everything together. A summary table including title, author, publication date and key findings is a useful feature to present in your review (see Table 1 for an example).
Background Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence. Information specialists and review teams ...
Literature Review. The structure of a literature review should include the following: An overview of the subject, issue or theory under consideration, along with the objectives of the literature review, Division of works under review into themes or categories (e.g. works that support of a particular position, those against, and those offering ...
A literature review examines the body of work surrounding previous research. The thesis should focus on a specific aspect of that research and highlight what you are searching for. Make sure your research question is specific, but does not exclude outside sources with important information
Important aspects of a systematic literature review (SLR) include a structured method for conducting the study and significant transparency of the approaches used for summarizing the literature (Hiebl, 2023).The inspection of existing scientific literature is a valuable tool for (a) developing best practices and (b) resolving issues or controversies over a single study (Gupta et al., 2018).
Literature search—finding materials relevant to the subject being explored; Data evaluation—determining which literature makes a significant contribution to the understanding of the topic; Analysis and interpretation—discussing the findings and conclusions of pertinent literature; Remember, this is a process and not necessarily a linear one.
The process of formulating a good research question can be challenging and frustrating. While a comprehensive literature review is compulsory, the researcher usually encounters methodological difficulties in the conduct of the study, particularly if the primary study question has not been adequately selected in accordance with the clinical dilemma that needs to be addressed.
Data continue to accumulate indicating that many systematic reviews are methodologically flawed, biased, redundant, or uninformative. Some improvements have occurred in recent years based on empirical methods research and standardization of appraisal tools; however, many authors do not routinely or consistently apply these updated methods. In addition, guideline developers, peer reviewers, and ...