Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 15 September 2022
- Pavel Kolkhir ORCID: orcid.org/0000-0001-5380-8132 1 , 2 ,
- Ana M. Giménez-Arnau 3 ,
- Kanokvalai Kulthanan ORCID: orcid.org/0000-0002-7618-821X 4 ,
- Jonny Peter ORCID: orcid.org/0000-0002-2658-0723 5 , 6 ,
- Martin Metz ORCID: orcid.org/0000-0002-4070-9976 1 , 2 &
- Marcus Maurer ORCID: orcid.org/0000-0002-4121-481X 1 , 2
Nature Reviews Disease Primers volume 8 , Article number: 61 ( 2022 ) Cite this article
49k Accesses
88 Citations
120 Altmetric
Metrics details
- Chronic inflammation
- Skin diseases
Urticaria is an inflammatory skin disorder that affects up to 20% of the world population at some point during their life. It presents with wheals, angioedema or both due to activation and degranulation of skin mast cells and the release of histamine and other mediators. Most cases of urticaria are acute urticaria, which lasts ≤6 weeks and can be associated with infections or intake of drugs or foods. Chronic urticaria (CU) is either spontaneous or inducible, lasts >6 weeks and persists for >1 year in most patients. CU greatly affects patient quality of life, and is linked to psychiatric comorbidities and high healthcare costs. In contrast to chronic spontaneous urticaria (CSU), chronic inducible urticaria (CIndU) has definite and subtype-specific triggers that induce signs and symptoms. The pathogenesis of CSU consists of several interlinked events involving autoantibodies, complement and coagulation. The diagnosis of urticaria is clinical, but several tests can be performed to exclude differential diagnoses and identify underlying causes in CSU or triggers in CIndU. Current urticaria treatment aims at complete response, with a stepwise approach using second-generation H1 antihistamines, omalizumab and cyclosporine. Novel treatment approaches centre on targeting mediators, signalling pathways and receptors of mast cells and other immune cells. Further research should focus on defining disease endotypes and their biomarkers, identifying new treatment targets and developing improved therapies.

Similar content being viewed by others
Cold-induced urticarial autoinflammatory syndrome related to factor XII activation
Jörg Scheffel, Niklas A. Mahnke, … Karoline Krause
Mathematical-based morphological classification of skin eruptions corresponding to the pathophysiological state of chronic spontaneous urticaria
Sungrim Seirin-Lee, Daiki Matsubara, … Michihiro Hide

Gut microbiota facilitate chronic spontaneous urticaria
Lei Zhu, Xingxing Jian, … Jie Li
Introduction
Urticaria is a common and heterogeneous inflammatory skin disorder, with a lifetime prevalence of up to 20% worldwide 1 , 2 . The disease results from the activation and degranulation of skin mast cells, followed by the release of histamine and other mediators that lead to sensory nerve activation, vasodilatation, plasma extravasation and cellular recruitment 3 , 4 . This process causes the development of the disease-defining signs and symptoms, itchy wheals (hives) and angioedema or both (Fig. 1 ).
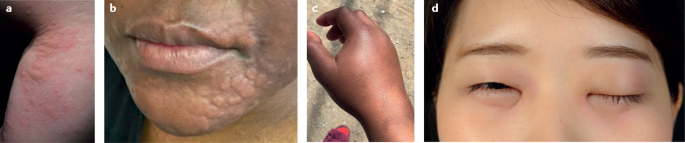
a , b | Wheals. c , d | Angioedema.
Urticaria is classified, first, based on its duration as acute urticaria (AU), lasting ≤6 weeks, or chronic urticaria (CU), lasting >6 weeks 4 . Urticaria is further divided into inducible and spontaneous forms. In inducible urticaria, the signs and symptoms are induced by a subtype-specific and definite trigger, for example cold in cold urticaria (ColdU). In spontaneous urticaria, the signs and symptoms appear unprompted, and there are no definite triggers, although stress, infections and other aggravators can increase disease activity in some patients. Spontaneous urticaria is more common than inducible urticaria and both can coexist in the same patient 4 , 5 , 6 (Tables 1 and 2 ).
Most cases of AU resolve within 1 week, and <40% of cases become chronic (see Supplementary Table 1 ). CU often lasts for several years before spontaneous remission occurs (see Supplementary Tables 2 and 3 ). AU is mostly spontaneous and of unknown cause, although infections and the intake of drugs or foods are thought to be relevant in some patients (see Supplementary Table 4 ). The underlying causes of chronic inducible urticaria (CIndU) remain unknown, whereas two causes of chronic spontaneous urticaria (CSU) (previously known as chronic idiopathic urticaria) are recognized: autoallergy (also called type I autoimmunity) with IgE autoantibody involvement, and type IIb autoimmunity with IgG autoantibody involvement 7 , 8 . These mast cell-activating autoantibodies along with cell infiltration, coagulation and complement activation are thought to be key drivers of the pathogenesis of CSU 9 , 10 .
The burden of CU for patients and society is substantial. CU manifestations have a strong effect on health-related quality of life, including sleep impairment, diminished physical and emotional well-being and poor performance at school and work 11 , 12 . Current treatment options are still limited and are not effective in around one-third of patients with CU. Novel targeted treatments are needed, and several promising drugs are already under development 13 .
In this Primer, we discuss the current knowledge of urticaria epidemiology, diagnosis, screening, management and quality of life, and highlight recent advances in the understanding of disease pathogenesis and targeted treatment. Other pathophysiologically and clinically distinct conditions that present with wheals and/or angioedema, for example urticarial vasculitis, autoinflammatory syndromes and bradykinin-mediated angioedema, are briefly discussed as differential diagnoses. Detailed tables of study findings relating to epidemiology and diagnosis, with accompanying references, can be found in the Supplementary information .
Epidemiology
Prevalence and incidence.
For 2017, the prevalence of urticaria was estimated at 86 million cases and the annual incidence at 160 million cases globally 14 . However, each urticaria subtype has its own prevalence within different populations. The prevalence of AU is highest in children <5 years of age 14 , 15 , 16 , whereas CU, especially CSU, is most prevalent in women >30 years old 6 , 17 , 18 , 19 , 20 , 21 . Adult patients with CSU are older than adult patients with CIndU (average age ~30–70 years versus ~20–40 years) and have older age of disease onset (~30–50 years versus ~20–35 years). In adults, all types of urticaria are more prevalent in women than in men, except for cholinergic urticaria (CholU), which is more prominent in both male adults and children; female predominance is absent or is less prominent in younger children 21 (Table 1 ; see Supplementary Tables 2 and 3 ).
All ethnicities are affected; however, the prevalence of AU and CU was higher in non-white patients in some studies 15 , 19 , 22 but not all 23 . The lifetime prevalence of all types of urticaria and AU is 3–22% and 6–19%, respectively (see Supplementary Table 5 ). The overall lifetime prevalence of CU is 4.4% 22 , and the point prevalence of CU (1-year prevalence in most studies) ranges from ≤1.5% in the USA and Europe to 3–4% in Mexico, Korea and China (Fig. 2 ; see Supplementary Table 5 ).
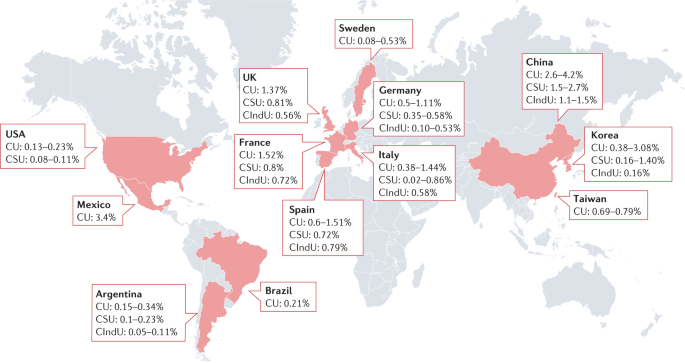
A point prevalence of chronic urticaria (CU) in children, adults and/or both is presented where available. The lowest prevalence rates were observed in the USA and Europe, whereas the highest rates were reported in Mexico, China and Korea. Detailed information can be found in Supplementary Table 5 . CIndU, chronic inducible urticaria; CSU, chronic spontaneous urticaria.
No significant changes in the global prevalence, incidence and the years of life lived with disability were seen for urticaria between 1990 and 2017 (ref. 14 ). The consistent increase in CU prevalence in South Korea 21 , 24 , Italy 25 , 26 and Taiwan 27 may be linked to country-specific demographic, environmental and behavioural factors and changes.
CIndU is less prevalent than CSU 6 , 28 . The pooled rate of all CIndU subtypes was 13% 29 and that of CSU was ~60–90% across all cases of CU (see Supplementary Table 6 ). The most prevalent types of CIndU are symptomatic dermographism, CholU and ColdU, in both adults and children 21 , 30 , whereas aquagenic urticaria, solar urticaria, heat urticaria, vibratory angioedema and contact urticaria are rare, that is, are seen in <2–3% of all CU cases (Table 2 ; see Supplementary Table 6 ). Delayed pressure urticaria is rarely seen as an isolated disorder but present in combination with CSU in up to 36% of patients with CU (Table 2 ; see Supplementary Table 6 ).
Natural course
The average duration of AU is ≤1 week. The rates of progression of AU to CU are between 5% and 39% in most studies (see Supplementary Table 1 ). CSU is of shorter duration than CIndU, with a mean or median disease duration of ~1–4 years in most studies (see Supplementary Table 2 ), and the cumulative weighted average estimates for spontaneous remission at 1, 5 and 20 years are 17%, 45% and 73%, respectively 31 . CSU relapses in 6–31% of patients (see Supplementary Table 2 ). The mean or median duration of CIndU and its three most common subtypes (symptomatic dermographism, CholU and ColdU) was 2–12 years, 2–5 years, 3–8 years and 2–9 years, respectively. Remission of CIndU within 5 years occurs in only around one-third of patients, with the highest rates in symptomatic dermographism and the lowest in CholU and ColdU 30 (see Supplementary Table 3 ). Various factors and markers have been found to be associated with urticaria natural course, phenotypes, endotypes, clinical and laboratory characteristics and response to treatment (see Supplementary Box 1 ).
Risk factors
Risk factors reported for AU include high population density 15 and personal 32 and parental history of allergic diseases 2 , 33 . Higher prevalence and/or higher risk of having AU may be associated with poverty and lower socio-economic status 14 , 15 , whereas risk for CU was linked to high income and socio-economic status in some studies 2 , 23 , 34 but not all 35 , 36 . In studies involving twins, genetic factors could partly explain susceptibility to urticaria 32 . The role of polymorphisms of several genes, including TNFRS11A, TBXA2R and PLA2G4A 37 , has been suggested in susceptibility to AU and/or angioedema induced by multiple NSAIDs.
Genetic predisposition to CU has been associated with gene polymorphisms of IFNγ, IL-6, IL-17RA, IL-10, TGFβ, IL-6, tumour necrosis factor (TNF), PTPN22, IL-1, IL-2 and HLA class I and II alleles 38 . Some of these genes, such as PTPN22 , and HLA alleles, for example HLA-DR4 allele, are also responsible for susceptibility to various autoimmune disorders. For example, HLA-DR4 was strongly associated with autoimmune CSU defined by a positive basophil histamine release assay (BHRA) 39 and other autoimmune diseases, for example rheumatoid arthritis and type 1 diabetes mellitus. CSU, especially in middle-aged women with autoimmune CSU 40 , is associated with an increased risk of developing autoimmune disease within 10 years after the diagnosis of CSU 41 . For example, women with CSU were 23 and 20 times more likely to also have hypothyroidism and rheumatoid arthritis, respectively, compared with the control group 41 . Diagnosis of CSU was made in ~80% and ~20% of patients before and after diagnosis of autoimmune diseases, respectively, including rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus and coeliac disease 41 . Patients with autoimmune thyroid diseases, especially female patients, had a considerably higher risk of CSU development 42 . Up to 25% of patients with CSU, especially those with positive markers of autoimmune urticaria 43 , 44 , 45 , had a family history of CSU (see Supplementary Table 7 ). In addition, female patients with peptic ulcer disease 46 and abnormal uterine bleeding 47 have been shown to have a higher CU risk.
Geographical differences in frequency of CholU and ColdU were reported suggesting that some environmental factors, for example temperature and altitude, might increase the risk of developing CIndU 48 .
Healthcare use and annual treatment costs
Urticaria, especially CU, is associated with considerable healthcare utilization and economic burden, including both regular and unanticipated healthcare visits, costs due to laboratory expenses and indirect costs due to the absence from work or reduced efficiency 11 , 49 . Patients with CU, especially those with angioedema, more frequently visit physicians, mostly family doctors, allergists and dermatologists, and emergency rooms, and are more frequently hospitalized than individuals without urticaria 12 , 49 , 50 . Patients with CIndU were more often hospitalized than patients with CSU (paediatric, 15.5% versus 9.9%; adult, 7.8% versus 4.6%; respectively) 6 . Average annual economic costs for a patient with CSU ranged from PPP$907 (purchasing power parity dollars) in Italy to PPP$2,984 in France, mostly due to therapies and inpatient visits 12 .
Mechanisms/pathophysiology
Skin mast cells have a central role in urticaria pathogenesis and are found in the upper papillary dermis as well as the deep dermis and subcutis, mostly around cutaneous blood vessels and sensory nerves. Their activation with subsequent degranulation drives the development of itchy wheals and/or angioedema 3 .
Acute urticaria
The pathogenesis of AU is poorly investigated. Acute spontaneous urticaria, as well as wheals and/or angioedema in patients with anaphylaxis, have been described to result from type I hypersensitivity reactions to foods, drugs and other allergens 51 . Type I hypersensitivity, also known as an immediate IgE-mediated reaction, describes interaction between exoallergen and a pre-existing complex of an IgE antibody bound to its high-affinity receptor, FcεRI, on mast cells and basophils that leads to cell activation and degranulation 52 . NSAID‐induced urticaria and/or angioedema is IgE-mediated or T cell-mediated, or due to the pharmacological inhibition of cyclooxygenase 1 (COX1) and increased levels of cysteinyl leukotrienes 53 . Acute contact urticaria can develop in response to direct contact with allergens with previous sensitization 54 and urticariogenic substances without prior sensitization, for example after touching stinging nettles 4 .
Chronic spontaneous urticaria
The development of wheals and angioedema in CSU is dependent on mast cell-activating signals and receptors, signalling pathways, inhibitory receptors and mediators, which are targets of current and future therapy 3 , 10 , 13 (Fig. 3 ). Mast cells express many activating receptors including FcεRI, MRGPRX2, C5aR, PAR1, PAR2, chemoattractant receptor-homologous molecule expressed on T helper 2 cells (T H 2 cells) (CRTh2) and cytokine receptors, which can be activated by various signals 13 . Interaction of stem cell factor (SCF), produced by fibroblasts, endothelial cells and mast cells, with its receptor KIT (CD117) on mast cells is a major driver of mast cell differentiation, migration, proliferation, survival and apoptosis 55 . Activation of FcεRI involves several cytoplasmic signalling proteins, for example. LYN, spleen tyrosine kinase (SYK) and Bruton’s tyrosine kinase (BTK), which phosphorylate downstream signalling targets and induce mast cell activation and degranulation 56 The first step in FcεRI-mediated signalling is the phosphorylation of the FcεRI β-chain and γ-chain by LYN followed by activation of SYK and BTK. The cytosolic tyrosine kinase BTK is the central positive regulator of FcεRI-mediated mast activation and cytokine production 56 , 57 . In addition to its role in mast cell activation, BTK is also required for B cell receptor (BCR) signalling 56 , 57 . In addition to activating receptors, mast cells express a few inhibitory receptors, such as sialic acid-binding immunoglobulin-like lectin 8 (Siglec 8), CD200R, CD300a and FcγRIIb 58 , which can silence mast cells and block mast cell activation upon interaction with their ligands.
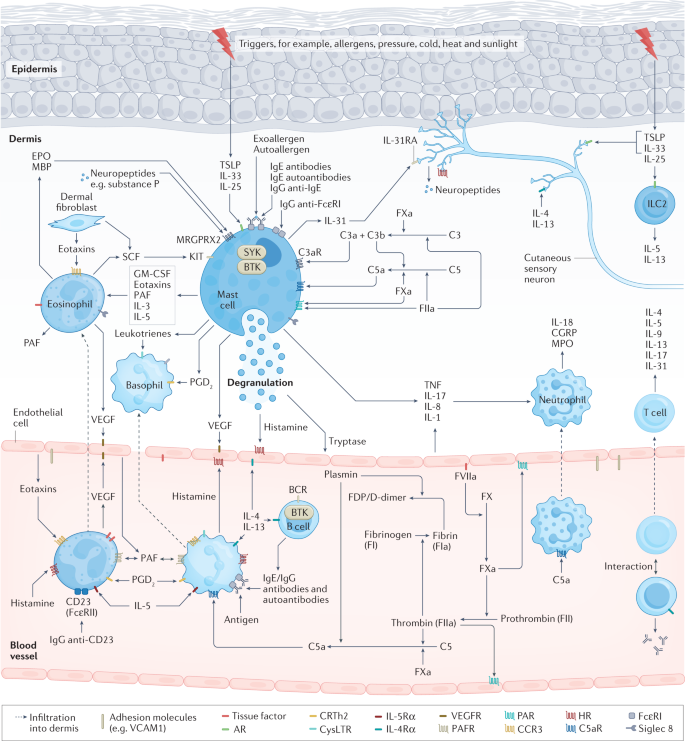
Activation and degranulation of mast cells leads to the development of signs and symptoms of urticaria due to the release of histamine and other mediators, which activate sensory skin nerves (itch), dilate skin blood vessels (erythema) and induce plasma extravasation (oedema and influx of other immune cells). In allergic urticaria, release of alarmins, TSLP, IL-33 and IL-25, by the epithelium, activation of skin-resident group 2 innate lymphoid cells (ILC2), polarization of T cells (mostly T helper 2 cells (T H 2 cells)) with release of T H 2 cytokines, for example IL-4, IL-5 and IL-13, and allergen-specific IgE production by B cells enable subsequent cross-linking of IgE–FcεRI complexes on the surface of mast cells by allergens, which triggers mast cell activation. Chronic spontaneous urticaria (CSU) can appear due to a chain of complex multistep interlinked events including cell infiltration (mostly eosinophils, basophils, neutrophils and T cells), autoimmunity (for example, IgE/IgG histamine-releasing autoantibodies), neurogenic inflammation (via histamine-dependent and histamine-independent itch signalling pathways mediated by cutaneous pruriceptive sensory nerves), activation of the complement cascade (for example, via production of anaphylatoxin C5a) and activation of tissue factor-initiated extrinsic pathway of the coagulation cascade. Here, receptors (for example, FcεRI, C5aR, MRGPRX2, Siglec 8, KIT and IL-4Rα), signalling pathways (for example, BTK and SYK) and mediators (for example, histamine, tryptase, IL-5, IL-17 and IL-31) of mast cells, eosinophils, basophils and/or other immune cells involved in urticaria, as well as activating signals (for example, IgE anti-autoallergens), are the targets for current therapy and drugs in development. In chronic inducible urticaria (CIndU), similar mechanisms of mast cell activation including autoallergy and/or autoimmunity may have a role. AR, alarmin receptors; BCR, B cell receptor; BTK, Bruton’s tyrosine kinase; CGRP, calcitonin gene-related peptide; CRTh2, chemoattractant receptor-homologous molecule expressed on T H 2 cells; EPO, eosinophil peroxidase; FDP, fibrin degradation products; FXa, factor Xa; GM-CSF, granulocyte–macrophage colony-stimulating factor; HR, histamine receptors; MBP, major basic protein; MPO, myeloperoxidase; MRGPRX2, Mas-related G-protein-coupled receptor X2; PAF, platelet-activating factor; PAR, protease-activated receptor; PGD 2 , prostaglandin D2; SCF, stem cell factor; Siglec 8, sialic acid-binding immunoglobulin-like lectin 8; SYK, spleen tyrosine kinase; TNF, tumour necrosis factor; TSLP, thymic stromal lymphopoietin; VEGF, vascular endothelial growth factor.
CSU symptoms occur mainly due to the release of histamine, but also by a broad range of other produced and secreted mediators, including tryptase, prostaglandin D2 (PGD 2 ), TNF, IL-4, IL-5, IL-13, IL-17 and IL-31, which can exert effects on resident skin cells and other recruited target cells, for example T cells, eosinophils and basophils 3 , 13 .
Cell infiltrate
In CSU, perivascular and interstitial inflammatory cellular infiltrates, resembling an allergic late-phase reaction, include eosinophils, neutrophils, lymphocytes and basophils 9 , 59 , 60 , 61 . Infiltrating cells are thought to migrate from the blood into the skin in response to chemotactic factors, for example eotaxins , MCP3, RANTES, IL-5, C3a, C5a, TNF, IL-17 and platelet-activating factor (PAF), released by mast cells, activated endothelial cells, T H 2 cells, dermal fibroblasts and other cells 9 . Cell adhesion molecules, for example P-selectin, E-selectin, ICAM, VCAM and PECAM, are upregulated on the surface of endothelial cells in the lesional skin of patients with CSU due to action of histamine, thrombin, TNF and other factors 9 .
Blood basopenia and eosinopenia that are observed in ~10–15% of patients with CSU may reflect the transport of cells into the skin, and are associated with CSU activity, presence of autoantibodies and poor response to H1 antihistamines (H1-AH) and omalizumab 62 , 63 . Perivascular eosinophils and neutrophils appeared in wheals 30 min after intradermal injection of autologous serum and, along with T lymphocytes, increased in numbers over the following 2 h with a decrease by 48 h (neutrophils) or later (eosinophils and lymphocytes) 64 . Blood and skin-migrated basophils can contribute to the CSU pathogenesis by releasing histamine, leukotrienes and cytokines via activation of FcεRI and C5aR 65 , 66 . Eosinophil granule proteins such as major basic protein (MBP) were detected in the wheals of patients with CSU 67 , 68 . MBP was able to induce mast cell activation and degranulation, representing a putative mechanism of crosstalk between eosinophils and mast cells in CSU 67 . Activation of eosinophils can occur due to mast cell mediators, namely IL-5, TNF, PAF and eotaxin, and also IgG autoantibodies to the low-affinity IgE receptor 69 . Activated eosinophils can also release SCF, a growth factor for mast cells. Finally, eosinophils might be key players in activation of the coagulation cascade and of Mas-related G-protein-coupled receptor X2 (MRGPRX2) on mast cells via expression of tissue factor and release of MRGPRX2 agonists, respectively 67 , 69 .
T H 2 cells are a predominant type of lymphocyte in CSU skin biopsy samples but T H 1 cells and T H 17 cells are also present 9 . T H 2 cells are highly involved in allergic diseases, releasing a broad range of cytokines, stimulating IgE production and mast cell, basophil and eosinophil activation. In patients with CSU, cytokine levels and/or expression were increased in the blood and/or lesional skin, for example IFNγ, TNF, TGFβ, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-13, IL-17, IL-23, IL-24, IL-31 and IL-33, and correlated with disease activity, for example TNF, IL-6, IL-17, IL-23 and IL-24, and autologous serum skin test (ASST) positivity, for example IL-17 (ref. 9 ).
Coagulation cascade and complement
Eosinophils and dermal microvascular endothelial cells can express a large amount of tissue factor on their surface in response to tissue factor inducers, for example histamine, vascular endothelial growth factor (VEGF), LPS, TNF, IL-6, IL-33 and IL-1β 70 . Tissue factor can activate the extrinsic coagulation cascade leading to the production of activated coagulation factors, such as factor Xa (FXa) and FIIa (thrombin) 71 , 72 .
Coagulation factors, histamine, VEGF 73 , bradykinin, PAF and/or other molecules can lead to gap formation between vascular endothelial cells via protease-activated receptor 1 (PAR1), other specific receptors and/or a direct action on endothelial cells 9 . This results in the leakage of plasma that can contain autoantibodies to IgE or FcεRI, and/or autoantigens for specific IgE bound to mast cells in the skin with subsequent activation of mast cells and wheal and flare formation 9 . In addition, thrombin and FXa may induce mast cell degranulation via action on PAR1 and PAR2, respectively 10 , 74 . Furthermore, complement component C5a is thought to be produced following the activation of extrinsic coagulation, fibrinolysis and/or binding of IgG anti-FcεRI to FcεRI on mast cells and basophils 10 . Activated coagulation factors (FXa and FIIa) and plasmin can produce C5a and C5b from C5, and/or C3a and C3b from C3. C3a and C5a in leaked plasma can activate mast cells and basophils via C3aR and C5aR, respectively 10 , 75 . Finally, functional specific IgE antibodies to tissue factor were reported to release leukotriene C4 by tissue factor-stimulated peripheral basophils 76 .
Activation of coagulation and fibrinolysis in patients with CSU is reflected in increased mean platelet volume, levels of D-dimer, fibrin and fibrinogen degradation products, prothrombin fragment 1 + 2, FVIIa and other molecules 41 , 71 . An increase in blood markers of thrombin generation and fibrinolysis, for example D-dimer, was linked to severe CSU and a decrease was observed during CSU remission 77 . Although activation of the coagulation cascade happens in CSU, it is considered mostly a local process with active fibrinolysis without increased risk for thrombotic events 71 .
In patients with CSU, increased C-reactive protein (CRP) levels correlated with increased D-dimer, IL-6, C3 and C4 levels as well as CSU activity and ASST positivity 78 , 79 . This finding supports a close link between autoimmunity, inflammation, complement and coagulation activation pathways in CSU pathogenesis that may lead to maintenance and amplification of urticarial inflammation.
Autoantibodies
Antibodies of IgE, IgA, IgM and IgG classes are thought to be critically involved in the pathogenesis of CSU 80 , 81 , 82 . IgE binds to the α-subunit of its high-affinity receptor, FcɛRI, on mast cells and basophils and, if present at high concentrations, can activate these cells regardless of the specific antigen 83 . In addition, the cross-linking of IgE by their respective allergens, autoallergens and IgG anti-IgE antibodies can lead to mast cell and basophil activation and mediator release in allergic, autoallergic and autoimmune urticaria, respectively 8 , 82 , 84 , 85 .
Clinically relevant allergy as a cause of CSU is rare 84 , 86 . IgE antibodies to autoantigens, for example thyroid peroxidase (TPO), eosinophil peroxidase (EPO), double-stranded DNA, tissue factor, ECP, FcεRI, thyroglobulin and IL-24, are found in up to two-thirds of patients with CSU and some of these antibodies, for example IgE anti-IL-24 and IgE anti-TPO, were shown to activate mast cells and/or basophils in vitro 8 , 87 . Furthermore, evidence in support of IgE autoantibodies as a driver of CSU includes high rates of positive skin prick tests with TPO in patients with CSU with increased levels of IgE anti-TPO and successful experiments of passive transfer of IgE anti-TPO 88 . Cross-reactivity between proteins, for example between TPO (not present in the skin) and EPO (present in the skin) 89 , and expression of autoallergens in the skin, for example IL-24, might explain why IgE–autoallergen interaction leads to activation of mast cells in skin rather than in other organs 90 .
IgG autoantibodies to IgE and FcɛRI were the first autoantibodies described in the context of autoimmune CSU. Most studies report that 20–50% of patients with CSU have these autoantibodies 91 . Since 2013, when a taskforce position paper was published, autoimmune CSU associated with these autoantibodies is defined by triple positivity for presence of IgG autoantibodies by immunoassay, functionality of autoantibodies assessed by basophil tests (basophil activation test and/or BHRA) and skin autoreactivity assessed by ASST 92 . If these strict criteria are applied, only 8% of patients with CSU are diagnosed with autoimmune CSU 7 .
It is still a matter of debate whether clearly defined and distinct auto-IgE and auto-IgG endotypes exist. Although real overlap rates are still unknown, there is growing evidence of co-expression of IgG autoantibodies and other types of autoantibodies, specifically IgE, IgM and IgA autoantibodies, in the same patient 80 , 81 . IgE autoantibodies might appear in blood first, followed by other types of autoantibodies over the course of the disease.
Neurogenic inflammation
In CSU, the bidirectional interaction of mast cells, other immune cells and sensory nerves is thought to exist through release of histamine, IL-31, neuropeptides and other mediators 67 , 93 , 94 . This vicious cycle causes vasodilatation, plasma extravasation, neurogenic inflammation, pruritus and other urticaria symptoms, and might be supported via MRGPRX2-mediated activation of mast cells 95 . MRGPRX2 is a receptor for a broad range of exogenous and endogenous substances and is responsible for IgE-independent activation of mast cells 96 . MBP and EPO induced histamine release from human skin mast cells via MRGPRX2 (ref. 67 ). Levels of substance P, a neuropeptide and MRGPRX2 agonist, were elevated in CSU, correlated with disease activity in some studies 97 , and provocation with MRGPRX2 agonists revealed an increased skin reactivity in patients with CSU 98 . Lastly, the number of MRGPRX2-expressing mast cells has been found to be increased in patients with CSU 67 .
Chronic inducible urticaria
The pathogenesis of CIndU is largely unknown. Autoallergic IgE-mediated mast cell activation was suggested in symptomatic dermographism, ColdU, solar urticaria and CholU by passive transfer experiments and/or the efficacy of omalizumab 99 . IgE autoantibodies might be produced to a skin-derived protein released upon stimulation, for example by cold 100 . In solar urticaria, molecular alteration of chromophores by solar electromagnetic radiation and their binding to surface-bound IgE on mast cells, leading to their activation, have been suggested 101 . In CholU, blockade of the sweat gland duct might lead to sweat reflux and leakage into the dermis inducing the symptoms of CholU due to production of a sweat antigen. Specific IgE to sweat antigen MGL_1304 have been identified in sera of some patients with CholU 102 . Another proposed mechanism in CholU is reduced expression of cholinergic receptor M3 (CHRM3) in eccrine sweat gland epithelial cells, which leads to escape of acetylcholine with acetylcholine-mediated degranulation of adjacent mast cells 102 . IgG and/or IgM directed to IgE were shown in ColdU 100 . In patients with heat urticaria, few cases of a positive reaction on intradermal testing with heated autologous serum, presumably containing denatured IgE and inactivated complement, were described 103 . Hereditary vibratory angioedema can present as an autosomal dominant variant due to a gain-of-function mutation in adhesion G-protein-coupled receptor E2 (ADGRE2), located on mast cells, which might decrease inhibitory interaction between the α-subunit and the β-subunit of this receptor leading to sensitization of mast cells to vibration-induced degranulation 104 . Delayed pressure urticaria is thought to occur via a non-immunologic mechanism with involvement of many pro-inflammatory mediators other than histamine, for example IL-1, IL-6, IL-3 and TNF 105 . In contrast to other CIndU, delayed pressure urticaria showed a substantial leukocyte infiltration in the dermis perhaps due to a sustained initiating stimulus 105 . Contact urticaria can be classified as an immunological reaction, that is IgE-mediated or T cell-mediated, or a non-immunological reaction 106 . Hypotheses explaining pathogenesis of aquagenic urticaria include the synthesis of a mast cell-degranulating substance due to interaction between water and a component in or on the skin or sebum; changes in osmotic pressure surrounding hair follicles and increased passive diffusion of water; the existence of water-soluble antigens in the epidermis; and a histamine-independent mechanism 107 .
Diagnosis, screening and prevention
The clinical presentation of urticaria is very similar in all age groups, ethnicities and genders. Wheals and angioedema occur with the same anatomical distributions across all skin tones. However, erythema related to whealing is more difficult to detect in pigmented skin 108 . A detailed history and physical examination are the essential first steps in the diagnostic workup of all patients with urticaria. However, as wheals and angioedema are transient and may not be present at physical examination, physicians should also review patients’ pictures or their documentation of signs and symptoms (Fig. 4 ). Diagnosis of urticaria is usually straightforward irrespective of its type or subtype 4 (see Supplementary Fig. 1 ).
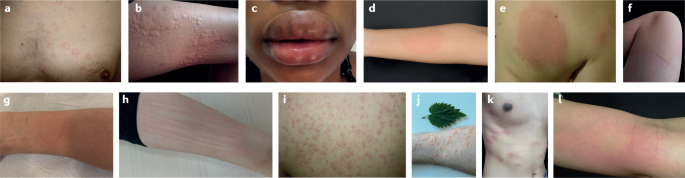
a | Acute urticaria (AU). b | Chronic spontaneous urticaria (CSU) (wheals). c | CSU (angioedema). d | Cold urticaria (ColdU). e | Delayed pressure urticaria. f | Solar urticaria. g | Heat urticaria. h | Symptomatic dermographism. i | Cholinergic urticaria (CholU). j | Contact urticaria. k | Aquagenic urticaria. l | Vibratory angioedema. Part e , image courtesy of A. Kasperska-Zajac. Part g , image courtesy of L. F. C. Ensina. Part l , image courtesy of K. Brockow.
By definition, AU is self-limiting and does not require routine or extensive diagnostic tests unless strongly suggested by patient history 4 . For example, in patients with a history of type I food allergy or drug hypersensitivity, urticaria symptoms can appear immediately after a contact with respective allergens and allergy tests may help avoid re-exposure to relevant causative factors.
Most patients with CSU present with only wheals (~57%), whereas the occurrence of wheals and angioedema (~37%) or only angioedema (~6%) is less common (see Supplementary Table 8 ). The signs and symptoms of CSU can occur spontaneously at any time of the day but commonly during the evening and night 109 , which may reflect circadian variations in mast cell activation 110 and differences in the underlying pathogenesis. For example, the presence of nocturnal symptoms has been associated with an autoimmune endotype of CSU 111 . Wheals can occur anywhere, but favour the arms and legs 109 . Angioedema appears most commonly on the face, for example lips and eyelids, but also on hands and feet and other body parts 1 . In most patients with moderate or severe CSU, wheals and/or angioedema occur on a daily or nearly daily basis or show an intermittent–recurrent course 4 .
In some patients with CSU, stress, foods, drugs (mostly NSAIDs) or infections can lead to exacerbation. NSAID hypersensitivity is observed in up to 30% of patients with CSU and is classified as NSAID‐exacerbated cutaneous disease 53 . CSU exacerbation occurs within minutes or hours after the intake of COX1 inhibitors (selective COX2 inhibitors are usually tolerated) 112 and can be confirmed by oral drug provocation testing 53 .
The current international urticaria guideline has introduced the 7C concept, that is, seven aims of the diagnostic workup in patients with CSU 4 . The 7C concept includes ruling out differential diagnoses (confirm); looking for markers of autoimmune urticaria (cause); identifying potential triggers (cofactors); checking for autoimmunity, mental health disorders and other comorbidities; identifying problems with sleep, distress, sexual health and social performance (consequences); assessing potential biomarkers or predictors of treatment response (components); and monitoring CSU activity, impact and control (course).
Basic tests include a differential blood count and the erythrocyte sedimentation rate (ESR) and/or CRP in all patients with CSU, and measurements of IgG anti-TPO and total serum IgE levels in patients with CSU in specialist care. The latter two can help in the diagnosis of autoimmune CSU and elevated IgG anti-TPO levels point to concomitant autoimmune thyroiditis 7 , 40 , 113 . Further tests should only be performed if indicated by patient history and are rarely needed 4 .
In patients with CIndU, wheals are often of shorter duration (≤1 h) than in those with CSU (up to 24 h) 4 , 106 . High-frequency trigger exposure and a low trigger threshold are linked to high disease activity 4 , 106 . Even though the lesions are usually confined to areas of skin exposed to the trigger stimuli, systemic reactions including anaphylaxis may occur and adrenaline autoinjectors should be prescribed to high-risk patients 114 . During pregnancy, one-half of patients experience improvement of their CU, whereas exacerbation occurs in approximately one-third of patients 115 . In particular, patients with CIndU or both CSU and CIndU showed a twofold increase of disease exacerbation during pregnancy. Disease activity changes during pregnancy might be linked to changes in trigger exposure and/or hormonal and immunological changes that promote mast cell activation.
The diagnosis of CIndU is based on a thorough history and the results of provocation testing . The aims of provocation testing are to determine the relevant triggers and to assess trigger thresholds, which are useful for measuring disease activity and monitoring treatment responses. For most CIndU subtypes, validated tools for provocation testing are available (Table 2 ; see Supplementary Table 9 and Supplementary Fig. 2 ).
Comorbidities
Infection, mainly upper respiratory tract infection, is considered the most common comorbidity and aetiological factor of acute spontaneous urticaria (~30–40% of AU cases), although the prevalence of infectious aetiologies decreases as the age of patients increases (see Supplementary Table 4 ). Less frequently, reactions can be triggered by drugs, mostly NSAIDs and antibiotics, inhalant allergens (for example, pollen, dust mites), foods, stress, Hymenoptera stings and physical factors (see Supplementary Table 4 ). Drugs such as NSAIDs are often used when infection is present, and this makes it challenging to differentiate between medications or infection as the cause of urticaria. Inducible factors are considered to be a rare cause of AU in children but can lead to AU in <15% of all adult AU cases 116 (see Supplementary Table 4 ). The cause of AU was more frequently detected in patients 0–6 years of age than in those 7–18 years of age 51 . Foods and infection caused AU more often in children <13 years of age than in children 13–18 years of age 51 . AU usually disappears after resolution or eradication of infection, or avoidance of drugs and other causative triggers.
CIndU is a frequent comorbidity of CSU, with most studies reporting rates >10% (see Supplementary Table 6 ). Among patients with CU, 29–93% had CSU only, 6–35% had CIndU only and 1–43% had both CSU and CIndU (see Supplementary Table 6 ). Of 245 patients with CSU, 36% had CIndU confirmed by a positive challenge test, mostly symptomatic dermographism (25%) and ColdU (13%) 48 . Multiple types of CIndU may coexist in the same patient with CSU 117 .
Autoimmune diseases are present in 28% of patients with CSU, most frequently autoimmune thyroid diseases (~25%, mostly Hashimoto’s thyroiditis with or without hypothyroidism), vitiligo, rheumatoid arthritis, autoimmune gastritis and diabetes mellitus (1–2%) 40 , 118 .
Bacterial infection including Helicobacter pylori and focal bacterial infections, for example dental infection, have been reported in up to 77% of patients with CSU 5 . However, the evidence for the causal link between bacterial infection and CSU development is still weak and conflicting 5 . The clinical relevance of viral infection, for example viral hepatitis and HIV infection, and fungal infection is still unknown and these infections are unlikely to contribute to the development of CSU 5 . In one study, CU did not affect the course of COVID-19, whereas COVID-19 induced CU exacerbation in one-third of patients, especially in patients with severe COVID-19 (ref. 119 ). CSU improved in one-third of patients after the treatment of confirmed infection with helminths or protozoa with antiparasitic drugs, although the link between CSU and parasites is still ill-characterized 5 .
The evidence for the increased prevalence of allergic diseases in patients with CSU is inconsistent 5 . Three large studies suggest that individuals with CSU are considerably more likely to have allergic diseases compared with the general population or control subjects without urticaria 34 , 120 , 121 . Type I hypersensitivity to allergens as a cause of CSU should be ruled out in patients with intermittent symptoms, a temporal relationship to a particular allergen and possible symptoms due to other allergic diseases, for example asthma.
Cancer, mostly non-haematologic, was reported in 0–9% of patients with CSU 5 , 26 and CSU resolved in some cases once patients were in remission 122 . CSU development in patients with cancer might be related to cancer-induced immune dysregulation including activation of complement and coagulation cascade 123 , and return to normal homeostatic conditions might parallel CSU improvement after cancer treatment. Mental health disorders, mainly depression and anxiety, are present in up to 60% of patients with CSU and are associated with considerably impaired quality of life 5 . Prevalence of one or several components of metabolic syndrome, that is, central obesity, dyslipidaemia, hyperglycaemia and hypertension, was considerably increased in patients with CU including CSU compared with control subjects without CU/CSU 5 .
CIndU can coexist with CSU and other CIndU forms. CSU was reported in 71%, 25% and 10% of patients with symptomatic dermographism 124 , CholU 125 and ColdU 114 , respectively. Among patients with CU, symptomatic dermographism alone or in combination with CSU is more prevalent than other CIndU, followed by ColdU and CholU (see Supplementary Table 6 ). Allergic diseases were reported to be a frequent comorbidity (up to 26–48%) of CholU 126 , solar urticaria 127 , ColdU 128 and symptomatic dermographism 129 .
Differential diagnosis
Patients with certain diseases other than urticaria can present with wheals and/or angioedema as associated or prodromic signs 130 (Fig. 5 ; see Supplementary Table 10 ). The differential diagnoses of CSU are guided by the history and physical examination and supported by exploratory tests.
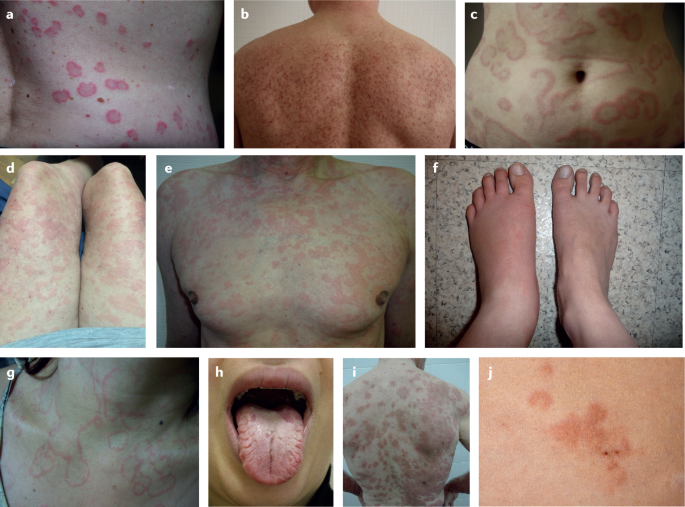
a | Urticarial vasculitis. b | Mastocytosis in the skin. c | Erythema annularis centrifugum. d | Cryopyrin-associated autoinflammatory syndrome e | Schnitzler syndrome. f | Hereditary angioedema. g | Erythema marginatum in a patient with hereditary angioedema. h | Melkersson–Rosenthal syndrome (oedema of the upper lip and fissured tongue are seen). i | Erythema multiforme. j | Hypereosinophilic syndrome.
In patients who exclusively develop wheals but not angioedema, autoinflammatory disorders, such as Schnitzler syndrome or cryopyrin-associated periodic syndromes, should be considered differential diagnoses 4 . Wheals in patients with autoinflammatory disorders are refractory to antihistamines and can present a distinct entity called neutrophilic urticarial dermatosis with a dense perivascular and interstitial infiltrate of neutrophils with leukocytoclasia but without vessel wall necrosis 131 .
In urticarial vasculitis, both long-lasting wheals (>24 h) and angioedema can occur and the diagnosis is confirmed by a combination of three histological criteria: leukocytoclasia, fibrin deposits and extravasated erythrocytes 132 , 133 . By contrast, CSU histopathology usually shows dermal oedema with an inflammatory infiltrate consisting of lymphocytes, eosinophils, neutrophils and nuclear dust, without evidence of vasculitis 60 , 132 .
When patients show recurrent angioedema without wheals, bradykinin-mediated angioedema including angiotensin-converting enzyme inhibitor-induced angioedema and hereditary angioedema should be excluded 4 .
Disease activity and control
Several patient-reported outcome measures, global and validated tools, were developed to assess CU activity and control and are used both in clinical care and in trials 134 (Box 1 ). The Urticaria Activity Score (UAS) is a gold standard to assess disease activity (wheals number and itch intensity) in CSU and is usually used prospectively during seven consecutive days (UAS7) 4 , 135 . Similarly, the Angioedema Activity Score (AAS) is a prospective, diary-type tool that enables clinicians to measure angioedema activity in patients with all forms of recurrent angioedema 136 . In CIndU, the Cholinergic Urticaria Activity Score (CholUAS; not yet validated) and the Cold Urticaria Activity Score (ColdUAS), modified versions of the UAS, collect data on a daily basis assessing the intensity of wheals and pruritus as well as the intensity of exposure to specific triggers with a recall period of 24 h 134 , 137 , 138 . The Urticaria Control Test (UCT) 139 and the Angioedema Control Test (AECT) 140 are retrospective tools with a recall period of 4 weeks used to assess disease control in all forms of CU.
Box 1 Established disease-specific instruments to assess activity and control of chronic urticaria and patient quality of life
Disease activity
Urticaria Activity Score (UAS) 4 , 135
Angioedema Activity Score (AAS) 136
Cholinergic Urticaria Activity Score (CholUAS) 138
Cold Urticaria Activity Score (ColdUAS) 137
Disease control
Urticaria Control Test (UCT) 139
Angioedema Control Test (AECT) 140
Patient quality of life
Chronic Urticaria-Quality of Life Questionnaire (CU-Q 2 oL) 223
Angioedema-Quality of Life Questionnaire (AE-QoL) 225
Cholinergic Urticaria-Quality of Life Questionnaire (CholU-QoL) 224
No screening methods are available for urticaria in the general population. Patients with autoimmune diseases, especially autoimmune thyroiditis, should be asked for signs and symptoms of CSU 40 , 42 , 118 . Similarly, patients with CSU should be regularly checked for autoimmune thyroid disease by palpation of the thyroid, thyroid function tests and anti-TPO and anti-thyroglobulin antibodies 4 , 40 , 118 .
In CSU, basic screening tests (differential blood count, ESR and/or CRP) and screening tests performed in specialist care (total IgE and IgG anti-TPO) can help in identifying comorbid and underlying diseases, for example chronic infection, autoimmune disease and allergy, as well as differential diagnoses, for example urticarial vasculitis or autoinflammatory disorder 4 . Mental health disorders, namely depression and anxiety, common comorbidities and consequences of CSU should be checked for in every patient with CSU 4 .
No primary prevention exists for urticaria with the possible exception of allergic urticaria, in which general preventive measures for allergic diseases may apply (especially in children with high risk of allergy). A population-based cross-sectional study in China found that breastfeeding >6 months after birth can lower the risk of urticaria 33 . Secondary prevention measures include avoidance of exposure to a relevant trigger, for example wearing of tight-fitting clothes by a patient with delayed pressure urticaria or intake of particular food or drug by a patient with allergic urticaria 4 . Migration to different geographic regions with different ambient temperatures might decrease the risk of development of CIndU such as ColdU. Finally, tertiary prevention is possible with treatment of symptoms using guideline-recommended options, for example antihistamines and omalizumab 4 , and might be possible with induction of long-lasting remission with disease-modifying treatments, for example allergen-specific immune therapy in patients with allergic urticaria 84 , cyclosporine in patients with autoimmune CSU 141 , 142 and novel mast cell-reducing therapies 143 .
The management of urticaria has progressed substantially over the past two decades, with disease control possible for at least two-thirds of patients 144 , 145 , 146 . Increased understanding of disease pathogenesis underpins an expanding pipeline of targeted therapies with improved adverse effect profiles compared with traditional immunosuppressants (Table 3 ). Structured assembly of the evidence for pharmacological and non-pharmacological agents using GRADE methodologies (Grading of Recommendations Assessment, Development and Evaluation methodologies), together with publication of up-to-date international consensus guidelines, has led to the establishment and regular review of an international standard of care for urticaria 4 . The overall goal of treatment is safe and effective attainment of complete disease control (UAS7 = 0 and UCT = 16) with a normal quality of life. Figure 6 provides the current consensus algorithm for urticaria treatment 4 .
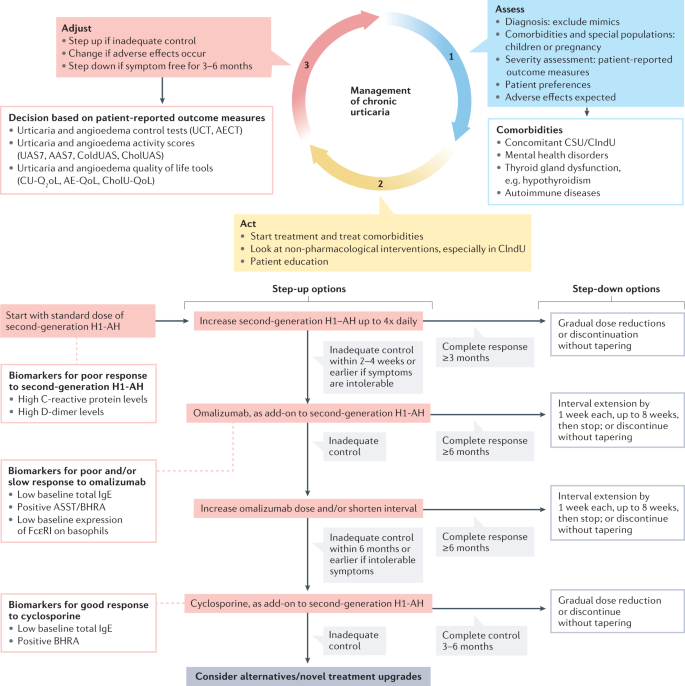
The 3A management approach of assess, act and adjust recognizes the importance of several comorbidities associated with urticaria; and the wide range and dynamic nature of disease severity across time, with the need to step up and step down therapies to achieve complete disease control while limiting cost and adverse effects. Only some biomarkers of response to treatment are shown 246 , 259 and other biomarkers are listed in Supplementary Box 1 . AAS7, Angioedema Activity Score used prospectively during 7 consecutive days; AECT, Angioedema Control Test; AE-QoL, Angioedema-Quality of Life Questionnaire; ASST, autologous serum skin test; BHRA, basophil histamine release assay; CholUAS, Cholinergic Urticaria Activity Score; CholU-QoL, Cholinergic Urticaria-Quality of Life Questionnaire; CIndU, chronic inducible urticaria; ColdUAS, Cold Urticaria Activity Score; CSU, chronic spontaneous urticaria; CU-Q 2 oL, Chronic Urticaria-Quality of Life Questionnaire; H1-AH, H1 antihistamines; UAS7, Urticaria Activity Score used prospectively during 7 consecutive days; UCT, Urticaria Control Test. Adapted with permission from ref. 4 , Wiley.
Elimination of causes and avoidance of triggers
AU or CSU with an intermittent–recurrent course with an identifiable exogenous trigger, for example an NSAID, is effectively managed with avoidance of the trigger and cross-reacting molecules 4 , 147 . Inducible urticaria, by definition, has an eliciting factor, or group of factors, and exposure reduction methods are a mainstay of therapy; however, avoidance might be hard to achieve, for example avoiding cold ambient temperatures, or might negatively affect quality of life 4 . Autoimmunity underlying CSU cannot be eliminated, although direct autoantibody removal through plasmapheresis has been successful, with high financial cost, in a limited number of patients with severe treatment resistance 148 , 149 . Removal of inciting antigens, either spontaneously (for example, resolution of an acute viral or bacterial infection) or through treatment of an underlying condition (for example, solid cancer surgery), can lead to resolution of urticaria 122 . Disappointingly, especially in CU, the treatment of underlying or associated factors often fails to alter the trajectory of CU. Eradication therapies for chronic infections, such as H. pylori , are a good example, and an analysis of studies based on the GRADE approach demonstrated weak and conflicting evidence between H. pylori eradication and improvement of CU 150 . Thyroid supplementation or anti-thyroid drugs in patients with CSU with Hashimoto’s thyroiditis or Graves’ disease, respectively, is another example when associated disease treatments have limited efficacy in reducing urticaria 118 , 151 . In most of these instances, no randomized controlled trial data are available and the evidence quality is low 4 , 151 . Thus, the intensive and costly general screening programmes for causes of CSU should not be performed, unless indicated by the patient’s clinical history, physical examination and/or initial CSU workup. In addition, the treatment of associated thyroid autoimmunity and other comorbidities should be individualized and based on disease-specific complications, for example hypothyroidism 4 .
Second-generation H1 antihistamines
Multiple effective and affordable second-generation antihistamines (sgAHs) that block the H 1 receptor are available and are the standard and first step in urticaria treatment 152 , 153 . They do not antagonize the binding of histamine but act as inverse agonists and have the opposite effect on the receptor to histamine, therefore shifting the equilibrium towards the inactive state 154 . Most guidelines recommend against the use of first-generation H1-AH due to sedative and anticholinergic effects, and drug–drug interactions 4 , 155 . Most, but not all, sgAHs have been evaluated and shown effective in urticaria at standard doses 4 . Studies have also confirmed the safety and efficacy of using off-label high-dose sgAH therapy (up to fourfold the recommended daily dose), including for bilastine, cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine and rupatadine 145 , 156 , 157 , 158 , 159 , 160 However, the use of updosed sgAHs is associated with a higher risk of somnolence than the use of standard dose of sgAHs (relative risk 3.28; 95% confidence interval 1.55–6.95; p = 0.002) 161 . Of note, British urticaria guidelines from 2021 recommend against updosing on mizolastine 162 . High-dose sgAHs are now considered part of the first step in urticaria treatment, if the standard dose is not sufficient to control symptoms 4 . The optimal timing and approach to step up or step down antihistamine therapy are still based on expert opinion, and include both tapering or immediate discontinuation. Based on half-life considerations, a 2-week period is generally considered sufficient to observe the effect of antihistamine changes in CU 163 .
Around 61% of patients with CSU do not respond to the licensed doses of sgAHs and only ~63% of these non-responders benefit from updosed sgAHs 145 . The second-line therapy with omalizumab as an add-on to sgAHs is indicated in patients with CSU who do not respond to increased doses of sgAHs 4 .
Omalizumab is the first in-class anti-IgE monoclonal antibody (mAb) and binds and lowers free IgE, with subsequent downregulation of FcεRI on basophils and mast cells 164 , 165 , 166 , 167 . Lowered FcεRI expression may render the cells less susceptible to activation by IgE and IgG anti-FcεRI and prevent histamine release and inflammation 167 . As the step-up treatment from antihistamines, omalizumab, as an add-on therapy, has the strongest evidence base and recommendations of all possible treatments for CSU in patients ≥12 years of age 146 . A meta-analysis of 67 real-life CSU studies reported complete and partial response rates of 72% and 18%, respectively, comparable with efficacy in clinical trials, with a mean adverse effect rate of 4% 168 . Omalizumab improves various aspects of quality of life in patients with CSU in both clinical trials and real-life studies 169 , 170 , 171 . It has also been shown to be effective (although has not yet been approved) in the management of CIndU, particularly symptomatic dermographism, ColdU, CholU and solar urticaria 166 , 172 , 173 , 174 , 175 . Poor and/or slow response to omalizumab was linked to several markers and features of autoimmune CSU, including BHRA positivity and low total IgE levels (see Supplementary Box 1 ).
Real-world practice continues to raise several questions around omalizumab use as monotherapy (without antihistamines): the initial treatment duration, updosing or a shortened dosing interval and discontinuation strategies 176 . Alternative approaches include combination therapy with other systemic immunosuppressives (cyclosporine, dapsone and colchine), although evidence is very limited, or a complete switch to cyclosporine, a third-line treatment of CU 4 , 164 , 177 , 178 .
Cyclosporine
Cyclosporine is a T cell immunosuppressive agent that also inhibits mediator release from basophils and mast cells 179 . It has been used for more than three decades for the treatment of CU 180 . Current guidelines recommend off-label use of cyclosporine as an add-on to sgAHs for patients with CSU with severe disease that is refractory to the combination of high-dose antihistamine and omalizumab 4 . Efficacy has been demonstrated in placebo-controlled randomized control trials 180 , 181 , 182 and open-label studies 183 , with meta-analysis data suggesting response rates up to 73% 182 . Adverse effects can be severe, particularly renal impairment, and occur in up to 50% of patients; hence, most guidelines do not recommend cyclosporine as standard treatment 4 . However, in many countries without access to omalizumab, cyclosprine is used as a step-up therapy from antihistamines, owing to a more favourable risk–benefit profile than long-term corticosteroid use 4 .
Alternative and specific treatments
Intravenous or oral systemic corticosteroids are effective for AU, and for rapid control of severe disease flares in CSU 4 . Guidelines and experts recommend limiting oral steroid therapy to a maximum of 10 days using the lowest effective dose, due to severe adverse effects associated with long therapy duration 4 . Other immunosuppressant or immunomodulatory therapies were used in CSU with various success rates, including dapsone, colchicine, sulfasalazine, methotrexate, interferon, phototherapy, intravenous immunoglobulin and plasmapheresis 4 , 184 . Few of these approaches have been studied in well-designed randomized control trials, with most data published as case series; hence, all have a low-quality evidence base 4 . Some immune therapies have shown efficacy including anti-TNF in CSU and delayed pressure urticaria 185 , 186 , and UVA, UVB and psoralen plus UVA (commonly termed PUVA) treatment in CSU, ColdU, CholU and symptomatic dermographism 187 , 188 , 189 .
Evidence for other therapies, such as H2 antihistamines, leukotriene receptor antagonists, diets, such as a pseudo-allergen-free diet, and mast cell stabilizers also remains low 4 . However, subgroups of patients with CSU might benefit from specific approaches, for example the use of leukotriene receptor antagonists in patients who are aspirin-intolerant 4 , 190 . Tolerance induction can be useful in selected patients with ColdU, CholU or solar urticaria 191 , but tolerance is lost without continuous daily exposure, making the therapy impractical for many patients, for example the need for daily cold baths or showers in patients with ColdU 4 .
Treatment of special populations
Pregnant women, breastfeeding women, children and geriatric populations require special consideration in CSU. The over-the-counter use of first-generation antihistamines and sgAHs for allergic disease makes it likely that, at least, early pregnancy exposure to these drugs is common 4 . However, safety in pregnancy has been shown for cetirizine and loratadine only, and these drugs are therefore preferred 192 , 193 . The use of sgAHs such as loratadine and cetirizine is also advised in breastfeeding women 4 , as nursing infants can develop sedation from first-generation antihistamines secreted into breast milk 194 . Omalizumab is reported safe in pregnancy 115 , 195 , 196 , 197 , 198 and in younger children, although current licensing is for individuals aged 12 years and older 146 .
In the paediatric population, several sgAHs have proven efficacy and safety: bilastine, cetirizine, desloratadine, fexofenadine, levocetirizine, loratadine and rupatadine 4 . The individual choice should consider country-specific availability of suitable preparations, such as syrups.
The older population is particularly sensitive to adverse effects from first-generation antihistamines, which should be avoided 199 . Data have shown efficacy and safety of standard doses of sgAHs and omalizumab in older patients 199 , 200 (≥65 years old). However, older populations may be particularly susceptible to the sedative action of some sgAHs, such as cetirizine and loratadine, when recommended doses are exceeded 199 . Also, sgAHs updosing might cause risks in some older patients with renal, hepatic and/or cardiac disorders.
Risk–benefit profiles for immunosuppressive therapies, including corticosteroids and cyclosporine, need to be carefully considered given the increased toxic effects in all three of these patient populations. Detailed reviews of all aspects of CSU management in these groups are available 115 , 199 , 201 , 202 , 203 .
Therapies in development
Various therapies, targeting mediators, receptors and signalling pathways of mast and other immune cells, are in preclinical and clinical development 13 , 164 , 204 , 205 , 206 (Fig. 3 ; all ongoing studies are listed in Table 3 ). Most therapies are focused on patients with antihistamine-refractory and/or omalizumab-refractory CSU.
Ligelizumab, a humanized anti-IgE mAb, had a greater affinity to IgE and showed higher efficacy than omalizumab in a phase IIb trial 207 but not in the phase III PEARL1 and PEARL2 trials 208 .
Fenebrutinib, a selective, reversible, oral BTK inhibitor, improved CSU within the first week and demonstrated a substantial benefit in patients with antihistamine-resistant CSU including patients with circulating anti-FcεRI autoantibodies; however, reversible grade 3 liver enzyme abnormalities were observed 209 . Another BTK inhibitor, remibrutinib, provided improvements in UAS7 versus placebo, increased patient quality of life 210 and had a favourable safety profile 211 ; a phase III trial is ongoing.
An anti-KIT mAb, barzolvolimab, was tested in CSU and CIndU 212 . In 95% of patients with antihistamine-resistant ColdU and symptomatic dermographism, a complete response (negative provocation testing) was observed after a single dose of barzolvolimab. Serum tryptase and skin mast cell depletion mirrored barzolvolimab clinical activity. Similar effects of multiple-dose treatment with barzolvolimab were seen in patients with CSU 213 and a phase II study is ongoing. Barzolvolimab was generally well tolerated and most adverse effects were mild or moderate in severity including urinary tract infections, headache, neutropenia and back pain in patients with CSU 214 and hair colour changes, mild infusion reactions and transient changes in taste in patients with CIndU 212 .
Benralizumab, an anti-IL-5Rα mAb, demonstrated sustained mean changes in UAS7 from baseline to week 24 in patients with CSU in a single-centre study: both components of the UAS7, pruritus severity and wheal size, decreased to a similar extent 215 . In a phase III trial, dupilumab, an anti-IL-4Rα mAb, reduced itch and wheals in patients with CSU who were omalizumab-naïve sgAH-resistant, but failed to meet primary end points in patients with poor response to omalizumab 216 .
Lirentelimab, an anti-Siglec 8 mAb, increased UCT scores across cohorts of patients with CSU and CIndU, with complete response rates of 92%, 36%, 82% and 40% in patients who are omalizumab-naïve, patients who are omalizumab-refractory, patients with CholU and patients with symptomatic dermographism, respectively 217 . Secukinumab, an anti-IL-17 mAb, was effective in eight patients with CSU who were omalizumab-refractory, but onset of action was slow 218 . Other promising targets include tryptase, SYK, C5aR, MRGPRX2, IL-33, thymic stromal lymphopoietin (TSLP) and H4R; for some of them, drugs are already in development (Table 3 and Fig. 3 ).
Quality of life
The quality of life of patients with urticaria, especially CU, is often severely impaired, with marked physical, psychological, social and emotional effects 11 . Many different instruments are available for the assessment of quality of life impairment in patients with urticaria, ranging from generic questionnaires, such as the Short Form-36 (SF-36) 219 or the Nottingham Health Profile (NHP) 220 , to organ-specific quality of life questionnaires, such as the Skindex-29 (ref. 221 ) or the Dermatology Life Quality Index (DLQI) 222 , to disease-specific instruments such as the Chronic Urticaria-Quality of Life Questionnaire (CU-Q 2 oL) 223 , Cholinergic Urticaria-Quality of Life Questionnaire (CholU-QoL) 224 and Angioedema-Quality of Life Questionnaire (AE-QoL) 225 (Box 1 ). These questionnaires are available in validated form for many countries and languages, and the disease-specific questionnaires are recommended by the current international guideline on the diagnosis and treatment of urticaria for all patients with CU 4 .
Owing to its short duration, AU has only a limited effect on patient quality of life, showing mean DLQI scores <1 for questions regarding social or leisure activities and personal relationships 226 . Patients with AU expressed greater satisfaction with life compared with patients with CU 227 . Patients with CIndU have a slightly better quality of life than patients with CSU, probably due to the transient nature of stimulus, whereas a combination of CSU and CIndU showed the highest DLQI scores, indicating the largest impact on quality of life 228 .
The two most important drivers of quality of life impairment in CSU are the unpredictable course of the disease with the sudden onset of wheals and angioedema, and the severe pruritus. Consequences of pruritus in patients with CSU include lack of sleep, fatigue and lack of concentration 12 , 18 , 109 , 229 , 230 . Many patients with CSU suffer from daily or almost daily occurrence of the symptoms 4 , 231 , which can lead to patients feeling loss of control over their lives 229 . The signs and symptoms of urticaria also result in embarrassment, frustration, sadness and anxiety in many patients 18 , 223 , 229 . These feelings are further exacerbated by the fact that the importance of urticaria is underestimated by others, including treating physicians 232 , 233 . In addition to reduced quality of life, CSU also leads to limitations in social interactions, work performance and functioning in daily life, including impairments in interpersonal relationships and sexual life 12 , 18 , 229 , 234 , 235 .
Few direct comparisons of quality of life impairment in CU with other skin diseases exist. Independent studies with large numbers of patients in Europe and the USA have shown that quality of life impairment in CU is comparable with that in patients with moderate to severe psoriasis 18 , 236 , 237 and atopic dermatitis 236 . Compared with non-dermatologic conditions, social quality of life impairments in patients with CU were shown to be similar to those in patients with coronary artery disease who were about to undergo arterial bypass surgery 229 and worse than in patients with type I diabetes mellitus 238 .
Untreated CU has substantial negative effects on patient quality of life, but response to therapy is associated with a corresponding improvement in quality of life: the more effective the therapy, the better the quality of life 239 , 240 , 241 , 242 .
Personalized treatment
AU is a disease with transient manifestations and without long-standing impairment of quality of life. However, for CU, no cure exists and the burden of disease is substantial. Future research should focus on the aetiology and pathogenesis of CU, which are currently poorly investigated and understood. Patients with CU often present with the same type of skin rash but differ in genotype, endotype and phenotype 38 , 91 , 243 .
Genetic contributions to urticaria pathogenesis should be elucidated in high-throughput genetic studies, including whole-genome sequencing. Presence of comorbid diseases along with the phenotypes of CU subtypes are factors that contribute to the heterogeneity of the disease and can influence the variable therapeutic response and adverse effects to available drugs. Differences in CU phenotypes have been reported, including better response to antihistamines in patients with exclusive CIndU and less psychiatric comorbidities in patients with isolated CIndU or recurrent CSU 243 . Defining disease endotypes and their biomarkers is a major unmet need and will enable the identification of novel therapeutic targets, refine disease management via optimization of prevention and treatment with the available drugs, and assign newly developed drugs to those patients who will have the best benefit to risk ratio. Ultimately, this will also contribute to reducing healthcare costs, with improved quality of life for patients with CU. In this regard, mast cell, basophil and eosinophil activating factors have to be identified to better characterize the aetiology and pathogenesis of CU. For example, commercially available in vitro tests for detecting serum autoantibodies need to be developed.
A multi-omics approach, using data from genomics, transcriptomics and proteomics, can help identify relevant biomarkers to objectively measure characteristics of the disease. Reliable biomarkers, either alone or in combination, can help stratify patients with CU according to phenotypes and endotypes of disease, predict progression from AU to CU and measure disease activity, relapse and response to treatment, all of which would lead to a more precise treatment approach. Markers of CSU activity are already emerging, including increased levels of CRP, IL-6 and D-dimer 78 , 79 , 244 . The autoimmune endotype of CSU is associated with basopenia, eosinopenia, elevated IgG anti-TPO and low total IgE levels, which were linked to poor response to treatment with antihistamines and/or omalizumab. By contrast, higher baseline levels of total IgE were associated with better clinical responses to omalizumab 245 , whereas low total IgE levels and a positive BHRA were linked to good response to cyclosporine 246 . Further promising markers exist (see Supplementary Box 1 ). Nevertheless, prospective multicentre studies are needed to further investigate these markers and identify new biomarkers, as well as to study pathogenesis in antihistamine-resistant urticaria and angioedema.
Drugs that failed to meet the primary end points in phase II/III trials in the classic one-size-fits-all approach may still be promising candidates in certain subpopulations of patients. Finally, drugs that have the potential to modify the disease course and induce sustained remission, especially in patients with CSU who are non-responders to sgAHs and omalizumab, are urgently needed.
Multidisciplinary approach including patients
A multidisciplinary approach should involve patients, dermatologists, allergists, rheumatologists, geneticists, pharmacologists, immunologists and researchers in these fields. Patient-reported outcome measures require more explicit recognition both in trials and in clinical practice. Specific quality of life instruments for ColdU, symptomatic dermographism and CholU already exist or are under development, but tools for the other forms of CIndU are missing and should be developed.
Patients with CU are interested in using mobile applications to monitor their disease activity and control 247 . For example, several mobile applications for the self-evaluation of CU and angioedema are available including UrCare, Urticaria, UrticariApp-Control Urticaria, SymTrac HIVES and TARGET My Hives 248 . However, existing mobile applications are limited in function and geographical reach 248 . The Chronic Urticaria Self Evaluation App (CRUSE) was developed in 2022 by the CRUSE core team and CRUSE advisory board, an international team of expert dermatologists and allergists, based on validated patient-reported outcome measures to help patients assess CSU activity and control and effects of CSU on quality of life. Patient’s data can be sent directly to the treating physician and included in the Chronic Urticaria Registry (CURE). Initially launched in Germany, the CRUSE team aims to cover patients with CSU worldwide in the near future. Patients with CU are increasingly seeking information from information and communications technologies with web browsers being the preferred platform to obtain general health information 249 , followed by email to contact physicians and WhatsApp for communicating with other patients 250 . The educational needs of patients can be addressed by urticaria-related websites and groups on social media, webinars and urticaria seminars.
Global approach
All urticaria stakeholders, including those in basic science, medical specialists, the pharmaceutical industry and health authorities together with patients, should contribute to creating solid evidence that enables identifying, managing and treating urticaria early with the aim of minimizing its effects on individuals and society. Awareness of up-to-date urticaria management should be increased worldwide to decrease diagnostic and therapeutic delays (Box 2 ). Several global initiatives have been launched to address these objectives.
First, international guidelines for diagnosis and treatment of urticaria endorsed by 50 national and international societies from 31 countries are revised every 4 years 4 . Second, networks of Urticaria and Angioedema Centers of Reference and Excellence (UCARE and ACARE, respectively) 251 , 252 have been launched by the Global Allergy and Asthma European Network (GA 2 LEN) with the aim to provide excellence in urticaria and angioedema management and to increase the knowledge of urticaria and angioedema through research and education. Third, the CURE 253 is the first international academia-driven registry to collect high-quality, real-life data on CU including patient characteristics, course of disease including cause, comorbidities, treatment response, quality of life impairment and healthcare data. Fourth, UCARE initiatives (UCARE LevelUp for physicians and UCARE 4U for patients) present up-to-date urticaria-related educational webinars. Finally, the Global Burden of Disease initiative can help assess urticaria prevalence, incidence and disability-adjusted life years 14 . However, as yet, these data do not differentiate between AU and CU, and therefore CU prevalence data for some countries and continents, for example Africa, are still lacking 14 , 22 .
A global approach together with mobile health and personalized medicine should help implement prevention and control of urticaria in a cost-effective manner with each patient getting the best available treatment.
Box 2 Global variations in diagnosis and management of urticaria
Approaches to the diagnosis and treatment of urticaria, as well as healthcare resource utilization, differ worldwide 260 , 261 . For example, patients residing in Europe compared with those in Central/South America have higher incidence of general practitioner visits and higher rates of controlled disease and treatment, including higher frequency of escalation to omalizumab 261 . This disparity can be explained by differences in awareness of the use of best available treatment options, differences in availability of diagnostic tests, treatment options and economic resources.
The mean time from chronic urticaria (CU) onset to proper diagnosis is ~2–4 years and considerably varies across countries 11 , 12 , 262 . Adherence to best-practice international urticaria guidelines has a direct affect on the quality of care of patients with urticaria and is associated with quicker diagnosis and treatment, and better treatment efficacy 12 , 260 , 263 . Approximately 60% of specialists follow international urticaria guidelines 260 and discrepancies in physicians’ awareness of the guidelines among countries exist 263 . Although national and international urticaria guidelines usually agree on most points regarding urticaria management, some expert-based recommendations differ (mostly because of weak scientific evidence) that can also affect the final patient outcome 264 .
One-fifth of physicians think that some of the guidelines’ recommendations cannot be implemented in the physician’s country of residency 260 . Availability of drugs and diagnostic tests, economic considerations, differences in coverage and payment for healthcare among different regions influence the choice of diagnostic and treatment strategies. Omalizumab is unavailable in some countries or its cost is high and not covered by health insurance programmes 265 ; thus, systemic corticosteroids and first-generation H1 antihistamines (H1-AH), which are cheaper than omalizumab and second-generation H1-AH, may be preferred 263 . Similarly, some diagnostic tests, for example the basophil histamine release test and basophil activation test for diagnosis of autoimmune chronic spontaneous urticaria (CSU) or TempTest for diagnosis of cold and heat urticaria, are not standardized, are expensive and/or are available only at some urticaria centres, which may contribute to diagnosis delay 92 .
Zuberbier, T., Balke, M., Worm, M., Edenharter, G. & Maurer, M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin. Exp. Dermatol. 35 , 869–873 (2010).
CAS PubMed Google Scholar
Lee, S. J. et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy Asthma Immunol. Res. 9 , 212–219 (2017).
PubMed PubMed Central Google Scholar
Church, M. K., Kolkhir, P., Metz, M. & Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 282 , 232–247 (2018).
Zuberbier, T. et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 77 , 734–766 (2022). This update and revision of the international guideline for urticaria developed by 64 experts from 31 countries covers the definition and classification of urticaria and outlines expert-guided and evidence-based diagnostic and therapeutic approaches for the different subtypes of urticaria.
PubMed Google Scholar
Kolkhir, P. & Maurer, M. in Urticaria and Angioedema (eds Zuberbier, T., Grattan, C. & Maurer, M.) 77–107 (Springer International, 2021).
Weller, K. et al. Epidemiology, comorbidities, and healthcare utilization of patients with chronic urticaria in Germany. J. Eur. Acad. Dermatol. Venereol. 36 , 91–99 (2022).
Schoepke, N. et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST study. Allergy 74 , 2427–2436 (2019). This international multicentre study characterizes for the first time the subpopulation of patients with autoimmune CSU diagnosed by triple positivity of the ASST, basophil tests and immunoassay.
Schmetzer, O. et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 142 , 876–882 (2018). In this study, IL-24 is a common, specific and functional autoantigen of IgE antibodies in patients with CSU, suggesting autoallergic mechanism in many patients with CSU.
Giménez-Arnau, A. M. et al. The pathogenesis of chronic spontaneous urticaria: the role of infiltrating cells. J. Allergy Clin. Immunol. Pract. 9 , 2195–2208 (2021).
Yanase, Y., Takahagi, S., Ozawa, K. & Hide, M. The role of coagulation and complement factors for mast cell activation in the pathogenesis of chronic spontaneous urticaria. Cells 10 , 1759 (2021).
CAS PubMed PubMed Central Google Scholar
Goncalo, M. et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 184 , 226–236 (2021).
Maurer, M. et al. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy 72 , 2005–2016 (2017).
Kolkhir, P., Elieh-Ali-Komi, D., Metz, M., Siebenhaar, F. & Maurer, M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 22 , 294–308 (2022).
Peck, G. et al. Global epidemiology of urticaria: increasing burden among children, females and low-income regions. Acta Derm. Venereol. 101 , adv00433 (2021).
Jadhav, R., Alcala, E., Sirota, S. & Capitman, J. Risk factors for acute urticaria in central California. Int. J. Environ. Res. Public Health 18 , 3728 (2021).
Eun, S. J., Lee, J. Y., Kim, D.-Y. & Yoon, H.-S. Natural course of new-onset urticaria: results of a 10-year follow-up, nationwide, population-based study. Allergol. Int. 68 , 52–58 (2019).
Parisi, C. A., Ritchie, C., Petriz, N., Torres, C. M. & Gimenez-Arnau, A. Chronic urticaria in a health maintenance organization of Buenos Aires, Argentina — new data that increase global knowledge of this disease. Bras. Dermatol. 93 , 76–79 (2018).
Google Scholar
Balp, M. M. et al. Burden of chronic urticaria relative to psoriasis in five European countries. J. Eur. Acad. Dermatol. Venereol. 32 , 282–290 (2018).
Wertenteil, S., Strunk, A. & Garg, A. Prevalence estimates for chronic urticaria in the United States: a sex- and age-adjusted population analysis. J. Am. Acad. Dermatol. 81 , 152–156 (2019).
Tayefi, M. et al. Chronic urticaria: a swedish registry-based cohort study on population, comorbidities and treatment characteristics. Acta Derm. Venereol. 102 , adv00624 (2022).
Seo, J.-H. & Kwon, J.-W. Epidemiology of urticaria including physical urticaria and angioedema in Korea. Korean J. Intern. Med. 34 , 418–425 (2019).
Fricke, J. et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy 75 , 423–432 (2020). This systematic review and meta-analysis reports prevalence of CU on a global level and shows considerable regional differences.
Xiao, Y. et al. The prevalence of atopic dermatitis and chronic spontaneous urticaria are associated with parental socioeconomic status in adolescents in China. Acta Derm. Venereol. 99 , 321–326 (2019).
Kim, Y. S. et al. Prevalence and incidence of chronic spontaneous urticaria in the entire Korean adult population. Br. J. Dermatol. 178 , 976–977 (2018).
Cantarutti, A. et al. Epidemiology of frequently occurring skin diseases in Italian children from 2006 to 2012: a retrospective, population-based study. Pediatr. Dermatol. 32 , 668–678 (2015).
Lapi, F. et al. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br. J. Dermatol. 174 , 996–1004 (2016).
Chu, C.-Y., Cho, Y.-T., Jiang, J.-H., Lin, E. I.-C. & Tang, C.-H. Epidemiology and comorbidities of patients with chronic urticaria in Taiwan: a nationwide population-based study. J. Dermatol. Sci. 88 , 192–198 (2017).
Balp, M.-M. et al. Prevalence and clinical characteristics of chronic spontaneous urticaria in pediatric patients. Pediatr. Allergy Immunol. 29 , 630–636 (2018).
Trevisonno, J., Balram, B., Netchiporouk, E. & Ben-Shoshan, M. Physical urticaria: review on classification, triggers and management with special focus on prevalence including a meta-analysis. Postgrad. Med. 127 , 565–570 (2015).
Bal, F., Kahveci, M., Soyer, O., Sekerel, B. E. & Sahiner, U. M. Chronic inducible urticaria subtypes in children: clinical features and prognosis. Pediatr. Allergy Immunol. 32 , 146–152 (2021).
Balp, M.-M. et al. Clinical remission of chronic spontaneous urticaria (CSU): a targeted literature review. Derm. Ther. 12 , 15–27 (2022).
Thomsen, S. F., van der Sluis, S., Kyvik, K. O. & Backer, V. Urticaria in monozygotic and dizygotic twins. J. Allergy 2012 , 125367 (2012).
Yabin, H. et al. Breastfeeding duration modified the effects of neonatal and familial risk factors on childhood asthma and allergy: a population-based study. Respir. Res. 22 , 41 (2021).
Rosman, Y. et al. Characterization of chronic urticaria and associated conditions in a large population of adolescents. J. Am. Acad. Dermatol. 81 , 129–135 (2019).
Gaig, P. et al. Epidemiology of urticaria in Spain. J. Investig. Allergol. Clin. Immunol. 14 , 214–220 (2004).
Ferrer, M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J. Investig. Allergol. Clin. Immunol. 19 , 21–26 (2009).
Jurado-Escobar, R. et al. Genetic variants in cytosolic phospholipase A 2 associated with nonsteroidal anti-inflammatory drug-induced acute urticaria/angioedema. Front. Pharmacol. 12 , 667824 (2021).
Losol, P., Yoo, H.-S. & Park, H.-S. Molecular genetic mechanisms of chronic urticaria. Allergy Asthma Immunol. Res. 6 , 13 (2014).
O’Donnell, B. F. et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br. J. Dermatol. 140 , 853–858 (1999).
Kolkhir, P. et al. Autoimmune diseases are linked to type IIb autoimmune chronic spontaneous urticaria. Allergy Asthma Immunol. Res. 13 , 545–559 (2021).
Confino-Cohen, R. et al. Chronic urticaria and autoimmunity: associations found in a large population study. J. Allergy Clin. Immunol. 129 , 1307–1313 (2012). This study involving more than 12,000 patients with CU points to a strong association between CU and major autoimmune diseases.
Kim, Y. S. et al. Increased risk of chronic spontaneous urticaria in patients with autoimmune thyroid diseases: a nationwide, population-based study. Allergy Asthma Immunol. Res. 9 , 373–377 (2017).
Asero, R. Chronic idiopathic urticaria: a family study. Ann. Allergy Asthma Immunol. 89 , 195–196 (2002).
Sahiner, U. M. et al. Chronic urticaria: etiology and natural course in children. Int. Arch. Allergy Immunol. 156 , 224–230 (2011).
Irinyi, B. et al. Clinical and laboratory examinations in the subgroups of chronic urticaria. Int. Arch. Allergy Immunol. 144 , 217–225 (2007).
Chen, C.-M., Huang, W.-T., Chang, L.-J., Hsu, C.-C. & Hsu, Y.-H. Peptic ulcer disease is associated with increased risk of chronic urticaria independent of Helicobacter pylori infection: a population-based cohort study. Am. J. Clin. Dermatol. 22 , 129–137 (2021).
Chen, T.-L. et al. Risk of chronic spontaneous urticaria in reproductive-aged women with abnormal uterine bleeding: a population-based cohort study. J. Dermatol. 48 , 1754–1762 (2021).
Sánchez, J. et al. Prevalence of inducible urticaria in patients with chronic spontaneous urticaria: associated risk factors. J. Allergy Clin. Immunol. Pract. 5 , 464–470 (2017).
Vietri, J. et al. Effect of chronic urticaria on US patients: analysis of the National Health and Wellness Survey. Ann. Allergy Asthma Immunol. 115 , 306–311 (2015).
Balp, M.-M., Vietri, J., Tian, H. & Isherwood, G. The impact of chronic urticaria from the patient’s perspective: a survey in five European countries. Patient 8 , 551–558 (2015).
Techasatian, L., Phungoen, P., Chaiyarit, J. & Uppala, R. Etiological and predictive factors of pediatric urticaria in an emergency context. BMC Pediatrics 21 , 92 (2021).
Galli, S. J. & Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 18 , 693 (2022).
Doña, I. et al. Progress in understanding hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Allergy 75 , 561–575 (2020).
Asero, R. Peach-induced contact urticaria is associated with lipid transfer protein sensitization. Int. Arch. Allergy Immunol. 154 , 345–348 (2011).
Okayama, Y. & Kawakami, T. Development, migration, and survival of mast cells. Immunol. Res. 34 , 97–115 (2006).
Gilfillan, A. M. & Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6 , 218–230 (2006).
Mendes-Bastos, P. et al. Bruton’s tyrosine kinase inhibition — an emerging therapeutic strategy in immune-mediated dermatological conditions. Allergy https://doi.org/10.1111/all.15261 (2022).
Article PubMed Google Scholar
Karra, L., Berent-Maoz, B., Ben-Zimra, M. & Levi-Schaffer, F. Are we ready to downregulate mast cells? Curr. Opin. Immunol. 21 , 708–714 (2009).
Kay, A. B. et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br. J. Dermatol. 171 , 505–511 (2014).
Batista, M. et al. Histopathology of chronic spontaneous urticaria with occasional bruising lesions is not significantly different from urticaria with typical wheals. J. Cutan. Pathol. 48 , 1020–1026 (2021).
Ying, S., Kikuchi, Y., Meng, Q., Kay, A. B. & Kaplan, A. P. T H 1/T H 2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J. Allergy Clin. Immunol. 109 , 694–700 (2002).
Kolkhir, P. et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J. Allergy Clin. Immunol. Pract. 8 , 318–325 (2020).
MacGlashan, D. Jr, Saini, S. & Schroeder, J. T. Response of peripheral blood basophils in subjects with chronic spontaneous urticaria during treatment with omalizumab. J. Allergy Clin. Immunol. 147 , 2295–2304 (2021).
Grattan, C. E., Boon, A. P., Eady, R. A. & Winkelmann, R. K. The pathology of the autologous serum skin test response in chronic urticaria resembles IgE-mediated late-phase reactions. Int. Arch. Allergy Appl. Immunol. 93 , 198–204 (1990).
Saini, S. S. Basophil responsiveness in chronic urticaria. Curr. Allergy Asthma Rep. 9 , 286 (2009).
Ferrer, M. Immunological events in chronic spontaneous urticaria. Clin. Transl Allergy 5 , 30 (2015).
Fujisawa, D. et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 134 , 622–6339 (2014).
Caproni, M. et al. Infiltrating cells and related cytokines in lesional skin of patients with chronic idiopathic urticaria and positive autologous serum skin test. Exp. Dermatol. 12 , 621–628 (2003).
Altrichter, S. et al. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 145 , 1510–1516 (2020).
Cugno, M. et al. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int. Arch. Allergy Immunol. 148 , 170–174 (2009).
Tedeschi, A. et al. Chronic urticaria and coagulation: pathophysiological and clinical aspects. Allergy 69 , 683–691 (2014).
Cugno, M., Borghi, A., Garcovich, S. & Marzano, A. V. Coagulation and skin autoimmunity. Front. Immunol. 10 , 1407 (2019).
Tedeschi, A. et al. Plasma levels and skin-eosinophil-expression of vascular endothelial growth factor in patients with chronic urticaria. Allergy 64 , 1616–1622 (2009).
Dugina, T. N., Kiseleva, E. V., Chistov, I. V., Umarova, B. A. & Strukova, S. M. Receptors of the PAR family as a link between blood coagulation and inflammation. Biochemistry 67 , 65–74 (2002).
Yanase, Y. et al. Coagulation factors induce human skin mast cell and basophil degranulation via activation of complement 5 and the C5a receptor. J. Allergy Clin. Immunol. 147 , 1101–1104 (2021).
Cugno, M. et al. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy 73 , 2408–2411 (2018).
Farres, M. N. et al. Activation of coagulation in chronic urticaria in relation to disease severity and activity. Allergol. Immunopathol. 43 , 162–167 (2015).
CAS Google Scholar
Kolkhir, P., Altrichter, S., Hawro, T. & Maurer, M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy 73 , 940–948 (2018).
Kasperska-Zajac, A., Sztylc, J., Machura, E. & Jop, G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin. Exp. Allergy 41 , 1386–1391 (2011).
Asero, R. et al. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin. Exp. Immunol. 200 , 242–249 (2020).
Altrichter, S. et al. IgM and IgA in addition to IgG autoantibodies against FcɛRIα are frequent and associated with disease markers of chronic spontaneous urticaria. Allergy 75 , 3208–3215 (2020).
Hide, M. et al. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 328 , 1599–1604 (1993). In this report, functional histamine-releasing IgG autoantibodies to the α-subunit of the high-affinity IgE receptor are detected in the circulation of some patients with CU, suggesting an involvement of these autoantibodies in the pathogenesis of CU.
Kawakami, T. & Kitaura, J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J. Immunol. 175 , 4167–4173 (2005).
Shi, C.-R. et al. IgE-mediated allergy: a rare cause of chronic spontaneous urticarial with allergen-specific immunotherapy as treatment option — a systematic review with meta-analysis from China. J. Eur. Acad. Dermatol. Venereol. 26 , 533–544 (2012).
Sabroe, R. A. et al. Chronic idiopathic urticaria: comparison of the clinical features of patients with and without anti-FcεRI or anti-IgE autoantibodies. J. Am. Acad. Dermatol. 40 , 443–450 (1999).
Augey, F. et al. Chronic spontaneous urticaria is not an allergic disease. Eur. J. Dermatol. 21 , 349–353 (2011).
Shin, Y. S., Suh, D.-H., Yang, E.-M., Ye, Y.-M. & Park, H.-S. Serum specific IgE to thyroid peroxidase activates basophils in aspirin intolerant urticaria. J. Korean Med. Sci. 30 , 705–709 (2015).
Sánchez, J., Sánchez, A. & Cardona, R. Causal relationship between anti-TPO IgE and chronic urticaria by in vitro and in vivo tests. Allergy Asthma Immunol. Res. 11 , 29–42 (2019).
Sánchez, J. et al. Presence of IgE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in severe chronic spontaneous urticaria and atopic dermatitis. Allergy Asthma Immunol. Res. 13 , 746–761 (2021).
de Montjoye, L. et al. Increased expression of IL-24 in chronic spontaneous urticaria. Allergy 74 , 1811–1813 (2019).
Kolkhir, P. et al. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 149 , 1819–1831 (2022).
Konstantinou, G. N. et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy 68 , 27–36 (2013).
Siiskonen, H. & Harvima, I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front. Cell. Neurosci. 13 , 422 (2019).
Raap, U. et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp. Dermatol. 19 , 464–466 (2010).
Meixiong, J. et al. Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50 , 1163–11715 (2019).
McNeil, B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519 , 237–241 (2015).
Kühn, H. et al. Mas-related G protein-coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 147 , 456–469 (2021).
Shtessel, M. et al. MRGPRX2 activation causes increased skin reactivity in patients with chronic spontaneous urticaria. J. Invest. Dermatol. 141 , 678–681.e2 (2021).
Newcomb, R. W. & Nelson, H. Dermographia mediated by immunoglobulin E. Am. J. Med. 54 , 174–180 (1973).
Maltseva, N. et al. Cold urticaria — what we know and what we do not know. Allergy 76 , 1077–1094 (2021).
McSweeney, S. M., Sarkany, R., Fassihi, H., Tziotzios, C. & McGrath, J. A. Pathogenesis of solar urticaria: classic perspectives and emerging concepts. Exp. Dermatol. 31 , 586–593 (2022).
Fukunaga, A. et al. Cholinergic urticaria: epidemiology, physiopathology, new categorization, and management. Clin. Auton. Res. 28 , 103–113 (2018).
Pezzolo, E., Peroni, A., Schena, D. & Girolomoni, G. Preheated autologous serum skin test in localized heat urticaria. Clin. Exp. Dermatol. 39 , 921–923 (2014).
Kulthanan, K. et al. Vibratory angioedema subgroups, features, and treatment: results of a systematic review. J. Allergy Clin. Immunol. Pract. 9 , 971–984 (2021).
Cassano, N., Mastrandrea, V., Vestita, M. & Vena, G. A. An overview of delayed pressure urticaria with special emphasis on pathogenesis and treatment. Dermatol. Ther. 22 , 1 (2009).
Magerl, M. et al. The definition, diagnostic testing, and management of chronic inducible urticarias — the EAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy 71 , 780–802 (2016).
Rujitharanawong, C. et al. A systematic review of aquagenic urticaria — subgroups and treatment options. J. Allergy Clin. Immunol. Pract. https://doi.org/10.1016/j.jaip.2022.04.033 (2022).
Lehloenya, R. J., Phillips, E. J., Pasieka, H. B. & Peter, J. Recognizing drug hypersensitivity in pigmented skin. Immunol. Allergy Clin. North. Am. 42 , 219–238 (2022).
Maurer, M., Ortonne, J. P. & Zuberbier, T. Chronic urticaria: an internet survey of health behaviours, symptom patterns and treatment needs in European adult patients. Br. J. Dermatol. 160 , 633–641 (2009).
Nakao, A. & Nakamura, Y. Time will tell about mast cells: circadian control of mast cell activation. Allergol. Int. https://doi.org/10.1016/j.alit.2022.06.008 (2022).
Marcelino, J. et al. What basophil testing tells us about CSU patients — results of the CORSA study. Front. Immunol. 12 , 742470 (2021).
Sánchez-Borges, M., Caballero-Fonseca, F. & Capriles-Hulett, A. Tolerance of nonsteroidal anti-inflammatory drug-sensitive patients to the highly specific cyclooxygenase 2 inhibitors rofecoxib and valdecoxib. Ann. Allergy Asthma Immunol. 94 , 34–38 (2005).
Kolkhir, P. et al. Autoimmune chronic spontaneous urticaria detection with IgG anti-TPO and total IgE. J. Allergy Clin. Immunol. Pract. 9 , 4138–41468 (2021).
Bizjak, M. et al. Risk factors for systemic reactions in typical cold urticaria: results from the COLD-CE study. Allergy 77 , 2185–2219 (2022).
Kocatürk, E. et al. Effects of pregnancy on chronic urticaria: results of the PREG-CU UCARE study. Allergy 76 , 3133–3144 (2021).
Sánchez-Borges, M., Capriles-Hulett, A. & Caballero-Fonseca, F. Demographic and clinical profiles in patients with acute urticaria. Allergol. Immunopathol. 43 , 409–415 (2015).
Skander, D., Allenova, A., Maurer, M. & Kolkhir, P. Omalizumab is effective in patients with chronic spontaneous urticaria plus multiple chronic inducible urticaria. Eur. Ann. Allergy Clin. Immunol. 53 , 91–93 (2021).
Kolkhir, P., Metz, M., Altrichter, S. & Maurer, M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy 72 , 1440–1460 (2017).
Kocatürk, E. et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy 76 , 816–830 (2021).
Shalom, G. et al. Chronic urticaria and atopic disorders: a cross-sectional study of 11 271 patients. Br. J. Dermatol. 177 , 96–97 (2017).
Kim, B. R., Yang, S., Choi, J. W., Choi, C. W. & Youn, S. W. Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population-based study. J. Dermatol. 45 , 10–16 (2018).
Larenas-Linnemann, D., Saini, S. S., Azamar-Jácome, A. A. & Maurer, M. Chronic urticaria can be caused by cancer and resolves with its cure. Allergy 73 , 1562–1566 (2018).
Bauer, A. T., Gorzelanny, C., Gebhardt, C., Pantel, K. & Schneider, S. W. Interplay between coagulation and inflammation in cancer: limitations and therapeutic opportunities. Cancer Treat. Rev. 102 , 102322 (2022).
Liu, L., Wang, X., Wang, W., Wang, B. & Li, L. Symptomatic dermographism in Chinese population: an epidemiological study of hospital-based multicenter questionnaire survey. Curr. Med. Res. Opin. 38 , 131–137 (2022).
Rujitharanawong, C., Tuchinda, P., Chularojanamontri, L., Chanchaemsri, N. & Kulthanan, K. Cholinergic urticaria: clinical presentation and natural history in a tropical country. Biomed. Res. Int. 2020 , 7301652 (2020).
Asady, A. et al. Cholinergic urticaria patients of different age groups have distinct features. Clin. Exp. Allergy 47 , 1609–1614 (2017).
Monfrecola, G. et al. Solar urticaria: a report on 57 cases. Am. J. Contact Dermat. 11 , 89–94 (2000).
Möller, A., Henning, M., Zuberbier, T. & Czarnetzki-Henz, B. M. Epidemiology and clinical aspects of cold urticaria [German]. Hautarzt 47 , 510–514 (1996).
Schoepke, N., Młynek, A., Weller, K., Church, M. K. & Maurer, M. Symptomatic dermographism: an inadequately described disease. J. Eur. Acad. Dermatol. Venereol. 29 , 708–712 (2015).
Peter, J., Krause, K., Staubach, P., Wu, M. A. & Davis, M. Chronic urticaria and recurrent angioedema: clues to the mimics. J. Allergy Clin. Immunol. Pract. 9 , 2220–2228 (2021).
Gusdorf, L. & Lipsker, D. Neutrophilic urticarial dermatosis: an entity bridging monogenic and polygenic autoinflammatory disorders, and beyond. J. Eur. Acad. Dermatol. Venereol. 34 , 685–690 (2020).
Puhl, V. et al. A novel histopathological scoring system to distinguish urticarial vasculitis from chronic spontaneous urticaria. Clin. Transl Allergy 11 , e12031 (2021).
Marzano, A. V. et al. Urticarial vasculitis: clinical and laboratory findings with a particular emphasis on differential diagnosis. J. Allergy Clin. Immunol. 149 , 1137–1149 (2022).
Weller, K. et al. Clinical measures of chronic urticaria. Immunol. Allergy Clin. North. Am. 37 , 35–49 (2017).
Młynek, A. et al. How to assess disease activity in patients with chronic urticaria? Allergy 63 , 777–780 (2008).
Weller, K. et al. Development, validation, and initial results of the Angioedema Activity Score. Allergy 68 , 1185–1192 (2013).
Ahsan, D. M. et al. Development of the Cold Urticaria Activity Score. Allergy 77 , 2509–2519 (2022).
Koch, K., Weller, K., Werner, A., Maurer, M. & Altrichter, S. Antihistamine updosing reduces disease activity in patients with difficult-to-treat cholinergic urticaria. J . Allergy Clin. Immunol. 138 , 1483–1485 (2016).
Weller, K. et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J. Allergy Clin. Immunol. 133 , 1365–1372 (2014).
Weller, K. et al. Validation of the Angioedema Control Test (AECT) — a patient-reported outcome instrument for assessing angioedema control. J. Allergy Clin. Immunol. Pract. 8 , 2050–20574 (2020).
Alves, F., Calado, R., Relvas, M., Gomes, T. & Gonçalo, M. Short courses of ciclosporin can induce long remissions in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 36 , 645–646 (2022).
Kolkhir, P., Pogorelov, D. & Kochergin, N. Chronic spontaneous urticaria associated with vitiligo and thyroiditis (autoimmune polyglandular syndrome IIIC): case series. Int. J. Dermatol. 56 , 89–90 (2017).
Alvarado, D. et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy https://doi.org/10.1111/all.15262 (2022). This publication describes systemic mast cell depletion associated with administration of an anti-KIT mAb that may represent a safe and novel approach to treat mast cell-driven disorders.
Maurer, M. et al. Unmet clinical needs in chronic spontaneous urticaria. A GA²LEN task force report. Allergy 66 , 317–330 (2011).
Guillen-Aguinaga, S., Jauregui Presa, I., Aguinaga-Ontoso, E., Guillen-Grima, F. & Ferrer, M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br. J. Dermatol. 175 , 1153–1165 (2016).
Agache, I. et al. EAACI biologicals guidelines — omalizumab for the treatment of chronic spontaneous urticaria in adults and in the paediatric population 12–17 years old. Allergy 77 , 17–38 (2022).
Doña, I. et al. NSAID-induced urticaria/angioedema does not evolve into chronic urticaria: a 12-year follow-up study. Allergy 69 , 438–444 (2014).
Grattan, C. E., Francis, D. M., Slater, N. G., Barlow, R. J. & Greaves, M. W. Plasmapheresis for severe, unremitting, chronic urticaria. Lancet 339 , 1078–1080 (1992).
Aleksandraviciute, L., Malinauskiene, L., Cerniauskas, K. & Chomiciene, A. Plasmapheresis: is it a potential alternative treatment for chronic urticaria? Open Med. (Wars.) 17 , 113–118 (2022).
Shakouri, A., Compalati, E., Lang, D. M. & Khan, D. A. Effectiveness of Helicobacter pylori eradication in chronic urticaria: evidence-based analysis using the grading of recommendations assessment, development, and evaluation system. Curr. Opin. Allergy Clin. Immunol. 10 , 362–369 (2010).
Gonzalez-Diaz, S. N. et al. Chronic urticaria and thyroid pathology. World Allergy Organ. J. 13 , 100101 (2020).
Sharma, M., Bennett, C., Cohen, S. N. & Carter, B. H1-antihistamines for chronic spontaneous urticaria. Cochrane Database Syst. Rev. 11 , Cd006137 (2014).
Zuberbier, T. & Maurer, M. Antihistamines in the treatment of urticaria. Adv. Exp. Med. Biol. 709 , 67–72 (2010).
Leurs, R., Church, M. K. & Taglialatela, M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin. Exp. Allergy 32 , 489–498 (2002).
Church, M. K. et al. Risk of first-generation H1-antihistamines: a GA(2)LEN position paper. Allergy 65 , 459–466 (2010).
Zuberbier, T. et al. Double-blind crossover study of high-dose cetirizine in cholinergic urticaria. Dermatology 193 , 324–327 (1996).
Staevska, M. et al. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J. Allergy Clin. Immunol. 125 , 676–682 (2010).
Siebenhaar, F., Degener, F., Zuberbier, T., Martus, P. & Maurer, M. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J. Allergy Clin. Immunol. 123 , 672–679 (2009).
Gimenez-Arnau, A., Izquierdo, I. & Maurer, M. The use of a responder analysis to identify clinically meaningful differences in chronic urticaria patients following placebo-controlled treatment with rupatadine 10 and 20 mg. J. Eur. Acad. Dermatol. Venereol. 23 , 1088–1091 (2009).
Cataldi, M., Maurer, M., Taglialatela, M. & Church, M. K. Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin. Exp. Allergy 49 , 1615–1623 (2019).
Zhou, P., Zeng, S., Fu, L., Chen, H. & Li, L. Efficacy and safety of intensive nonsedating antihistamines for chronic spontaneous urticaria: a meta-analysis of randomized controlled trials. Int. Arch. Allergy Immunol. 183 , 796–803 (2022).
Sabroe, R. A. et al. British Association of Dermatologists guidelines for the management of people with chronic urticaria 2021. Br. J. Dermatol. 186 , 398–413 (2022).
Türk, M. et al. Experience-based advice on stepping up and stepping down the therapeutic management of chronic spontaneous urticaria: where is the guidance? Allergy 77 , 1626–1630 (2022).
Gimenez-Arnau, A. M. & Salman, A. Targeted therapy for chronic spontaneous urticaria: rationale and recent progress. Drugs 80 , 1617–1634 (2020).
Maurer, M. et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N. Engl. J. Med. 368 , 924–935 (2013). This key study demonstrates omalizumab efficacy in patients with antihistamine-resistant CSU and highlights the importance of IgE–FcεRI-driven mast cell activation in this disease.
Maurer, M. et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J. Allergy Clin. Immunol. 141 , 638–649 (2018).
Kaplan, A. P., Giménez-Arnau, A. M. & Saini, S. S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 72 , 519–533 (2017).
Tharp, M. D. et al. Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “real-world” evidence. JAMA Dermatol. 155 , 29–38 (2019).
Finlay, A. Y. et al. Omalizumab substantially improves dermatology-related quality of life in patients with chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 31 , 1715–1721 (2017).
Buyukozturk, S. et al. Omalizumab markedly improves Urticaria Activity Scores and quality of life scores in chronic spontaneous urticaria patients: a real life survey. J. Dermatol. 39 , 439–442 (2012).
Salman, A., Demir, G. & Bekiroglu, N. The impact of omalizumab on quality of life and its predictors in patients with chronic spontaneous urticaria: real-life data. Dermatol. Ther. 32 , e12975 (2019).
Gastaminza, G. et al. Efficacy and safety of omalizumab (Xolair) for cholinergic urticaria in patients unresponsive to a double dose of antihistamines: a randomized mixed double-blind and open-label placebo-controlled clinical trial. J. Allergy Clin. Immunol. Pract. 7 , 1599–1609.e1 (2019).
Dressler, C. et al. Chronic inducible urticaria: a systematic review of treatment options. J. Allergy Clin. Immunol. 141 , 1726–1734 (2018).
Maurer, M. et al. Omalizumab is effective in symptomatic dermographism — results of a randomized placebo-controlled trial. J. Allergy Clin. Immunol. 140 , 870–873 (2017).
Metz, M. et al. Omalizumab is effective in cold urticaria — results of a randomized placebo-controlled trial. J. Allergy Clin. Immunol. 140 , 864–867 (2017).
Türk, M. et al. How to treat patients with chronic spontaneous urticaria with omalizumab: questions and answers. J. Allergy Clin. Immunol. Pract. 8 , 113–124 (2020).
Salman, A., Ergun, T. & Gimenez-Arnau, A. M. Real-life data on the effectiveness and safety of omalizumab in monotherapy or combined for chronic spontaneous urticaria: a retrospective cohort study. J. Dermatol. Treat. 31 , 204–209 (2020).
Rosenblum, J. D., Nassau, S., Fonacier, L. & Mawhirt, S. L. Concomitant treatment with omalizumab and cyclosporine for chronic spontaneous urticaria. Ann. Allergy Asthma Immunol. 125 , 111–112 (2020).
Harrison, C. A., Bastan, R., Peirce, M. J., Munday, M. R. & Peachell, P. T. Role of calcineurin in the regulation of human lung mast cell and basophil function by cyclosporine and FK506. Br. J. Pharmacol. 150 , 509–518 (2007).
Grattan, C. E. et al. Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br. J. Dermatol. 143 , 365–372 (2000). This paper shows efficacy of cyclosporine in patients with CSU including autoimmune/autoreactive CSU .
Vena, G. A. et al. Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 55 , 705–709 (2006).
Kulthanan, K. et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J. Allergy Clin. Immunol. Pract. 6 , 586–599 (2018).
Doshi, D. R. & Weinberger, M. M. Experience with cyclosporine in children with chronic idiopathic urticaria. Pediatr. Dermatol. 26 , 409–413 (2009).
Rutkowski, K. & Grattan, C. E. H. How to manage chronic urticaria ‘beyond’ guidelines: a practical algorithm. Clin. Exp. Allergy 47 , 710–718 (2017).
Magerl, M., Philipp, S., Manasterski, M., Friedrich, M. & Maurer, M. Successful treatment of delayed pressure urticaria with anti-TNF-α. J. Allergy Clin. Immunol. 119 , 752–754 (2007).
Bangsgaard, N., Skov, L. & Zachariae, C. Treatment of refractory chronic spontaneous urticaria with adalimumab. Acta Derm. Venereol. 97 , 524–525 (2017).
Hannuksela, M. & Kokkonen, E. L. Ultraviolet light therapy in chronic urticaria. Acta Derm. Venereol. 65 , 449–450 (1985).
Borzova, E., Rutherford, A., Konstantinou, G. N., Leslie, K. S. & Grattan, C. E. Narrowband ultraviolet B phototherapy is beneficial in antihistamine-resistant symptomatic dermographism: a pilot study. J. Am. Acad. Dermatol. 59 , 752–757 (2008).
Engin, B., Ozdemir, M., Balevi, A. & Mevlitoglu, I. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm. Venereol. 88 , 247–251 (2008).
Pacor, M. L., Di Lorenzo, G. & Corrocher, R. Efficacy of leukotriene receptor antagonist in chronic urticaria. A double-blind, placebo-controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid. Clin. Exp. Allergy 31 , 1607–1614 (2001).
Beissert, S., Stander, H. & Schwarz, T. UVA rush hardening for the treatment of solar urticaria. J. Am. Acad. Dermatol. 42 , 1030–1032 (2000).
Weber-Schoendorfer, C. & Schaefer, C. The safety of cetirizine during pregnancy. A prospective observational cohort study. Reprod. Toxicol. 26 , 19–23 (2008).
Schwarz, E. B., Moretti, M. E., Nayak, S. & Koren, G. Risk of hypospadias in offspring of women using loratadine during pregnancy: a systematic review and meta-analysis. Drug Saf. 31 , 775–788 (2008).
Ngo, E. et al. Antihistamine use during breastfeeding with focus on breast milk transfer and safety in humans: a systematic literature review. Basic Clin. Pharmacol. Toxicol. 130 , 171–181 (2022).
Namazy, J. et al. The Xolair pregnancy registry (EXPECT): the safety of omalizumab use during pregnancy. J. Allergy Clin. Immunol. 135 , 407–412 (2015).
Gonzalez-Medina, M., Curto-Barredo, L., Labrador-Horrillo, M. & Gimenez-Arnau, A. Omalizumab use during pregnancy for chronic spontaneous urticaria (CSU): report of two cases. J. Eur. Acad. Dermatol. Venereol. 31 , e245–e246 (2017).
Ghazanfar, M. N. & Thomsen, S. F. Successful and safe treatment of chronic spontaneous urticaria with omalizumab in a woman during two consecutive pregnancies. Case Rep. Med. 2015 , 368053 (2015).
Ensina, L. F., Cusato-Ensina, A. P., Camelo-Nunes, I. C. & Sole, D. Omalizumab as third-line therapy for urticaria during pregnancy. J. Investig. Allergol. Clin. Immunol. 27 , 326–327 (2017).
Ventura, M. T. et al. Management of chronic spontaneous urticaria in the elderly. Drugs Aging 32 , 271–282 (2015).
Nettis, E. et al. Omalizumab in elderly patients with chronic spontaneous urticaria: an Italian real-life experience. Ann. Allergy Asthma Immunol. 120 , 318–323 (2018).
Saini, S., Shams, M., Bernstein, J. A. & Maurer, M. Urticaria and angioedema across the ages. J. Allergy Clin. Immunol. Pract. 8 , 1866–1874 (2020).
Caffarelli, C. et al. Management of chronic urticaria in children: a clinical guideline. Ital. J. Pediatr. 45 , 101 (2019).
Cornillier, H. et al. Chronic spontaneous urticaria in children — a systematic review on interventions and comorbidities. Pediatr. Allergy Immunol. 29 , 303–310 (2018).
Kolkhir, P., Altrichter, S., Munoz, M., Hawro, T. & Maurer, M. New treatments for chronic urticaria. Ann. Allergy Asthma Immunol. 124 , 2–12 (2020).
Hon, K. L., Li, J. T. S., Leung, A. K. C. & Lee, V. W. Y. Current and emerging pharmacotherapy for chronic spontaneous urticaria: a focus on non-biological therapeutics. Expert Opin. Pharmacother. 22 , 497–509 (2021).
Wedi, B. Emerging treatments for chronic urticaria. Expert Opin. Investig. Drugs 31 , 281–290 (2022).
Maurer, M. et al. Ligelizumab for chronic spontaneous urticaria. N. Engl. J. Med. 381 , 1321–1332 (2019).
Novartis. Novartis provides an update on phase III ligelizumab (QGE031) studies in chronic spontaneous urticaria (CSU). Novartis https://www.novartis.com/news/media-releases/novartis-provides-update-phase-iii-ligelizumab-qge031-studies-chronic-spontaneous-urticaria-csu (2021).
Metz, M. et al. Fenebrutinib in H1 antihistamine-refractory chronic spontaneous urticaria: a randomized phase 2 trial. Nat. Med. 27 , 1961–1969 (2021). The results of this study indicate that fenebrutinib, a BTK inhibitor, can be effective in reducing disease activity in patients with antihistamine-refractory CSU including patients with refractory type IIb autoimmunity.
Maurer, M. et al. Remibrutinib treatment improves quality of life in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 149 , AB179 (2022).
Kaul, M. et al. Remibrutinib (LOU064): a selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial. Clin. Transl Sci. 14 , 1756–1768 (2021).
Terhorst-Molawi, D. et al. The anti-KIT antibody, CDX-0159, reduces mast cell numbers and circulating tryptase and improves disease control in patients with chronic inducible urticaria (CIndU). J. Allergy Clin. Immunol. 149 , AB178 (2022).
Bernstein, J. et al. Effects of multiple dose treatment with an anti-KIT antibody, CDX-0159, in chronic spontaneous urticaria [abstract 100097]. Presented at the EAACI Congress (2022).
Celldex. Celldex Therapeutics presents positive interim data from barzolvolimab phase 1b study in chronic spontaneous urticaria at EAACI 2022. GlobeNewswire https://www.globenewswire.com/news-release/2022/06/30/2472574/24180/en/Celldex-Therapeutics-Presents-Positive-Interim-Data-from-Barzolvolimab-Phase-1b-Study-in-Chronic-Spontaneous-Urticaria-at-EAACI-2022.html (2022).
Bernstein, J. A. et al. Benralizumab for chronic spontaneous urticaria. N. Engl. J. Med. 383 , 1389–1391 (2020). This is the first clinical study showing that benralizumab can be effective in patients with antihistamine-resistant CSU, supporting further investigation of this treatment approach in urticaria and other mast cell-driven diseases.
Maurer, M. et al. Dupilumab significantly reduces itch and hives in patients with chronic spontaneous urticaria: results from a phase 3 trial (LIBERTY-CSU CUPID study A). J. Allergy Clin. Immunol. 149 , AB312 (2022).
Altrichter, S. et al. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J. Allergy Clin. Immunol. 149 , 1683–1690 (2022). This study shows efficacy of lirentelimab, an anti-sialic acid-binding immunoglobulin-like lectin 8 mAb, in patients with CSU and CIndU.
Sabag, D. A. et al. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin. Exp. Allergy 50 , 799–804 (2020). This report demonstrates efficacy of the anti-IL-17 mAb, secukinumab, in patients with antihistamine-resistant and omalizumab-resistant CSU, suggesting the role of IL-17 in disease pathogenesis.
Ware, J. E. Jr & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30 , 473–483 (1992).
Hunt, S. M., McEwen, J. & McKenna, S. P. Measuring health status: a new tool for clinicians and epidemiologists. J. R. Coll. Gen. Pract. 35 , 185 (1985).
Chren, M. M., Lasek, R. J., Quinn, L. M., Mostow, E. N. & Zyzanski, S. J. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J. Invest. Dermatol. 107 , 707–713 (1996).
Finlay, A. Y. & Khan, G. K. Dermatology Life Quality Index (DLQI) — a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 19 , 210–216 (1994).
Baiardini, I. et al. A new tool to evaluate the impact of chronic urticaria on quality of life: Chronic Urticaria Quality of Life Questionnaire (CU-QoL). Allergy 60 , 1073–1078 (2005).
Ruft, J. et al. Development and validation of the Cholinergic Urticaria Quality-of-Life Questionnaire (CholU-QoL). Clin. Exp. Allergy 48 , 433–444 (2018).
Weller, K. et al. Development and construct validation of the Angioedema Quality of Life Questionnaire. Allergy 67 , 1289–1298 (2012).
Kulthanan, K., Chiawsirikajorn, Y. & Jiamton, S. Acute urticaria: etiologies, clinical course and quality of life. Asian Pac. J. Allergy Immunol. 26 , 1–9 (2008).
Zelić, S. B., Rubeša, G., Brajac, I., Peitl, M. V. & Pavlović, E. Satisfaction with life and coping skills in the acute and chronic urticaria. Psychiatr. Danub. 28 , 34–38 (2016).
Maurer, M. et al. Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ. J. 13 , 100460 (2020).
O’Donnell, B. F., Lawlor, F., Simpson, J., Morgan, M. & Greaves, M. W. The impact of chronic urticaria on the quality of life. Br. J. Dermatol. 136 , 197–201 (1997).
Mann, C., Dreher, M., Weess, H. G. & Staubach, P. Sleep disturbance in patients with urticaria and atopic dermatitis: an underestimated burden. Acta Derm. Venereol. 100 , adv00073 (2020).
Saini, S. S. et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J. Invest. Dermatol. 135 , 67–75 (2015).
Hoskin, B., Ortiz, B., Paknis, B. & Kavati, A. Exploring the real-world profile of refractory and non-refractory chronic idiopathic urticaria in the USA: clinical burden and healthcare resource use. Curr. Med. Res. Opin. 35 , 1387–1395 (2019).
Sussman, G. et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: analyses from ASSURE-CSU. Allergy 73 , 1724–1734 (2018).
Baiardini, I. et al. Quality of life and patients’ satisfaction in chronic urticaria and respiratory allergy. Allergy 58 , 621–623 (2003).
Ertas, R., Erol, K., Hawro, T., Yilmaz, H. & Maurer, M. Sexual functioning is frequently and markedly impaired in female patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. Pract. 8 , 1074–1082 (2020).
Grob, J. J., Revuz, J., Ortonne, J. P., Auquier, P. & Lorette, G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br. J. Dermatol. 152 , 289–295 (2005).
Mendelson, M. H. et al. Patient-reported impact of chronic urticaria compared with psoriasis in the United States. J. Dermatol. Treat. 28 , 229–236 (2017).
Brzoza, Z. et al. Chronic spontaneous urticaria and type 1 diabetes mellitus — does quality of life impairment always reflect health danger? J. Clin. Med. 9 , 2505 (2020).
PubMed Central Google Scholar
Gimenez-Arnau, A. et al. Ligelizumab improves sleep interference and disease burden in patients with chronic spontaneous urticaria. Clin. Transl Allergy 12 , e12121 (2022).
Maurer, M. et al. Antihistamine-resistant chronic spontaneous urticaria remains undertreated: 2-year data from the AWARE study. Clin. Exp. Allergy 50 , 1166–1175 (2020).
Nochaiwong, S. et al. Impact of pharmacological treatments for chronic spontaneous urticaria with an inadequate response to H1-antihistamines on health-related quality of life: a systematic review and network meta-analysis. J. Allergy Clin. Immunol. Pract. 10 , 297–308 (2022).
Di Agosta, E. et al. Quality of life in patients with allergic and immunologic skin diseases: in the eye of the beholder. Clin. Mol. Allergy 19 , 26 (2021).
Curto-Barredo, L., Pujol, R. M., Roura-Vives, G. & Gimenez-Arnau, A. M. Chronic urticaria phenotypes: clinical differences regarding triggers, activity, prognosis and therapeutic response. Eur. J. Dermatol. 29 , 627–635 (2019).
Asero, R. et al. Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy 63 , 176–180 (2008).
Marzano, A. V. et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J. Eur. Acad. Dermatol. Venereol. 33 , 918–924 (2019).
Fok, J. S., Kolkhir, P., Church, M. K. & Maurer, M. Predictors of treatment response in chronic spontaneous urticaria. Allergy 76 , 2965–2981 (2021).
Cherrez-Ojeda, I. et al. Chronic urticaria patients are interested in apps to monitor their disease activity and control: A UCARE CURICT analysis. Clin. Transl Allergy 11 , e12089 (2021).
Antó, A. et al. Automatic screening of self-evaluation apps for urticaria and angioedema shows a high unmet need. Allergy 76 , 3810–3813 (2021).
Maurer, M. et al. The usage, quality and relevance of information and communications technologies in patients with chronic urticaria: a UCARE study. World Allergy Organ. J. 13 , 100475 (2020).
Cherrez-Ojeda, I. et al. How are patients with chronic urticaria interested in using information and communication technologies to guide their healthcare? A UCARE study. World Allergy Organ. J. 14 , 100542 (2021).
Maurer, M. et al. Definition, aims, and implementation of GA 2 LEN Urticaria Centers of Reference and Excellence. Allergy 71 , 1210–1218 (2016). This publication describes the aims, the requirements and deliverables, the application process and the audit and accreditation protocol for Urticaria Centers of Reference and Excellence .
Maurer, M. et al. Definition, aims, and implementation of GA 2 LEN/HAEi Angioedema Centers of Reference and Excellence. Allergy 75 , 2115–2123 (2020).
Weller, K. et al. The Chronic Urticaria Registry: rationale, methods and initial implementation. J. Eur. Acad. Dermatol. Venereol. 35 , 721–729 (2021). This report describes the rationale, methods and implementation of the CURE .
Mao, M. et al. Clinical difference between single subtype and mixed subtype chronic urticaria: a retrospective study. Indian J. Dermatol. Venereol. Leprol. 88 , 171–176 (2022).
Prosty, C. et al. Prevalence, management, and anaphylaxis risk of cold urticaria: a systematic review and meta-analysis. J. Allergy Clin. Immunol. Pract. 10 , 586–5964 (2022).
Maul, J.-T. et al. Canakinumab lacks efficacy in treating adult patients with moderate to severe chronic spontaneous urticaria in a phase II randomized double-blind placebo-controlled single-center study. J. Allergy Clin. Immunol. Pract. 9 , 463–4683 (2021).
Oliver, E. T. et al. Effects of an oral CRTh2 antagonist (AZD1981) on eosinophil activity and symptoms in chronic spontaneous urticaria. Int. Arch. Allergy Immunol. 179 , 21–30 (2019).
Dickson, M. C. et al. Effects of a topical treatment with spleen tyrosine kinase inhibitor in healthy subjects and patients with cold urticaria or chronic spontaneous urticaria: results of a phase 1a/b randomised double-blind placebo-controlled study. Br. J. Clin. Pharmacol. 87 , 4797–4808 (2021).
Gericke, J. et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 139 , 1059–1061.e1 (2017).
Kolkhir, P. et al. Management of chronic spontaneous urticaria: a worldwide perspective. World Allergy Organ. J. 11 , 14 (2018).
Maurer, M. et al. Differences in chronic spontaneous urticaria between Europe and Central/South America: results of the multi-center real world AWARE study. World Allergy Organ. J. 11 , 32 (2018).
Wagner, N. et al. Patients with chronic urticaria remain largely undertreated: results from the DERMLINE online survey. Dermatol. Ther. 11 , 1027–1039 (2021).
Cherrez, A. et al. Knowledge and management of chronic spontaneous urticaria in Latin America: a cross-sectional study in Ecuador. World Allergy Organ. J. 10 , 21 (2017).
Zuberbier, T. & Bernstein, J. A. A comparison of the United States and international perspective on chronic urticaria guidelines. J. Allergy Clin. Immunol. Pract. 6 , 1144–1151 (2018).
Wilches, P., Wilches, P., Calderon, J. C., Cherrez, A. & Cherrez Ojeda, I. Omalizumab for chronic urticaria in Latin America. World Allergy Organ. J. 9 , 1–5 (2016).
Download references
Acknowledgements
The authors thank H. Bonnekoh, K. Brockow, T. Buttgereit, L. Ensina, M. Hide, T. Jacob, A. Kasperska-Zając, L. Kiefer, E. Kocatürk, O. M. E. Calderón LLosa and P. Pyatilova for providing clinical pictures during the writing of this publication.
Author information
Authors and affiliations.
Urticaria Center of Reference and Excellence (UCARE), Institute of Allergology, Charité — Universitätsmedizin Berlin, Berlin, Germany
Pavel Kolkhir, Martin Metz & Marcus Maurer
Allergology and Immunology, Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Berlin, Germany
Urticaria Center of Reference and Excellence (UCARE), Department of Dermatology, Hospital del Mar, Institut Mar d’Investigacions Mediques, Universitat Autònoma, Barcelona, Spain
Ana M. Giménez-Arnau
Urticaria Center of Reference and Excellence (UCARE), Department of Dermatology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Kanokvalai Kulthanan
Urticaria Center of Reference and Excellence (UCARE), Division of Allergy and Clinical Immunology, Department of Medicine, University of Cape Town, Cape Town, South Africa
Jonny Peter
Urticaria Center of Reference and Excellence (UCARE), Allergy and Immunology Unit, University of Cape Town, Lung Institute, Cape Town, South Africa
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (P.K., A.M.G.-A., K.K., M. Maurer); Epidemiology (P.K., K.K., M. Maurer); Mechanisms/pathophysiology (P.K., M. Metz, M. Maurer); Diagnosis/screening/prevention (P.K., A.M.G.-A., K.K., M. Maurer); Management (P.K., A.M.G.-A., J.P., M. Maurer); Quality of life (P.K., M. Metz, M. Maurer); Outlook (P.K., A.M.G.-A., J.P., M. Maurer); Overview of the Primer (P.K., M. Maurer).
Corresponding authors
Correspondence to Pavel Kolkhir or Marcus Maurer .
Ethics declarations
Competing interests.
P.K. received speakers fees, honoraria or travel support from Novartis and Roche. A.M.G.-A. is/was a medical adviser for Uriach Pharma/Neucor, Genentech, Novartis, FAES, GSK, Sanofi–Regeneron, Amgen, Thermo Fisher Scientific, Almirall, Celldex and LEO Pharma; received research grants supported by Uriach Pharma, Novartis and Instituto Carlos III — FEDER; and participated in educational activities for Uriach Pharma, Novartis, Genentech, Menarini, LEO Pharma, GSK, MSD, Almirall, Sanofi and Avene. K.K. received grants/research support from Novartis and honoraria or consultation fees from Novartis, Menarini and Sanofi. J.P. received speakers fees, honoraria or travel support from Takeda, CSL Behring, Janssen, Novartis and Sanofi and Pharming, and educational research grants from Takeda and Novartis. M. Metz has received honoraria (advisory board, speaker) from Amgen, ArgenX, Celldex, Moxie, Novartis, Roche and Sanofi. M. Maurer is or recently was a speaker and/or adviser for and/or has received research funding from Astria, Allakos, Alnylam, Amgen, Aralez, ArgenX, AstraZeneca, BioCryst, Blueprint, Celldex, Centogene, CSL Behring, Dyax, FAES, Genentech, GIInnovation, GSK, Innate Pharma, Kalvista, Kyowa Kirin, LEO Pharma, Lilly, Menarini, Moxie, Novartis, Pfizer, Pharming, Pharvaris, Roche, Sanofi/Regeneron, Shire/Takeda, Third Harmonic Bio, UCB and Uriach.
Peer review
Peer review information.
Nature Reviews Disease Primers thanks Y. M. Ye, Z. K. Brzoza, A. Marzano, K. V. Godse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent.
The authors affirm that human research participants provided written informed consent for publication of the images in Figs. 1 and 4.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
The process by which cytoplasmic granules (originating from mast cells or other granulocytes) release their contents including preformed mediators such as histamine.
Intermittent, circumscribed, superficial, central swellings, often surrounded by reflex erythema, that cause itching or burning sensations.
An intermittent, localized and self-limiting swelling of the subcutaneous or submucosal tissue, due to a temporary increase in vascular permeability, causing tingling, burning, tightness and, sometimes, pain.
Immune cells of the myeloid lineage that contain many granules rich in histamine and other mediators and are present in connective tissues throughout the body, especially skin, near blood vessels, lungs and intestines.
A family of inflammatory lipid mediators synthesized from arachidonic acid by various cells including mast cells, eosinophils and basophils.
A CC chemokine subfamily of potent eosinophil chemoattractants including CCL11 (eotaxin 1), CCL24 (eotaxin 2) and CCL26 (eotaxin 3).
(ASST). Intradermal injection of autologous serum to assess autoreactivity associated with autoantibodies and other histamine-releasing factors; defined as a serum-induced wheal response with a diameter ≥1.5 mm compared with that of the saline-induced response.
The high-affinity receptor expressed on eosinophils, epithelial cells and other cell types, and cofactor for factor VII (FVII)/FVIIa important for initiation of blood coagulation.
The gold standard for diagnosis of NSAID-mediated reactions including identification of a culprit drug, confirming or excluding cross-reactivity, and finding the most likely tolerated alternative drug.
A method to confirm the diagnosis of chronic inducible urticaria (CIndU) using established protocols.
(Grading of Recommendations Assessment, Development and Evaluation methodologies). A transparent framework for rating the quality of evidence and grading the strength of recommendations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Kolkhir, P., Giménez-Arnau, A.M., Kulthanan, K. et al. Urticaria. Nat Rev Dis Primers 8 , 61 (2022). https://doi.org/10.1038/s41572-022-00389-z
Download citation
Accepted : 03 August 2022
Published : 15 September 2022
DOI : https://doi.org/10.1038/s41572-022-00389-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The european network for ige-mediated autoimmunity and autoallergy (enigma) initiative.
- Pavel Kolkhir
- Sabine Altrichter
- Marcus Maurer
Nature Medicine (2024)
- Xingxing Jian
Nature Communications (2024)
Pressure and Skin: A Review of Disease Entities Driven or Influenced by Mechanical Pressure
- Wei-Chen Chien
- Tsen-Fang Tsai
American Journal of Clinical Dermatology (2024)
Welche Augmentations- und Triggerfaktoren sind bei der Urtikaria relevant?
- Julia Zarnowski
- Regina Treudler
Die Dermatologie (2024)
Spontanremission der Urtikaria: Gibt es das, und wenn ja, wann?
- Mathias Sulk
- Christoph M. Hammers
- Guido Heine
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Scientific landscape and trend analysis of chronic urticaria: a two-decade bibliometric review
Affiliations.
- 1 Department of Dermatology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China, Eight-Year MD Program, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China.
- 2 Department of Dermatology, Beijing Tongren Hospital, Capital Medical University, Beijing, China.
- 3 Department of Dermatology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China.
- PMID: 37823491
- DOI: 10.1684/ejd.2023.4536
Background: In the past 20 years, an increasing number of studies have advanced our understanding of the pathogenic mechanism of chronic urticaria (CU), providing new treatment options.
Objectives: This bibliometric study aimed to evaluate published reports of CU-related studies from a number of different angles, review the research trends of the studies, and provide future perspectives of CU.
Materials & methods: Publications related to CU from 2001-2022 were searched in the Web of Science Core Collection. The database file was imported to Excel and analysed using bibliometric software, including VOSviewer and BiblioShiny.
Results: A total of 4,452 publications of CU were included. The number of publications related to CU has increased steadily over time. The journal with the most published articles was Allergy, and the countries with the most publications were the United States, Germany, and Italy. The most productive author was Maurer Marcus from Germany. There was close co-authorship between authors and countries (and regions) across the world. Recent studies have focused more on the pathogenesis and treatment of CU. Future hotspots may include emerging biologics for treatment.
Conclusion: This study shows the research development of CU over the past two decades, which may provide beneficial reference for publication and future trends in CU research.
Keywords: VOSviewer; bibliometric analysis; chronic urticaria; scientific landscape.
- Bibliometrics
- Biological Products*
- Chronic Urticaria*
- Databases, Factual
- Biological Products
Advertisement
Pathophysiology, Diagnosis, and Management of Chronic Spontaneous Urticaria: A Literature Review
- Published: 01 September 2022
- Volume 63 , pages 381–389, ( 2022 )
Cite this article
- Benjamin Greiner ORCID: orcid.org/0000-0001-7604-2539 1 , 3 ,
- Savannah Nicks 2 ,
- Michael Adame 1 , 3 &
- Jennifer McCracken 1 , 3
2008 Accesses
5 Citations
Explore all metrics
Chronic spontaneous urticaria (CSU) is characterized by recurring wheals that last 6 weeks or longer without an identifiable cause. The estimated point prevalence of CSU worldwide is 1%. Furthermore, it has a significant impact on quality of life in both adults and pediatric patients and their families. Although it is most often a self-limited disease, some patients have urticaria refractory to first-line treatment: second-generation H1 antihistamines. In these patients, the use of targeted monoclonal antibodies is necessary. While omalizumab is the only Food and Drug Administration-approved monoclonal antibody for CSU, others, including ligelizumab, dupilumab, benralizumab, and several orally administered Bruton’s tyrosine kinase inhibitors, are also promising therapeutics for reducing the morbidity of CSU. Novel therapies, among others discussed here, are rapidly being developed with new trials and therapeutics being released nearly monthly. Thus, we performed a scoping literature review of randomized controlled trials studying targeted therapies for CSU. We also discuss the pathophysiology, diagnosis, prognosis, and future research directions in CSU.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
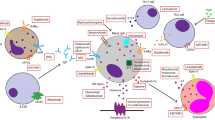
Current and Emerging Therapies for Chronic Spontaneous Urticaria: A Narrative Review
Gil Yosipovitch, Georgia Biazus Soares & Omar Mahmoud

Targeted Therapy for Chronıc Spontaneous Urtıcarıa: Ratıonale and Recent Progress
Ana M. Giménez-Arnau & Andaç Salman
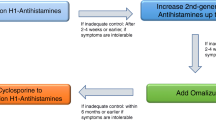
Review and Perspectives of the Recent International Guidelines on Treatment of Chronic Urticaria
Shazia Lutfeali & David A. Khan
Maurer M, Abuzakouk M, Bérard F et al (2017) The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy 72(12):2005–2016. https://doi.org/10.1111/all.13209
Article CAS PubMed Google Scholar
Fricke J, Ávila G, Keller T et al (2020) Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy 75(2):423–432. https://doi.org/10.1111/all.14037
Article PubMed Google Scholar
Balp MM, Weller K, Carboni V et al (2018) Prevalence and clinical characteristics of chronic spontaneous urticaria in pediatric patients. Pediatr Allergy Immunol 29(6):630–636. https://doi.org/10.1111/pai.12910
Saini SS, Kaplan AP (2018) Chronic spontaneous urticaria: the devil’s itch. J Allergy Clin Immunol Pract 6(4):1097–1106. https://doi.org/10.1016/j.jaip.2018.04.013
Article PubMed PubMed Central Google Scholar
Sharma VK, Gupta V, Pathak M, Ramam M (2017) An open-label prospective clinical study to assess the efficacy of increasing levocetirizine dose up to four times in chronic spontaneous urticaria not controlled with standard dose. J Dermatol Treat 28(6):539–543. https://doi.org/10.1080/09546634.2016.1246705
Article CAS Google Scholar
Sotés PI, Armisén M, Usero-Bárcena T et al (2021) Efficacy and safety of up-dosing antihistamines in chronic spontaneous urticaria: a systematic review of the literature. J Investig Allergol Clin Immunol 31(4):282–291. https://doi.org/10.18176/jiaci.0649
Zuberbier T, Abdul Latiff AH, Abuzakouk M et al (2022) The international EAACI/GA 2 LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 77(3):734–766. https://doi.org/10.1111/all.15090
Bernstein JA, Lang DM, Khan DA et al (2014) The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 133(5):1270–1277. https://doi.org/10.1016/j.jaci.2014.02.036
Endo T, Toyoshima S, Kanegae K et al (2019) Identification of biomarkers for predicting the response to cyclosporine A therapy in patients with chronic spontaneous urticaria. Allergol Int 68(2):270–273. https://doi.org/10.1016/j.alit.2018.09.006
Sussman G, Hébert J, Gulliver W et al (2020) Omalizumab re-treatment and step-up in patients with chronic spontaneous urticaria: OPTIMA trial. J Allergy Clin Immunol Pract 8(7):2372-2378.e5. https://doi.org/10.1016/j.jaip.2020.03.022
Kaplan A, Ledford D, Ashby M et al (2013) Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol 132(1):101–109. https://doi.org/10.1016/j.jaci.2013.05.013
Nettis E, Cegolon L, Di Leo E et al (2018) Omalizumab in chronic spontaneous urticaria: efficacy, safety, predictors of treatment outcome, and time to response. Ann Allergy Asthma Immunol 121(4):474–478. https://doi.org/10.1016/j.anai.2018.06.014
Bernstein JA, Kavati A, Tharp MD et al (2018) Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of “real-world” evidence. Expert Opin Biol Ther 18(4):425–448. https://doi.org/10.1080/14712598.2018.1438406
Bernstein JA, Singh U, Rao MB, Berendts K, Zhang X, Mutasim D (2020) Benralizumab for chronic spontaneous urticaria. N Engl J Med 383(14):1389–1391. https://doi.org/10.1056/NEJMc2016395
Kaul M, End P, Cabanski M et al (2021) Remibrutinib (LOU064): a selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial. Clin Transl Sci. https://doi.org/10.1111/cts.13005
Giménez-Arnau AM, Salman A (2020) Targeted therapy for chronic spontaneous urticaria: rationale and recent progress. Drugs 80(16):1617–1634. https://doi.org/10.1007/s40265-020-01387-9
Kocatürk E, Maurer M, Metz M, Grattan C (2017) Looking forward to new targeted treatments for chronic spontaneous urticaria. Clin Transl Allergy 7:1. https://doi.org/10.1186/s13601-016-0139-2
Article CAS PubMed PubMed Central Google Scholar
Altrichter S, Frischbutter S, Fok JS et al (2020) The role of eosinophils in chronic spontaneous urticaria. J Allergy Clin Immunol 145(6):1510–1516. https://doi.org/10.1016/j.jaci.2020.03.005
Bansal CJ, Bansal AS (2019) Stress, pseudoallergens, autoimmunity, infection and inflammation in chronic spontaneous urticaria. Allergy Asthma Clin Immunol 15:56. https://doi.org/10.1186/s13223-019-0372-z
Bracken SJ, Abraham S, MacLeod AS (2019) Autoimmune theories of chronic spontaneous urticaria. Front Immunol 10:627. https://doi.org/10.3389/fimmu.2019.00627
Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW (1993) Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med 328(22):1599–1604. https://doi.org/10.1056/NEJM199306033282204
Grattan CE, Francis DM, Hide M, Greaves MW (1991) Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin Exp Allergy 21(6):695–704. https://doi.org/10.1111/j.1365-2222.1991.tb03198.x
Dabija D, Tadi P (2021) Chronic urticaria. In: StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/pubmed/32310370 . Accessed 29 Sept 2021
Gu H, Li L, Gu M, Zhang G (2015) Association between Helicobacter pylori infection and chronic urticaria: a meta-analysis. Gastroenterol Res Pract 2015:486974. https://doi.org/10.1155/2015/486974
Gaig P, García-Ortega P, Enrique E, Papo M, Quer JC, Richard C (2002) Efficacy of the eradication of Helicobacter pylori infection in patients with chronic urticaria. A placebo-controlled double blind study. Allergol Immunopathol 30(5):255–258. https://doi.org/10.1016/s0301-0546(02)79133-7
Yanase Y, Takahagi S, Hide M (2018) Chronic spontaneous urticaria and the extrinsic coagulation system. Allergol Int 67(2):191–194. https://doi.org/10.1016/j.alit.2017.09.003
Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R (2009) Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol 148(2):170–174. https://doi.org/10.1159/000155748
Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP (2002) TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol 109(4):694–700. https://doi.org/10.1067/mai.2002.123236
Toyoda M, Maruyama T, Morohashi M, Bhawan J (1996) Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol 18(1):49–57. https://doi.org/10.1097/00000372-199602000-00008
Hon KL, Leung AKC, Ng WGG, Loo SK (2019) Chronic urticaria: an overview of treatment and recent patents. Recent Pat Inflamm Allergy Drug Discov 13(1):27–37. https://doi.org/10.2174/1872213X13666190328164931
Church MK, Maurer M, Simons FER et al (2010) Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy 65(4):459–466. https://doi.org/10.1111/j.1398-9995.2009.02325.x
Phinyo P, Koompawichit P, Nochaiwong S, Tovanabutra N, Chiewchanvit S, Chuamanochan M (2021) Comparative efficacy and acceptability of licensed dose second-generation antihistamines in chronic spontaneous urticaria: a network meta-analysis. J Allergy Clin Immunol Pract 9(2):956-970.e57. https://doi.org/10.1016/j.jaip.2020.08.055
Staevska M, Popov TA, Kralimarkova T et al (2010) The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol 125(3):676–682. https://doi.org/10.1016/j.jaci.2009.11.047
Siebenhaar F, Degener F, Zuberbier T, Martus P, Maurer M (2009) High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol 123(3):672–679. https://doi.org/10.1016/j.jaci.2008.12.008
van den Elzen MT, van Os-Medendorp H, van den Brink I et al (2017) Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clin Transl Allergy. https://doi.org/10.1186/s13601-017-0141-3
Nayak AS, Berger WE, LaForce CF et al (2017) Randomized, placebo-controlled study of cetirizine and loratadine in children with seasonal allergic rhinitis. Allergy Asthma Proc 38(3):222–230. https://doi.org/10.2500/aap.2017.38.4050
Gupta S, Khalilieh S, Kantesaria B, Banfield C (2007) Pharmacokinetics of desloratadine in children between 2 and 11 years of age. Br J Clin Pharmacol 63(5):534–540. https://doi.org/10.1111/j.1365-2125.2006.02810.x
Meltzer EO, Scheinmann P, Rosado Pinto JE et al (2004) Safety and efficacy of oral fexofenadine in children with seasonal allergic rhinitis—a pooled analysis of three studies. Pediatr Allergy Immunol 15(3):253–260. https://doi.org/10.1111/j.1399-3038.2004.00167.x
Pampura AN, Papadopoulos NG, Spičák V, Kurzawa R (2011) Evidence for clinical safety, efficacy, and parent and physician perceptions of levocetirizine for the treatment of children with allergic disease. Int Arch Allergy Immunol 155(4):367–378. https://doi.org/10.1159/000321181
Novák Z, Yáñez A, Kiss I et al (2016) Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol 27(5):493–498. https://doi.org/10.1111/pai.12555
Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P, Neo-I-30 Study Group (2006) Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol 55(4):705–709. https://doi.org/10.1016/j.jaad.2006.04.078
Deacock SJ (2008) An approach to the patient with urticaria. Clin Exp Immunol 153(2):151–161. https://doi.org/10.1111/j.1365-2249.2008.03693.x
Agache I, Rocha C, Pereira A et al (2021) Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: a systematic review for the EAACI Biologicals Guidelines. Allergy 76(1):59–70. https://doi.org/10.1111/all.14547
Saini SS, Bindslev-Jensen C, Maurer M et al (2015) Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol 135(3):925. https://doi.org/10.1038/jid.2014.512
Maurer M, Rosén K, Hsieh HJ et al (2013) Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 368(10):924–935. https://doi.org/10.1056/NEJMoa1215372
Maurer M, Kaplan A, Rosén K et al (2018) The XTEND-CIU study: long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol 141(3):1138-1139.e7. https://doi.org/10.1016/j.jaci.2017.10.018
Ocak M, Soyer O, Buyuktiryaki B, Sekerel BE, Sahiner UM (2020) Omalizumab treatment in adolescents with chronic spontaneous urticaria: efficacy and safety. Allergol Immunopathol 48(4):368–373. https://doi.org/10.1016/j.aller.2020.03.011
Arm JP, Bottoli I, Skerjanec A et al (2014) Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy 44(11):1371–1385. https://doi.org/10.1111/cea.12400
Maurer M, Giménez-Arnau AM, Sussman G et al (2019) Ligelizumab for chronic spontaneous urticaria. N Engl J Med 381(14):1321–1332. https://doi.org/10.1056/NEJMoa1900408
Maurer M, Giménez-Arnau A, Bernstein JA et al (2022) Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: a one-year extension study. Allergy. https://doi.org/10.1111/all.15175
Harb H, Chatila TA (2020) Mechanisms of dupilumab. Clin Exp Allergy 50(1):5–14. https://doi.org/10.1111/cea.13491
Lee JK, Simpson RS (2019) Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract 7(5):1659-1661.e1. https://doi.org/10.1016/j.jaip.2018.11.018
Goodman B, Jariwala S (2021) Dupilumab as a novel therapy to treat adrenergic urticaria. Ann Allergy Asthma Immunol 126(2):205–206. https://doi.org/10.1016/j.anai.2020.06.034
Hom S, Pisano M (2017) Reslizumab (Cinqair): an interleukin-5 antagonist for severe asthma of the eosinophilic phenotype. P T 42(9):564–568. https://www.ncbi.nlm.nih.gov/pubmed/28890642 . Accessed 29 Sept 2021
Wechsler ME, Akuthota P, Jayne D et al (2017) Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 376(20):1921–1932. https://doi.org/10.1056/NEJMoa1702079
Ortega HG, Liu MC, Pavord ID et al (2014) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 371(13):1198–1207. https://doi.org/10.1056/NEJMoa1403290
Roufosse F, Kahn JE, Rothenberg ME et al (2020) Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 146(6):1397–1405. https://doi.org/10.1016/j.jaci.2020.08.037
Hata D, Kawakami Y, Inagaki N et al (1998) Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med 187(8):1235–1247. https://doi.org/10.1084/jem.187.8.1235
Maurer M, Berger W, Giménez-Arnau A et al (2021) Remibrutinib (LOU064) versus placebo in patients with chronic spontaneous urticaria: a randomised, double-blind, phase 2b dose-finding study. SSRN Electron J. https://doi.org/10.2139/ssrn.3968885
Article Google Scholar
Metz M, Sussman G, Gagnon R et al (2021) Fenebrutinib in H antihistamine-refractory chronic spontaneous urticaria: a randomized phase 2 trial. Nat Med 27(11):1961–1969. https://doi.org/10.1038/s41591-021-01537-w
Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM (1999) Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol 103(2 Pt 1):307–314. https://doi.org/10.1016/s0091-6749(99)70506-3
Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL (2011) Treatment of refractory chronic urticaria with tumor necrosis factor–alfa inhibitors. J Am Acad Dermatol 64(6):1221–1222. https://doi.org/10.1016/j.jaad.2009.10.043
Silverman GJ, Weisman S (2003) Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum 48(6):1484–1492. https://doi.org/10.1002/art.10947
Combalia A, Losno RA, Prieto-González S, Mascaró JM (2018) Rituximab in refractory chronic spontaneous urticaria: an encouraging therapeutic approach. Skin Pharmacol Physiol 31(4):184–187. https://doi.org/10.1159/000487402
Kitajima M, Lee HC, Nakayama T, Ziegler SF (2011) TSLP enhances the function of helper type 2 cells. Eur J Immunol 41(7):1862–1871. https://doi.org/10.1002/eji.201041195
Shikotra A, Choy DF, Ohri CM et al (2012) Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 129(1):104-111.e1−e9. https://doi.org/10.1016/j.jaci.2011.08.031
Sabag DA, Matanes L, Bejar J et al (2020) Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin Exp Allergy 50(7):799–804. https://doi.org/10.1111/cea.13616
Ferrer M, Giménez-Arnau A, Saldana D et al (2018) Predicting chronic spontaneous urticaria symptom return after omalizumab treatment discontinuation: exploratory analysis. J Allergy Clin Immunol Pract 6(4):1191-1197.e5. https://doi.org/10.1016/j.jaip.2018.04.003
Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M (2018) The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 73(3):705–712. https://doi.org/10.1111/all.13345
Ertas R, Ozyurt K, Ozlu E et al (2017) Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol 140(6):1749–1751. https://doi.org/10.1016/j.jaci.2017.08.007
Deza G, Bertolín-Colilla M, Sánchez S et al (2018) Basophil FcɛRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J Allergy Clin Immunol 141(6):2313-2316.e1. https://doi.org/10.1016/j.jaci.2018.02.021
Grieco T, Dies L, Sernicola A et al (2020) Potential clinical and serological predictors of chronic spontaneous urticaria relapse in patients under omalizumab treatment. Immunotherapy 12(16):1173–1181. https://doi.org/10.2217/imt-2020-0088
Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee (2021) Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326(7):621–627. https://doi.org/10.1001/jama.2021.13304
Warren RB, Marsden A, Tomenson B et al (2019) Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol 180(5):1069–1076. https://doi.org/10.1111/bjd.16776
Szefler SJ, Jerschow E, Yoo B et al (2021) Response to omalizumab in black and white patients with allergic asthma. J Allergy Clin Immunol Pract 9(11):4021–4028. https://doi.org/10.1016/j.jaip.2021.07.013
Download references
Dr. Greiner is supported by training grant T32 AI155385 from the U.S. National Institutes of Health. The funder had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author information
Authors and affiliations.
Department of Internal Medicine, Medical Branch, University of Texas, 301 University Blvd, Galveston, TX, USA
Benjamin Greiner, Michael Adame & Jennifer McCracken
Department of Otolaryngology, McLaren Oakland Hospital, Pontiac, MI, USA
Savannah Nicks
Division of Allergy & Immunology, University of Texas Medical Branch, Galveston, TX, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Benjamin Greiner .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Greiner, B., Nicks, S., Adame, M. et al. Pathophysiology, Diagnosis, and Management of Chronic Spontaneous Urticaria: A Literature Review. Clinic Rev Allerg Immunol 63 , 381–389 (2022). https://doi.org/10.1007/s12016-022-08952-y
Download citation
Accepted : 23 August 2022
Published : 01 September 2022
Issue Date : December 2022
DOI : https://doi.org/10.1007/s12016-022-08952-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic spontaneous urticaria
- Chronic idiopathic urticaria
- Targeted therapy
- Systematic review
- Find a journal
- Publish with us
- Track your research
A Guide To Urticaria
Home / Clinical Trials / A Guide to Urticaria and Clinical Trials
Match to Urticaria Clinical Trials
What is urticaria.
Urticaria is a common skin disorder also known as hives.
It can present in two main forms: Chronic Spontaneous Urticaria (CSU) describes a condition where hives are present for most days of the week for at least six weeks. Acute Urticaria is a similar condition, but it presents for fewer than six weeks.
Physical urticaria happens only when an irritating stimulus is applied to the skin; the hives fade when the stimulus is withdrawn. It is understood as an allergic reaction.
CSU and acute urticaria are known collectively as spontaneous urticaria and may require medical care.
What Conditions Are Associated With Urticaria?
Both CSU and acute urticaria can be debilitating due to the itching and pain the hives cause. The disorder is considered uncommonly difficult to treat among dermatological conditions. It is also a more emotionally demanding condition, which can cause great mental distress.
Flare-ups of urticaria are sudden and occur without warning. Itchy, burning, rash-like lesions can appear virtually anywhere on the skin. Individual lesions are called wheals. One characteristic of urticaria is the very short duration of the wheals, each fading within 24 hours.
Urticaria Recovery and Lifestyle Changes
The various forms of urticaria can be difficult to diagnose. This is because there can be several causes for any patient to develop wheals similar to urticaria. A complete diagnosis requires identifying any environmental factors that might contribute to the symptoms.
Some very effective treatments are available, but must be prescribed and used correctly. Most treatments center around non-sedating antihistamines. Antihistamines interfere with some of the body’s inflammatory responses and are also used to treat certain allergies.
Although the exact source of urticaria is not yet understood, doctors do know certain physical stimuli can lead to flare-ups. These include things like alcoholic drinks, over-the-counter NSAID medications like aspirin and ibuprofen, exercise, and extreme temperatures (both hot and cold.)
Patients can improve their symptom management by noticing which stimuli most often lead to their own flare-ups. By reducing or avoiding contact with these factors, many can substantially reduce the number of flare-ups they experience.
Recent Urticaria Medical Research
Nearly half of all cases of urticaria are associated with autoimmune dysfunction. The immune system can become too responsive to benign stimuli, leading to inflammation with no protective purpose. The course of treatment varies based on whether immune function is involved.
With that in mind, most medical research on urticaria is focused on finding targeted ways to counteract overactive immune function. Urticaria clinical studies also strive to uncover the other factors that might be involved in spontaneous urticaria, such as genetic factors.
Why Are Further Urticaria Clinical Studies Necessary?
Urticaria is unusual among dermatological conditions and has received plenty of attention. While urticaria clinical research has been successful so far, physicians have not been able to answer why some people get urticaria or isolate the risk factors involved.
Both acute and chronic spontaneous urticaria clinical trials are necessary if doctors are to fully understand these diseases and develop better treatments. Recent urticaria medical research led to promising findings, with several novel medications under development.
Current Urticaria Clinical Studies
The following list includes all urticaria clinical studies registered with ClinicalStudies.gov. To submit urticaria clinical studies to our list, please contact our team.
Although there are several treatments for spontaneous urticaria, many patients receive few benefits or have adverse reactions. Likewise, some specific forms of the disorder have few effective treatment options. Urticaria clinical studies are an essential piece in broadening the horizons of urticaria medical research and improving patients’ quality of life.
- https://www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/urticaria-acute-and-chronic-spontaneous-urticaria/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4622315/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3479225/
- https://www.webmd.com/skin-problems-and-treatments/chronic-skin-rash
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6557779/
Other Conditions
- Acute Aortic Dissection
- Ankylosing Spondylitis
- Alzheimer’s
- Auditory Loss and Deafness
- Autoimmune Hepatitis
- Bronchiectasis
- Breast Cancer
- Esophageal Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Compensation
- COPD Treatments
- Coronavirus
- Crohn’s Disease
- Diet And Nutrition
- Endometriosis
- Fibromyalgia
- Hepatitis B
- Hepatitis C
- Mindfulness
- Multiple Sclerosis
- Myasthenia Gravis
- Ophthalmology
- Osteoarthritis
- Parkinson’s Disease
- PBC and PSC
- Plant Based Diet
- Plastic Surgery
- Pulmonary Fibrosis
- Ulcerative Colitis
- Urinary Tract Infections
- Weight Loss
Paid Vaccine Trial For Women 16-17 Years Old
CMVictory: a vaccine trial for women between 16-17 years old.
Paid Clinical Trials Nationwide
Nationwide clinical trials offering up to $3,000 for participation and treatment.

Paid Vaccine Study For Teens
Vaccine study that aims to protect teens ages 12–17 against Epstein-Barr virus (EBV) and infectious mononucleosis (mono).
Paid Clinical Studies Nationwide
Sign up to receive email notifications on clinical trials for individual conditions in your local area.
Parents of son with myalgic encephalomyelitis/chronic fatigue syndrome want more research into disease
It has been four years since 35-year-old former builder Dan Harris has been able to get out of bed.
He lies for 24 hours a day in the dark, in silence, wearing an eye mask and earplugs.
Any variation to this is a strain on his body, incredibly fatiguing and painful.
Dan has the neurological disorder myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The disease affects many parts of the body — from the brain and muscles to the cardiac, nervous and digestive systems.
Heartbreaking condition
About 250,000 Australians have the disorder.
Most are women, and 25 per cent end up housebound or bed-bound.
Dan can no longer speak.
His mother Lynne, who is his primary carer, cannot touch or hug her son to comfort him — it's too much sensory information for his brain to process.
In the 1,400 days Dan has been confined to his bed, his parents Lynne and Michael Harris have watched their son lose his will to live.
"It's horrendous, it's horrible and it's heartbreaking to see someone that you love suffer so much," Lynne says.
"He has lost so much.
"He's lost his health, his ability to earn a living, all his relationships, his dignity, his freedom … he's lost everything.
"He's just existing. We feel like we're marking time."
Lynne says she fears her son would take his own life if he could, but he can't get out of bed.
His parents have had a conversation about voluntary assisted dying since it was permitted in South Australia on January 31 last year – but ME/CFS is not a fatal disease, so Dan doesn't qualify.
Instead, Lynne makes it through each day with the hope there will be a cure for ME/CFS, which was named in the 1980s.
There still is no diagnostic test for the disorder.
Lynne is angry there is no support from the government for research to improve the lives of people who have it.
Emerge Australia, the not-for-profit national patient support group and advocate for ME/CFS, shares those frustrations.
Chief executive Anne Wilson says the condition costs the Australian economy between $10.8 billion and $14.5 billion in lost productivity, and social security and National Disability Insurance Scheme costs each year.
Ms Wilson says the federal government granted $3 million for research in 2020, and since then no money has been allocated to ME/CFS.
"We have desperate calls from patients every day who can't find a doctor to treat them and they need help," she says.
"People like Lynne Harris and her son, they're just being ignored completely.
"There's grief and loss — these people suffer as a result of losing everything and no-one understands they've lost their lives."
Ms Wilson says the organisation's telehealth workers deal with people regularly wanting to end their lives due to the condition.
She says she hopes a focus on long-term COVID, which shares some similar symptoms including fatigue, will bring some research benefits.
A Department of Health spokesperson says the National Health and Medical Research Council committed $6.3 million towards research relevant to ME/CFS from 2000 to 2022.
"The government announced a further $50 million of funding for research into long COVID, which some consumers refer to as ME/CFS due to some similarities of symptoms," the spokesperson said.
Research money was also available under the Medical Research Future Fund with a commitment to provide $596.5 million over 10 years from 2022-23 for projects that address risk factors that contribute to chronic and complex diseases in Australia.
'These people are abandoned'
Emerge lead doctor Richard Schloeffel said the federal government's funding commitment to ME/CFS was "very little … hardly any … none, I'd say".
"There's around 250,000 people in Australia who have ME/CFS and now 45 per cent of people who have long COVID have developed ME/CFS according to the data that's coming from all around the world, so we've possibly got much more than 250,000," he said.
"These people are abandoned.
"Ninety per cent don't have a doctor, and because of those issues, most doctors aren't trained in diagnosing and treating ME/CFS.
"Most doctors would have difficulty recognising it, diagnosing it, and they definitely would have no idea of how to treat it."
Dr Schloeffel said he had treated nearly 6,000 ME/CFS patients.
"I teach doctors, I teach for the college of GPs, I teach at GP conferences … I'm doing research at the university and begging and borrowing money to pay for all the things we do and not getting funding, even though we apply for funding all the time and we don't get it," he said.
"We need guidelines, we need doctors trained, we need funding for research and we need funding of patients for carers, NDIS and support of the carers who look after them.
"I've got patients who've had it for 30 years to 40 years, their whole life almost.
"When they're very severe … the risk is they might die of it or they may suicide – they could end up with metabolic heart failure.
"I've had 35 of my patients die, including teenagers."
Dr Schloeffel said the condition was a response to a trigger.
"It can be an infection, it can be trauma or childbirth or some people are born with it … I've treated lots of children with it," he said.
"It's a cellular disorder.
"What happens is people get an infection such as glandular fever or they may have a chest infection, they may have a mosquito bite — what's called vector-borne diseases like Ross River fever, Barmah Forest virus; tick bites are known to cause it.
"As a result of that trigger, the body has a sort of autoimmune response which attacks individual cells."
Glandular fever link
Lynne believes Dan's condition could potentially have originated from the pathogen Epstein-Barr virus lying dormant in his body after a bout of glandular fever when he was about 19.
It came to the fore after he sustained a concussion at a gym while in Byron Bay, travelling with his then-fiance about five years ago.
The headache from the concussion never went away.
Lynne says Dan began to feel fatigued, but being a fit builder he tried to push through it – which research now shows is the worst thing to do, even though it was the medical advice at the time.
"It was like a slow deterioration," Lynne says.
"Then about 2019 it was like he fell off a cliff and he could not get up anymore.
"We all thought he would get better."
In 2020 he ended up back in Port Lincoln in his parents' care.
It has up-ended their lives too.
Lynne spends her time studying international research papers.
She is optimistic a focus on long COVID and its fatigue similarities to ME/CFS will find some answers.
"We're just waiting for a big medical breakthrough," she says.
The former junior primary school teacher gave up her job to care for Dan full-time.
On call 24/7
Lynne needs to be within 50 metres of her son so she can respond to his needs when the buzzer she carries goes off.
She only leaves the house once a day to walk the dog.
There have been no holidays, no weekends away, trips to the beach or even to a cafe for a coffee as a couple for Lynne and Michael.
"We haven't left the property together in four years," Lynne says.
Dan cannot drink, eat, use the toilet or care for himself without her help.
They communicate with Dan mouthing words and pointing with one finger, and his mother speaks softly in the dark using only two to three words.
Mother and son have a couple of chances to understand each other before Lynne has to leave the room because the communication becomes too much for Dan to process cognitively.
Any effort can cause post-exertional malaise (PEM), which worsens the condition.
Dan used to be able to write the occasional note but his condition deteriorated after the noise of helicopters and fire trucks that had to fight a bushfire that came within 10m of the house last year.
Lynne wants more funding to be invested in research into the syndrome.
She and her husband say they want the government to care about their son's life.
"If you could imagine yourself in a dark room with no sound ... not interacting with anybody for a day, times that by a thousand, because that’s how long he's been doing it," Lynne says.
- X (formerly Twitter)
Related Stories
Tim thought he had 'a little flu', but four years later he can no longer hold down a job.
Dee feels like a prisoner in her own body and wants more people to know the truth about her illness
Doctors warn of lasting effects of COVID-19 after struggling to recover from virus
'Potentially harmful and old-fashioned' chronic fatigue treatments under review
- Adelaide University
- Chronic Fatigue Syndrome
- Health Policy
- Medical Research
- Mental Health
- Port Lincoln
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 27, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
Research suggests physical activity can protect from chronic pain
by UiT The Arctic University of Norway

In 2023, researchers from UiT The Arctic University of Norway, the University Hospital of North Norway (UNN), and the Norwegian Institute of Public Health found that among more than 10,000 adults, those who were physically active had a higher pain tolerance than those who were sedentary; and the higher the activity level, the higher the pain tolerance.
After this finding, the researchers wanted to understand how physical activity could affect the chances of experiencing chronic pain several years later. And they wondered if this was related to how physical activity affects our ability to tolerate pain.
"We found that people who were more active in their free time had a lower chance of having various types of chronic pain 7-8 years later. For example, being just a little more active, such as going from light to moderate activity, was associated with a 5% lower risk of reporting some form of chronic pain later," says doctoral fellow Anders Årnes at UiT and UNN.
He is one of the researchers behind the study. He adds that for severe chronic pain in several places in the body, higher activity was associated with a 16% reduced risk.
Measured cold pain tolerance
The researchers found that the ability to tolerate pain played a role in this apparent protective effect. That explains why being active could lower the risk of having severe chronic pain, whether or not it is widespread throughout the body.
"This suggests that physical activity increases our ability to tolerate pain and may be one of the ways in which activity helps to reduce the risk of severe chronic pain," says Årnes.
The researchers included almost 7,000 people in their study, recruited from the large Tromsø survey, which has collected data on people's health and lifestyle over decades.
After obtaining information about the participants' exercise habits during their free time, the researchers examined how well the same people handled cold pain in a laboratory. Later, they checked whether the participants experienced pain that lasted for 3 months or more, including pain that was located in several parts of the body or pain that was experienced as more severe.
Among the participants, 60% reported some form of chronic pain, but only 5% had severe pain in multiple parts of the body. Few people experienced more serious pain conditions.
The research was recently published in the journal PAIN—Journal of the International Association for the Study of Pain .
When it comes to exercising if you already have chronic pain, the researcher says,
"Physical activity is not dangerous in the first place, but people with chronic pain can benefit greatly from having an exercise program adapted to help them balance their effort so that it is not too much or too little. Health care professionals experienced in treating chronic pain conditions can often help with this. A rule of thumb is that there should be no worsening that persists over an extended period of time, but that certain reactions in the time after training can be expected."
Explore further
Feedback to editors

Genetic causes of cerebral palsy uncovered through whole-genome sequencing
3 hours ago

Women with obesity do not need to gain weight during pregnancy, new study suggests
14 hours ago

Cancer-predisposition variants associated with adverse outcomes in rhabdomyosarcoma
16 hours ago

New study shows how the Crimean-Congo hemorrhagic fever virus enters our cells
COVID-19 antibody discovery could explain long COVID

Older brain cells linger unexpectedly before their death
17 hours ago
Researchers report clear shift in arterial diseases in diabetes
Researchers uncover potential treatment targets for Zika virus–related eye abnormalities
18 hours ago

Study discovers how a magnesium cellular transport 'pump' plays a vital role in cardiac function

Possible new biomarker for better detection of numerous inflammatory diseases
Related stories.

Pain in the pursuit of beauty: One in eight suffer chronic pain after cosmetic surgery, study finds
Mar 11, 2024

Physical activity may lessen pain intensity for cancer survivors
Feb 12, 2024

Physical activity linked to higher pain tolerance
May 24, 2023

Research suggests yoga benefits individuals with chronic back pain
Feb 21, 2024

All pain is not the same when it comes to MS
Jan 14, 2024
Different pain types in multiple sclerosis can cause difficulty staying active
Nov 1, 2023
Recommended for you

Cell phone video technology unveils new method for analyzing walking and gait
21 hours ago

Miscarriages linked to health risks in later pregnancies

Want to feel young? Protect your sleep, say researchers
Mar 27, 2024
Monitoring your own blood pressure can save money—and possibly your life

Dog-training program helps increase physical activity among kids with disabilities

Filters reduce arsenic levels by nearly 50% in Native American study participants with well water
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
COVID-19 antibody discovery could explain long COVID
UVA Health researchers have discovered a potential explanation for some of the most perplexing mysteries of COVID-19 and long COVID. The surprising findings could lead to new treatments for the difficult acute effects of COVID-19, long COVID and possibly other viruses.
Researchers led by UVA's Steven L. Zeichner, MD, PhD, found that COVID-19 may prompt some people's bodies to make antibodies that act like enzymes that the body naturally uses to regulate important functions -- blood pressure, for example. Related enzymes also regulate other important body functions, such as blood clotting and inflammation.
Doctors may be able to target these "abzymes" to stop their unwanted effects. If abzymes with rogue activities are also responsible for some of the features of long COVID, doctors could target the abzymes to treat the difficult and sometimes mysterious symptoms of COVID-19 and long COVID at the source, instead of merely treating the downstream symptoms.
"Some patients with COVID-19 have serious symptoms and we have trouble understanding their cause. We also have a poor understanding of the causes of long COVID," said Zeichner, a pediatric infectious disease expert at UVA Children's. "Antibodies that act like enzymes are called 'abzymes.' Abzymes are not exact copies of enzymes and so they work differently, sometimes in ways that the original enzyme does not. If COVID-19 patients are making abzymes, it is possible that these rogue abzymes could harm many different aspects of physiology. If this turns out to be true, then developing treatments to deplete or block the rogue abzymes could be the most effective way to treat the complications of COVID-19."
Understanding COVID-19 Abzymes
SARS-CoV-2, the virus that causes COVID, has protein on its surface called the Spike protein. When the virus begins to infect a cell, the Spike protein binds a protein called Angiotensin Converting Enzyme 2, or ACE2, on the cell's surface. ACE2's normal function in the body is to help regulate blood pressure; it cuts a protein called angiotensin II to make a derivative protein called angiotensin 1-7. Angiotensin II constricts blood vessels, raising blood pressure, while angiotensin 1-7 relaxes blood vessels, lowering blood pressure.
Zeichner and his team thought that some patients might make antibodies against the Spike protein that looked enough like ACE2 so that the antibodies also had enzymatic activity like ACE2, and that is exactly what they found.
Recently, other groups have found that some patients with long COVID have problems with their coagulation systems and with another system called "complement." Both the coagulation system and the complement system are controlled by enzymes in the body that cut other proteins to activate them. If patients with long COVID make abzymes that activate proteins that control processes such as coagulation and inflammation, that could explain the source of some of the long COVID symptoms and why long COVID symptoms persist even after the body has cleared the initial infection. It also may explain rare side effects of COVID-19 vaccination.
To determine if antibodies could be having unexpected effects in COVID patients, Zeichner and his collaborators examined plasma samples collected from 67 volunteers with moderate or severe COVID on or around day 7 of their hospitalization. The researchers compared what they found with plasma collected in 2018, prior to the beginning of the pandemic. The results showed that a small subset of the COVID patients had antibodies that acted like enzymes.
While our understanding of the potential role of abzymes in COVID-19 is still in its early stages, enzymatic antibodies have already been detected in certain cases of HIV, Zeichner notes. That means there is precedent for a virus to trigger abzyme formation. It also suggests that other viruses may cause similar effects.
Zeichner, who is developing a universal coronavirus vaccine, expects UVA's new findings will renew interest in abzymes in medical research. He also hopes his discovery will lead to better treatments for patients with both acute COVID-19 and long COVID.
"We now need to study pure versions of antibodies with enzymatic activity to see how abzymes may work in more detail, and we need to study patients who have had COVID-19 who did and did not develop long COVID," he said. "There is much more work to do, but I think we have made a good start in developing a new understanding of this challenging disease that has caused so much distress and death around the world. The first step to developing effective new therapies for a disease is developing a good understanding of the disease's underlying causes, and we have taken that first step."
Findings Published
The researchers have published their findings in the scientific journal mBio , a publication of the American Society for Microbiology. The research team consisted of Yufeng Song, Regan Myers, Frances Mehl, Lila Murphy, Bailey Brooks, and faculty members from the Department of Medicine, Jeffrey M. Wilson, Alexandra Kadl, Judith Woodfolk.
"It's great to have such talented and dedicated colleagues here at UVA who are excited about working on new and unconventional research projects," said Zeichner.
Zeichner is the McClemore Birdsong Professor in the University of Virginia School of Medicine's Departments of Pediatrics and Microbiology, Immunology and Cancer Biology; the director of the Pendleton Pediatric Infectious Disease Laboratory; and part of UVA Children's Child Health Research Center.
The abzyme research was supported by UVA, including the Manning Fund for COVID-19 Research at UVA; the Ivy Foundation; the Pendleton Laboratory Fund for Pediatric Infectious Disease Research; a College Council Minerva Research Grant; the Coulter Foundation; and the National Institutes of Health's National Institute of Allergy and Infection Diseases, grant R01 AI176515. Additional support came from the HHV-6 Foundation.
- COVID and SARS
- Infectious Diseases
- Hypertension
- Chronic Illness
- Diseases and Conditions
- Heart Disease
- Blood Clots
- West Nile virus
- Plantar wart
- Candidiasis
Story Source:
Materials provided by University of Virginia Health System . Note: Content may be edited for style and length.
Journal Reference :
- Yufeng Song, Regan Myers, Frances Mehl, Lila Murphy, Bailey Brooks, Jeffrey M. Wilson, Alexandra Kadl, Judith Woodfolk, Steven L. Zeichner. ACE-2-like enzymatic activity is associated with immunoglobulin in COVID-19 patients . mBio , 2024; DOI: 10.1128/mbio.00541-24
Cite This Page :
Explore More
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- What Controls Sun's Differential Rotation?
- Robot, Can You Say 'Cheese'?
- Researchers Turn Back the Clock On Cancer Cells
- Making Long-Term Memories: Nerve-Cell Damage
- A Solar Cell You Can Bend and Soak in Water
- 'Cosmic Cannibals': Fast-Moving Jets in Space
- Risk Factors for Faster Brain Aging
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Dermatol
- v.63(1); Jan-Feb 2018
Consensus Statement for the Diagnosis and Treatment of Urticaria: A 2017 Update
Kiran godse.
From the Department of Dermatology, D Y Patil Hospital, Navi Mumbai, Maharastra, India
Abhishek De
1 Department of Dermatology, Calcutta National Medical College, Kolkata, West Bengal, India
Vijay Zawar
2 Department of Dermatology, Skin Diseases Center, Nashik, India
3 Department of Dermatology, BJ Medical College, Ahmedabad, Gujarat, India
Mukesh Girdhar
4 Department of Dermatology, Max Super Speciality Hospital, New Delhi, India
Murlidhar Rajagopalan
5 Department of Dermatology, Apollo Hospital, Chennai, Tamil Nadu, India
D S Krupashankar
6 Department of Dermatology, Krupa Shankar Skin Care Center, Mallige Hospital, Bengaluru, Karnataka, India
This article is developed by the Skin Allergy Research Society of India for an updated evidence-based consensus statement for the management of urticaria, with a special reference to the Indian context. This guideline includes updated definition, causes, classification, and management of urticaria. Urticaria has a profound impact on the quality of life and causes immense distress to patients, necessitating effective treatment. One approach to manage urticaria is by identification and elimination of the underlying cause(s) and/or eliciting trigger(s) while the second one is by treatment for providing symptomatic relief. This guideline recommends the use of second-generation nonsedating H1-antihistamines as the first-line treatment. The dose can be increased up to four times to meet the expected results. In case patients still do not respond, appropriate treatment options can be selected depending on the associated medical condition, severity of the symptoms, affordability of the drugs, and accessibility of modern biologics such as omalizumab.
What was known?
Chronic urticaria (CU) is defined as daily or near-daily episodes of urticaria for more than 6 weeks. CU can be divided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CINDU). Treatment of urticaria is prolonged and often challenging.
Urticaria or hives is a common skin condition that affects population with a lifetime prevalence of up to 22% and point prevalence of 1%. Hives consist of three typical features; central swelling of variable size, surrounding reflex erythema, and associated symptoms of pruritus or burning. It usually resolves within a few hours and always by 24 h. Urticaria may or may not be associated with angioedema. Angioedema is typically characterized by sudden, pronounced swelling of the lower dermis and subcutis. Sometimes, it is associated with pain rather than pruritus. It frequently involves the mucous membranes and may take up to 72 h for resolution. Often both urticaria and angioedema coexist as relapsing and remitting episodes.[ 1 ]
Modern Classification of Urticaria
Classification of urticaria is based on its episodic nature, i.e., acute or chronic along with further identifiable eliciting factors or stimuli [ Table 1 ]. Chronic urticaria (CU) is defined as daily or near-daily episodes of urticaria for more than 6 weeks. CU can be divided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CINDU). CSU is when episodes of urticaria persist beyond 6 weeks without any external stimulus. CINDU is when symptoms are induced by a specific trigger, for example, temperature, pressure, and cholinergic stimulation.
Classification of urticaria

Consensus statement 1
Should the present classification be followed in urticaria?
There is strong recommendation to use this updated version of the classification.
Method of Preparing Urticaria Guideline
As members of the panel of Skin Allergy Research Society of India, the authors had prepared in advance their suggestions regarding the definition, classification, diagnosis, and treatment of urticaria. The resulting draft of the guideline took into account all available evidence in the literature (including Medline and Google Scholar searches) and was based on the existing consensus papers of different associations around the globe [ Table 2 ].
Existing consensus papers of different associations regarding the management of urticaria

A structured questionnaire was prepared and consensus was finally achieved during consensus meeting at the annual skin allergy society meeting held on March 26, 2017, in Chennai. The participation of urticaria specialists from different states across India ensured that this consensus included regional differences in viewpoint. The expression “we recommend” was used for strong recommendations and “we suggest” for weak recommendations.
The aim of this guideline is to provide an updated definition and classification of urticaria and to provide evidence-based diagnostic and therapeutic approach in Indian perspective. This guideline has involved experts from different parts of India and had also taken into account variations in patients, medical systems, and access to diagnosis and treatment across the country.
Epidemiology and Course of Urticaria
CSU is known to be the most common form of CU (66 to 93% of cases). Lifetime prevalence for urticaria is reported as 7.8–22.3%, with point prevalence being 0.5–1.0%. Approximately 4–33% of cases are reported to be physical urticaria and 1–7% of cases are cholinergic urticaria. The exact prevalence in India is not known.[ 3 , 4 ]
CSU is a chronic disease whose duration is estimated to be 1–5 years on an average. Of the diagnosed CSU patients, 50% resolves within 6 months of onset. Another, 20% resolves within 3 years. Further, 20% resolves within 5–10 years. However, 2% of CSU cases may take up to 25 years to resolve. There are reports to suggest in very rare situation; CSU can persist for up to 50 years.[ 5 ]
Pathophysiology
The pathogenesis of CSU is yet to be fully characterised. It is thought to be mediated by aberrant release of histamine and other inflammatory mediators from mast cells and basophils.[ 1 ] CSU skin lesions show recruitment of mast cells and also basophils, neutrophils, eosinophils, and T lymphocytes.[ 6 ] It is now recognised that urticaria is a mast cell-driven disease. Activated mast cells release histamine and other mediators. These mediators activate sensory nerves. However, mast cell-activating signals in urticaria are ill-defined and likely to be heterogeneous and diverse.[ 2 ] Mast cell activation in CSU may either be through autoimmune, allergic, or idiopathic mechanisms.
Degranulation of mast cells releases histamine and other inflammatory mediators, such as platelet-activating factor and pro-inflammatory cytokines, which ultimately activates sensory nerves and elicits local vasodilatation, plasma extravasation as well as leukocyte trafficking to urticarial lesions.[ 7 ] The oedema of the upper and mid-dermis in wheals is due to dilatation of the postcapillary venules and lymphatic vessels beneath the upper dermis, whereas in angioedema, lower dermis and the subcutis are involved. The oedematous skin lesions often involve upregulation of endothelial cell adhesion molecules and perivascular infiltrate of neutrophils and/or eosinophils, macrophages, and T-cells.[ 8 , 9 ]
As IgE is a key to the release of histamine and other pro-inflammatory mediators from mast cells and basophils following degranulation, it may play a role in CSU. Specific IgG antibodies against the FcεRIa subunit of IgE receptor also account for 30–50% of CU cases, and 5–10% of cases show IgG antibodies against IgE itself.[ 10 , 11 ] Most interestingly in some CU cases, elevated levels of antithyroglobulin or antithyroid antibodies in euthyroid participants are positively associated with urticarial flares.[ 12 , 13 ]
Around 15–20% of people have urticaria at least once during their lifetime.[ 14 ] Acute urticaria is rather common in young ages, mostly induced by Type I hypersensitivity allergic reactions to food, drug, insect sting, viral infections, or transfusion. Often anaphylaxis due to drugs such as opiates, vancomycin, and radiocontrast media is encountered in clinical practice, which needs to be differentiated from urticaria.
Assessment Tools of Urticaria Disease Activity
In spontaneous urticaria, disease activity is assessed by a robust and simple scoring system named as Urticaria Activity Score (UAS7). UAS7 [ Table 3 ] is a weekly composite sum of the pruritus and number of hives score, for measuring the disease activity.[ 1 ] It is a simple questionnaire-based system, which gives the dermatologist a semi-objective evaluation of pruritus and hives experienced by patients [ Table 2 ].[ 15 ] The resultant UAS7 score is the sum scores of seven consecutive days (0–42), which determines both the disease activity and efficacy of the ongoing interventions of CSU.
Urticaria Activity Score 7 scoring criteria for chronic spontaneous urticaria disease activity

Consensus statement 2
Should the UAS7 be used in assessing severity in urticaria?
There is a strong recommendation for the use the UAS7 to assess severity
The Angioedema Activity Score (AAS) is also a patient's self-reported evaluation of the activity of angioedema, which includes scoring of five key factors (duration, physical discomfort, impact on daily activities, impact on appearance, and overall severity) from 0 to 3 of each, respectively (thus a daily score of 0–15).[ 16 ] Daily AAS scores can be summed to give 7-day scores (AAS7), 4-week scores (AAS28), and 12-week scores (AAS84), respectively, for treating physician preference.
The diagnosis should be made by a specific ordered detailed patient history, physical examination, and also some routine laboratory investigations for ruling out any association of systemic autoimmune or autoinflammatory disease.[ 2 ] Patient history should include the following: (a) time of onset of disease; (b) presence of any precipitating factors; (c) association with angioedema; (d) persistence of individual wheal beyond 24 h; (e) use of drugs (nonsteroidal anti-inflammatory drugs [NSAIDs], angiotensin-converting enzyme [ACE] inhibitors, immunisations, hormones, and alternative remedies); (f) quality of life (QOL); and (g) treatment history, if any [ Table 4 ].
Diagnostic tests for urticaria

In cases of anaphylaxis, along with urticaria/angioedema signs, other symptoms of organ involvement such as pulmonary tract (wheezing and cough), gastrointestinal (GI) system (vomiting and diarrhoea), nervous system (dizziness), or cardiac system (changes in blood pressure or heart rate) should be evaluated. CSU can also be implicated from infectious status including viral infections (hepatitis B and C, herpes simplex virus),[ 17 ] Helicobacter pylori infections,[ 18 ] and also helminthic parasitic infections.[ 19 ]
Other systemic conditions should be ruled out in suspected cases, which include a wide range of disorders including cryoglobulinemia (chronic lymphocytic leukaemia), serum sickness, connective tissue diseases such as systemic lupus erythematosus, thyroid disease, neoplasms (particularly lymphoreticular malignancy and lymphoproliferative disorders), and other endocrine disorders.[ 20 , 21 ] Furthermore, in some cases, careful patient history along with laboratory findings may point toward the requirement of a skin biopsy to rule out dermatomyositis and vasculitis. Although a routine skin biopsy of CU lesions is not recommended, histopathology of urticaria lesion shows predominant lymphocytic infiltrate with polymorphonuclear cells.[ 22 ] Urticarial vasculitic lesions typically possess urticarial symptoms lasting >24 h, which is confirmed by a skin biopsy revealing the presence of leukocytoclastic vasculitis.[ 23 ] A positive correlation has been found between detectable thyroid autoantibodies and CU though routine assessment for thyroid autoantibodies is not recommended.[ 12 ] The CU should also be evaluated through proper patient history and complaints relating to any GI origin, which may apparently link to H. pylori infection, coeliac disease, helminthic infections, etc. Although relationship to such problems is weak and inconclusive, it may benefit in select case scenario.
Angioedema is essentially a clinical diagnosis presenting as nonitchy, brawny, nonpitting oedema also with typical undefined margins and without erythema.[ 16 ] In angioedema with the absence of coexisting urticaria, evaluation should also be focused for hereditary angioedema, acquired C1 inhibitor deficiency, or ACE inhibitor-associated angioedema. However, a detailed discussion of angioedema without urticaria is beyond the scope of the present article.
Management of Urticaria
General management.
The therapeutic approach should be based on elimination or avoidance of the cause or trigger/stimulus, symptomatic pharmacological treatment by reducing mast cell mediator release and/or the effect of these mediators at the target organ, and inducing tolerance. Identifying the cause of urticaria is not possible in most cases; however, good history to rule out causes of inducible urticaria will increase therapeutic efficiency. Avoidance of physical stimuli for the treatment of physical urticaria is suggested but may not always be possible. The aim of therapy for CU is quick and complete symptom control. The authors recommend aiming for complete symptom control in urticaria as safely as possible irrespective of the type of urticaria (CSU/CINDU). Drugs (e.g., nonsteroidal anti-inflammatory drug) causing nonallergic hypersensitivity reactions cannot only elicit but can also aggravate preexisting CSU; elimination of the drug wherever possible is suggested. CSU is often anecdotally reported to be associated with a variety of inflammatory or infectious diseases. These infections include those of the GI tract, such as H. pylori . Even though the association with urticaria is not clear and a meta-analysis shows overall low evidence, H. pylori should be eliminated if the treating physician feels in select cases.
First-line therapy
Second-generation nonsedating antihistamines
The mainstay of therapeutic options is directed upon symptomatic relief of urticaria by antagonising the specific actions of H1-receptor-mediated histamine actions upon endothelial cells (the wheal) and on sensory nerves (pruritus). The first-generation antihistamines are reported to have potent anticholinergic effects and sedative actions on central nervous system lasting longer than 12 h, with therapeutic actions only for 4–6 h. Most of them cross blood–brain barrier and interact with brain H1-receptor, leading to disturbed rapid eye movement sleep and cognitive functions.[ 24 ] Many drug-drug interactions were also reported for sedating antihistamines. Thus, first-generation antihistamines are no longer the choice in modern urticaria treatment, as currently, there is availability of a wide range of modern low-cost second-generation antihistamines with lesser side effects, without anticholinergic effect (no sedation and cognitive dysfunction) and also with higher efficacy and duration of action, thus better compliance.
Further progress with regard to drug safety was achieved by the development of the newer modern second-generation antihistamines, cetirizine (metabolite of hydroxyzine), loratadine, and fexofenadine, some of which are mostly nonsedating metabolites of earlier sedative antihistamines. More recently, a plethora of second-generation drugs came such as azelastine, desloratadine (the active metabolite of loratadine), ebastine, levocetirizine (the active enantiomer of cetirizine), and rupatadine. Two second-generation drugs, astemizole and terfenadine, were banned for various reports of cardiotoxic effects such as QT prolongation, ventricular arrhythmia, and torsade de pointes and metabolic interaction with ketoconazole or erythromycin. Many out of these second-generation antihistamines do not possess high level of evidence in effectiveness in treating urticaria, and also, considerable clinical differences between them exist. Only seven of them (cetirizine, desloratadine, fexofenadine, levocetirizine, loratadine, rupatadine, and bilastine) have been tested in details in urticaria. Three of the commonly used second-generation antihistamines in India (desloratadine, fexofenadine, and levocetirizine) were extensively evaluated in the management of urticaria for safety and efficacy even up to four-fold elevation of the standard doses.[ 25 , 26 , 27 ] Some reports indicate that according to the receptor occupancy, desloratadine is the most potent (Ki: 0.4 nM), followed by levocetirizine (Ki: 3 nM) and fexofenadine (Ki: 10 nM) (lower the concentration higher is the potency).[ 28 ] However, higher occupancy and affinity for H1-receptors should also be evaluated in respect with the clinical efficacy and safety. The second-generation drugs’ superior effectiveness is not only attributed to their antihistaminic activities but may also be due to other anti-inflammatory actions such as inhibition of cell adhesion molecules-1, endothelial leukocyte adhesion molecule-1 expression, generation and release of cytokines, and inhibition of eosinophil chemotaxis.[ 28 , 29 ] In a randomised clinical trial (RCT), it was shown that second-generation antihistamines such as bepotastine, cetirizine, fexofenadine, and olopatadine had similar efficacy in reducing histamine-induced flare when compared to placebo; however, bepotastine has significantly less sedative action when compared to others.[ 30 ]
So to sum up, modern second-generation antihistamines should always be considered as the first-line symptomatic treatment for urticaria because of their overall good safety profile, proven efficacy, and broad spectrum of controlling urticarial/angioedema pathological cascades.
Consensus statement 3
Are modern second generation H1-antihistamines to be preferred over first-generation H1-antihistamines in the treatment of urticaria?
There is a strong recommendation to prefer the use of modern second-generation H1-antihistamines over first-generation H1-antihistamines as first line of the treatment of urticaria.
Second-line therapy
Up-dosing of second-generation nonsedating antihistamines
Numerous clinical studies support the clinical benefit and safety of a higher dosage of antihistamines, even up to fourfold higher than recommended doses of desloratadine, fexofenadine, levocetirizine, etc., so strong recommendation had come from the latest updated GA 2 LEN/EDF/EAACI/WAO guidelines.[ 2 ] It is suggested that the majority of nonresponder urticaria patients will benefit from up-dosing of antihistamines. Thus, up-dosing of modern second-generation antihistamines up to four times of the respective licensed dose should be considered as the second-line treatment for CSU/CINDU.
Studies confirmed the absence of dose-related QT interval prolongation with high doses of fexofenadine as up to 800 mg once daily or 690 mg twice daily for 28 days establishing the safety of the drug in higher doses.[ 31 ] Apart from fexofenadine, other drugs with good safety and efficacy data on 4-time elevation standard doses are cetirizine, levocetirizine, and desloratadine.[ 25 ] Since levocetirizine is the active enantiomer of cetirizine, the present guideline recommends that only levocetirizine, fexofenadine, and desloratadine should be considered for 4-time elevation, till better safety and efficacy data are available for other molecules.
Before stepping up, we recommend to wait for 1–2 weeks for allowing maximum effectiveness of antihistamines to manifest. Similarly, once under control, a slow step down of antihistamine without losing the beneficial disease control is recommended.
Combining two antihistamines may not afford a simple additive or synergistic result on the antihistamine receptors as they have inverse agonist action on H1-receptors. The efficacy, safety, and drug-drug interaction are not well studied in different antihistamine combinations, but existing evidence suggests that there may not be adequate benefit in combining antihistamines. Rather up-dosing an antihistamine up to fourfold is extensively studied for many antihistamines, and it can be recommended on the virtue of evidence
Although at standard dosing, there is some evidence that the drug efficiency may differ between molecules as far as receptor-binding capacity and clinical efficiency in wheal control are concerned, there are not much evidence if the drugs differ in efficacy and safety parameters when up dosed. Hence, the present guidelines suggest against combining antihistamines or shifting between different antihistamines if the second line of treatment fails to give adequate disease control and recommend going to the third line of treatment instead.
Consensus statement 4
Is an increase in the dose to fourfold of modern second-generation H1-antihistamines useful as second-line treatment and to be preferred over other treatments in urticaria?
There is a strong recommendation of a trial of up to four-fold dose of modern second-generation H1-antihistamines as second line in the algorithm of treatment of urticaria.
Corticosteroids
Although oral corticosteroids are frequently used in CSU patients who are resistant to antihistamine therapy, controlled studies are lacking [ Table 5 ]. A retrospective analysis among 750 patients reports that 50% of patients with antihistamine-resistant CU were successfully treated with a single course of prednisone (25 mg/day for 3 days, tapered to 12.5 mg/day for 3 days, and then, 6.25 mg/day for 4 days).[ 32 ] In a case study among 10 Indian CSU patients, 2 months of methylprednisolone 16 mg BD along with levocetirizine 5 mg daily caused a significant reduction in mean UASs.[ 33 ] However, in view of the severe adverse effects associated with long-term corticosteroid therapy, systemic steroids are recommended to be used sparingly only for a short period for managing acute exacerbations when all other therapies have failed or there is an emergency.[ 57 ] Proper guidelines for dose and duration of oral corticosteroids in CU management are also lacking.
Comparison among pharmacotherapies in refractory chronic urticaria patients who are resistant to high dose or combination antihistamine therapy

Cyclosporine
Low-dose cyclosporine is often considered in severe unremitting cases of CSU/CINDU. Although T-cell-mediated action of cyclosporine is widely accepted in immunosuppression, inhibition of basophil activity and mast cell degranulation is also known.[ 32 , 58 ] In a double-blind RCT, cyclosporine at doses of 3–5 mg/kg/day for 16 weeks along with daily cetirizine was reported to significantly ameliorate symptoms of CSU patients.[ 34 ] Similarly, another RCT reported that cyclosporine (4 mg/kg/day) in 30 autologous serum skin test (ASST)-positive patients for 4-week therapy, significantly reduced UAS scores. However, there was a relapse after 6 weeks of drug holiday.[ 35 ] A study among ASST-positive Indian patients with cyclosporine at a dose of 3 mg/kg/day for 12 weeks also reveals substantial improvement in UAS scores within 2 weeks of therapy. There was complete remission in three out of four CSU patients in the study.[ 36 ] Efficacy of low-dose cyclosporine (1.5–2.5 mg/kg/day) over 5 months was found promising among 30 ASST-positive autoimmune urticaria patients.[ 37 ] After 1-year follow-up, 20 out of 23 patients were under complete remission and three relapsed.[ 37 ] Another study that compares cyclosporine (4 mg/kg/day) for 1-month versus 3-month therapy suggested that the results are equivalent in terms of clinical benefit. There was no significant difference in frequency of responses, reduction of UAS in either group.[ 38 ] Another RCT among 120 CU participants with cyclosporine (3 mg/kg) showed that 62% of the patients benefited in 3 months, 20% benefited after long-term therapy, but 18% did not get any response.[ 39 ] Cyclosporine therapy is also beneficial in elevated IgE levels associated CU, reported in a case series of over 21 patients.[ 40 ] However, potential renal impairment effects of cyclosporine (which may be reversible on stopping) and hypertension are often encountered; thus, continuous blood pressure and blood urea and creatinine monitoring are required during the course of therapy.
The US Food and Drug Administration and the European Medicines Agency have approved omalizumab for both adults and adolescents with refractory CSU. A growing number of study reports its benefit in standard therapy failure cases of urticaria and angioedema.[ 41 , 42 , 43 , 44 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ]
Omalizumab is a humanised monoclonal IgG antibody against IgE, with low immunogenicity. Omalizumab consists of 95% IgG1 kappa human framework and 5% mouse sequence, which is hidden from the immune system when omalizumab binds to IgE.[ 65 ] It inhibits binding of IgE to FceRI on the surface of mast cells and basophils. Omalizumab binds to the Ce3 domain of IgE, forming trimers or hexamers and preventing it from binding to FceRI on the surface of mast cells and basophils. However, omalizumab cannot bind to receptor-bound IgE.[ 45 ] Omalizumab binds to IgE and reduces free IgE levels, leading to downregulation of FceRI on mast cells and basophils.
Results from proof-of-concept X-CUISITE study supported the efficacy and safety of omalizumab.[ 41 ] Another Phase II study MYSTIQUE on omalizumab showed improvement in symptoms of CSU with 300 mg omalizumab, with no additional benefit for 600 mg omalizumab. Three Phase III studies were conducted ASTERIA I, ASTERIA II, and GLACIAL.[ 42 , 43 , 44 ] The studies established up to 71% of itch reduction in CSU with omalizumab after 12 weeks. Rapid itch reduction was seen with the first dose of 300 mg. Up to 44% achieved zero UAS 7 score with omalizumab at 12 weeks. About 78% Dermatology Life Quality Index (DLQI) reduction is seen from baseline at week 12. Overall safety profile was found to be good except for minor increase in upper respiratory tract infection, headache, and arthralgia.[ 59 ]
Numerous reports exist about its efficacy in cholinergic urticaria,[ 47 ] cold urticaria,[ 60 ] solar urticaria,[ 61 ] heat urticaria,[ 62 ] symptomatic dermographism,[ 63 ] and delayed pressure urticaria.[ 64 ] A double-blind RCT over 323 refractory urticaria patients with moderate-to-severe CSU demonstrated a high outcome with omalizumab (150 mg or 300 mg subcutaneous [SC] injection) at every 4 weeks apart for 12 weeks.[ 65 ] Safety data were also reported to be encouraging at that study. Although regarding safety data, it has been infrequently associated with anaphylaxis due to its immunological origin. Omalizumab efficacy is also validated in Indian population with CU not responding to other therapies.[ 45 ] Several more case series and reports conform omalizumab superiority in the treatment of refractory cases of CSU/CINDU.[ 46 , 47 , 48 , 49 , 50 , 51 ]
However, in Indian context, the cost of treatment and the requirement for SC administration in a clinician's office may limit its use.
Consensus statement 5
Are omalizumab and cyclosporine useful in the treatment of patients unresponsive to up dosing of H1-antihistamines as third-line treatment?
There is a strong recommendation to prefer the use of either omalizumab or cyclosporine over other drugs as third line of treatment of urticaria in patients who are unresponsive or incompletely responsive to up dosing of modern second-generation H1-antihistamines.
First-Generation Antihistamines
The consensus believes that though modern second-generation antihistamines should be preferred in the treatment, in select cases, hydroxyzine may be used in refractory cases, because of easy availability, cheaper costs, and long experience of the Indian doctors using the molecule.
H2-Blockers
A systematic Cochrane review concluded that combinations of H1- and H2-antihistamines in a smaller number of CU patients reported better outcome than H1-antihistamines alone but also pointed out the weak level of evidence.[ 52 ] An RCT among 45 CU patients also reported that adding ranitidine with terfenadine gave superior results to terfenadine alone in terms of itch, but effect on wheal or swellings was insignificant.[ 45 ] Most RCTs and other case studies show conflicting and disappointing results about benefit of adding H2-antihistamines.[ 54 , 55 ] The present guidelines recommend against random or routine use of H2-antihistamine.
Methotrexate
Some case studies and series are in support of methotrexate in relieving symptoms for corticosteroid-dependent CSU,[ 53 , 56 , 66 ] and also urticarial vasculitis.[ 67 ] An Indian RCT of small sample size concluded that adding methotrexate (15 mg weekly) for 3 months in refractory CU cases did not show any significant additional benefit over H1-antihistamines.[ 68 ] Though very limited data are available, the authors suggest that methotrexate may be considered as an alternative in selected cases of refractory urticaria especially in Indian perspective, for its low cost, easy availability, easy dosing schedule and wide acceptance.
Autologous Serum Therapy
A placebo-controlled trial over 56 CU patients suggested that ASST-positive CU could benefit from 8 weeks of aspartate transaminase (AST) but not in other CU subforms.[ 69 ] An Indian multicentre, prospective study also analysed that weekly AST injections for 9 weeks showed significant improvements in both ASST-positive and ASST-negative patients with a sustained action for 4-month follow-up period after the last dose.[ 70 ] Another RCT comparing AST and autologous whole blood injections in 88 CU patients found no statistically significant difference in terms of efficacy and QOL improvement.[ 71 ] Case series reported from India showed that AST is of only moderate efficacy in small number of the treated patients.[ 72 ] A recent RCT shows disappointing results of AST therapy as compared to saline injections as control group.[ 73 ] The present consensus suggests that AST may be tried in refractory urticaria for its low cost and good safety profile, but evidence for potential benefit is low.
Montelukast
Leukotriene antagonist montelukast at 10 mg/day was reportedly effective for the treatment of CU, both as monotherapy or in combination with H1-antihistamines although the treatment effect observed was small.[ 74 , 75 , 76 , 77 , 78 , 79 , 80 ] Numerous results of clinical studies have been inconsistent, some demonstrating a superior response[ 79 , 80 ] and others showing an inferior response with montelukast when compared to antihistamines.[ 74 ] Montelukast monotherapy has been especially found beneficial in food additives and NSAID-induced urticarial symptoms.[ 79 , 80 ] The present guidelines suggest that there is no added advantage of montelukast over standard antihistamines and therefore should not be considered as therapeutic option in regular basis and should only be reserved as an adjuvant in select refractory cases.
A small double-blind placebo controlled study on 22 patients clearly reflects about substantial efficacy of dapsone at dose of 100 mg/day for 6 weeks in CSU patients for controlling hives and itch.[ 81 ] Another RCT clearly shows that the combination of dapsone with antihistamine versus antihistamine alone caused a persistent decrease in UAS scores also with complete remission in some cases.[ 82 ] Moreover, some case studies also exist with reports of its prominent efficacy upon urticarial vasculitis[ 83 , 84 ] and idiopathic angioedema.[ 85 ] Dapsone is known to confer with side effects such as methaemoglobinaemia, peripheral neuropathy, hepatotoxicity. Ruling out G6PD deficiency is mandatory before initiation of dapsone. Due to the lack of evidence and possibility of serious side effects, the consensus guidelines recommend against the use of dapsone in the treatment of CSU/CINDU.
Doxepin is primarily a tricyclic antidepressant but possesses H1/H2-antagonistic action. In a double-blind cross-over study, doxepin (10 mg TDS) was found to be more efficacious with lesser sedation than diphenhydramine (25 mg TDS) in CU patients.[ 86 ] Another RCT results also support doxepin (10 mg TDS) compared to pheniramine (22.5 mg TDS) in terms of improvement and sedation.[ 87 ] Although there is a paucity of evidence, limited available RCT experience of the consensus group suggests that doxepin can be used as a third line of treatment in select cases of CSU/CINDU, especially when cyclosporine and omalizumab are unavailable, inaccessible, or contraindicated.
Hydroxychloroquine
Only one double-blind RCT exists, demonstrating that 18 CU patients for an intervention of hydroxychloroquine (200 mg/day) for 12 weeks lead to improvement in QOL scores. ASST reactivity had no correlation with its responsiveness.[ 88 ] The consensus guideline recommends against the use of hydroxychloroquine in urticaria.
Mycophenolate
In a small trial without control, mycophenolate mofetil (MMF) in doses of 1000 mg BD for 12 weeks was found to decrease UAS in refractory CU patients along with steroid-free disease activity control.[ 89 ] A retrospective analysis of 19 patients with autoimmune and chronic idiopathic urticaria, 89% got control over urticaria symptoms within 14 weeks of MMF (1000 mg to 6000 mg daily in two divided doses) but frequently reported GI adverse effects.[ 90 ] Moreover, case study depicts MMF to be successful in treating urticarial dermatitis[ 91 ] and cyclosporine intolerant CU patients.[ 92 ] However, because of low evidence, doubtful effect, high cost, and incidence of adverse effects present guideline suggests against using of MMF in CSU/CINDU.
Miscellaneous Treatments
Tumour necrosis factor-alpha antagonists in cases of delayed pressure urticaria and intravenous immunoglobulin in cases of refractory CSU were used successfully in odd case reports. UV-A1, PUVA, and narrowband UV-B were used as adjuvant with antihistamines for refractory urticaria.[ 93 ]
Consensus statement 6
Should hydroxyzine, autologous serum therapy, doxepin and methotrexate be used in the treatment of urticaria.
There is a suggestion to use hydroxyzine, autologous serum therapy, doxepin, and methotrexate in select cases of refractory urticaria especially when cyclosporine and omalizumab are unavailable, inaccessible, or contraindicated
Recommendation: [ Figure 1 ]

ALGORITHM of management of urticaria. *: Safety evidence of up-dosing is available for levocetirizine, desloratadine and fexofenadine, ++: May be considered in selected situation, $: Should be considered for not more than 2 months as limited data are available for long-term safety
- Our recommendation is to start with a second-generation antihistamine and assess the benefit for at least 7 days using the UAS7 criteria
- If effectiveness is not achieved by conventional dosage, then subsequently increase the dose up to 4 times the recommended one, but only in case of levocetirizine, fexofenadine, and desloratadine among the drugs currently available in India, the safety data are established with a high level of evidence
- A short course (not more than 5–7 days) of corticosteroids with a dose of 0.5 mg/kg/day of prednisolone equivalent may be given for decreasing the acute underline inflammation, mostly encountered with associated angioedema, breathing discomfort, wheezing, allergic rhinitis, etc. We strongly recommend against the long-term use of corticosteroids due to its potential adverse effect
- In patients whom even up to 4 times of up-dosing of second-generation antihistaminic do not control the symptoms, either omalizumab or cyclosporine can be considered. Majority of cases, antihistamine-refractory CSU patients, respond to omalizumab (anti-IgE) as per high-quality evidence of randomised controlled trials. Hence, omalizumab, a humanised monoclonal antibody against IgE, can be considered in select cases of CSU as the third line of treatment. However, due to its high cost, in Indian socioeconomic conditions, cost-to-benefit ratio is the major concern for practitioners as also for patients. Hence, we also recommend cyclosporine, which also has validated efficacy in refractory urticaria, to be also considered as the third line of therapy, for Indian scenario. However, due to a high incidence of adverse effects such as hypertension, nephrotoxicity, gingival hyperplasia, hypertrichosis, and altered lipid profile, the use of cyclosporine should be strictly monitored as per standard guidelines. Most reported cyclosporine doses in clinical trials to be effective were 4-5 mg/kg.[ 34 , 35 , 36 ] Although low-dose (2–3 mg/kg) therapy had also been shown to be effective in some reports.[ 37 ] We suggest against the continuous use of cyclosporine for more than 3–4 months and also to stick to the monitoring guidelines strictly
- The last options of pharmacological interventions are with molecules with low level of evidence, a first-generation antihistamine hydroxyzine, methotrexate, AST, and doxepin. Weekly once dosing of methotrexate at 10–15 mg may be cost-effective, have a steroid-sparing effect, as based on limited clinical data. AST has conflicting evidence and may be tried in some select scenario as the cost burden is very low to the patient. Although first-generation antihistamines are going out of favour globally due to high incidence of sedation and other side effects, the authors believe that first-generation antihistamine, especially hydroxyzine, still has place in the treatment of CSU, in select cases in Indian scenario.
In children
The use of first-generation sedative antihistamines in infants and children is strongly discouraged by most consensus reviews. Cetirizine, desloratadine, fexofenadine, levocetirizine, and loratadine have been well studied in children, and their long-term safety has been well established in the paediatric population. Hence, these drugs with proven efficacy and established long-term safety in paediatric population are recommended. Furthermore, further selection is also dependent upon available formulations, which are suitable for children.
Key concepts of urticaria management in children are:
- Elimination of underlying causes and/or eliciting triggers wherever possible
- Second-generation H1-antihistamines are the mainstay of treatment aimed at providing symptom relief. The safety of up-dosing has not been validated in children. First-generation H1-antihistamines should be avoided
- Difficult cases may require other therapeutic interventions, the risk-benefit ratio being carefully analysed, as there is a paucity of supporting evidence
- Corticosteroids should be avoided and if used, should strictly be limited for short periods only (3–7 days).[ 94 ]
In pregnancy and lactation
It is best to avoid all antihistamines in pregnancy, especially during the first trimester, although, till now, there is no reports of teratogenicity and birth defects in pregnant women using modern second-generation antihistamines. However, only small sample size studies are available for cetirizine and one large meta-analysis for loratadine. Loratadine and cetirizine are classified as the US Food and Drug Administration Pregnancy Category B drugs, implying that there is no evidence of harm to the foetus during pregnancy, although well-controlled studies in humans are not available to exclude harmful effects. Clinicians, because of its long safety record often choose chlorphenamine. Hydroxyzine is one antihistamine, which is mentioned to be contraindicated during the early stages of pregnancy in some manufacturers’ insert.[ 95 , 96 ] All H1-antihistamines are excreted in breast milk in low concentrations. The use of second-generation H1-antihistamines is advised as nursing infants may have sedation and impaired cognitive development from the old first-generation H1-antihistamines transmitted in breast milk. Cyclosporine is not teratogenic, but it is embryotoxic in animal models and is associated with preterm delivery and low birth weight in human infants. Hence, the use of cyclosporine should be judged carefully in CSU calculating the risk-benefit ratio. Long-term safety of omalizumab is not established in pregnancy and lactation.
In hepatic impairment
Mizolastine is contraindicated by significant hepatic impairment. Chlorphenamine and hydroxyzine should also be avoided in severe liver disease because their sedating effect is inappropriate. Other second-generation antihistamine may be used with caution weighing the risk benefit ratio.
Renal impairment
The dose of cetirizine, levocetirizine, and hydroxyzine should be halved in patients with impaired renal failure. Cetirizine and levocetirizine should not be used in severe renal impairment. Loratadine and desloratadine should be used with caution in severe renal impairment.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
What is new?
This guideline provides a comprehensive and evidence based analysis of evaluation and management of urticaria with an Indian perspective.
Acknowledgement
This guideline is the result of a systematic literature review and a structured consensus meeting held in Chennai on March 26, 2017, under the aegis of Skin Allergy Research Society of India. We also acknowledge the help of Mr. Dhrubojyoti Mukherjee in preparing the draft.

IMAGES
VIDEO
COMMENTS
Chronic urticaria differs from acute urticaria in characteristics and treatments. ... Research Summaries; ... Hawro T, Maurer M. New treatments for chronic urticaria. Ann Allergy Asthma Immunol ...
Abstract. Chronic urticaria is a common inflammatory skin disease affecting around 0.5-1% of the world's population. The disease has a chronic indolent course which significantly affects the patient's quality of life. Urticaria pathogenesis involves cross-linking of immunoglobulin E (IgE) on mast cells causing degranulation which occurs ...
Urticaria (hives) is a relatively common condition, with a point prevalence of about 0.5-1% 1. The peak incidence is in the range of 20-40 years. Urticaria is the general term for a cutaneous response characterized by wheals and swellings. A deeper localized swelling often associated with urticaria is called angioedema.
Many patients with chronic idiopathic urticaria (also called chronic spontaneous urticaria) do not have a response to therapy with H 1 -antihistamines, even at high doses. In phase 2 trials ...
Chronic urticaria (CU) is a common, heterogeneous, and debilitating disease. Antihistamines and omalizumab are the mainstay therapies of CU. Additional treatment options are needed. Here, we review the off and beyond label use of licensed drugs, novel treatments that are currently under development, and promising new targets.
Further research should focus on defining disease endotypes and their biomarkers, identifying new treatment targets and developing improved therapies. ... Chronic urticaria (CU) is either ...
Chronic urticaria (CU) is a common disease which represents a considerable burden for many patients. The current urticaria guideline describes the evidence-based diagnosis and treatment of CU. In addition, however, questions often arise in everyday practice that are not addressed by the guideline.
828 n engl j med 387;9 nejm.org September 1, 2022 The new england journal of medicine has not been encouraged.1,13,17 For patients with chronic urticaria who have an unremarkable comprehensive ...
Chronic spontaneous urticaria (CSU) is a condition in which wheals, angioedema, and pruritus occur spontaneously and recurrently for at least 6 weeks. The etiology of this disease is partially dependent on production of autoantibodies that activate and recruit inflammatory cells. Although the wheals can resolve within 24 h, symptoms have a significant detrimental impact on the quality of life ...
Background: In the past 20 years, an increasing number of studies have advanced our understanding of the pathogenic mechanism of chronic urticaria (CU), providing new treatment options. Objectives: This bibliometric study aimed to evaluate published reports of CU-related studies from a number of different angles, review the research trends of the studies, and provide future perspectives of CU.
Until 2014, only H1-antihistamines were approved for the treatment of chronic urticaria (CU); however, they do not sufficiently control symptoms in most patients. Approval of the anti-IgE mAb omalizumab in 2014 for chronic urticaria was a milestone. Up to 80% of patients are well controlled with this agent.
A new research paper describes a stepped-care treatment to help improve the quality of life for patients with chronic uticaria. Consult QD; Health Library; ... Chronic urticaria can profoundly impair quality of life, with random episodes of intense itching that disrupt sleep and interfere with physical, social, and emotional functioning. ...
Chronic spontaneous urticaria (CSU) is characterized by recurring wheals that last 6 weeks or longer without an identifiable cause. The estimated point prevalence of CSU worldwide is 1%. Furthermore, it has a significant impact on quality of life in both adults and pediatric patients and their families. Although it is most often a self-limited disease, some patients have urticaria refractory ...
3.3 Urticaria guidelines by health ministries or social security institutions are rare, and most do not provide guidance on stepping down CU treatment. Only 28.2% of the participants reported having a regulatory urticaria guideline issued by the ministry of health and/or social security institutions (Figure 2). All participants from Russia ...
Chronic urticaria, including chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU), is characterized by the activation and degranulation of skin mast cells. Approximately 20% of patients do not respond to the currently approved and guideline-recommended treatments.
Chronic spontaneous urticaria (CSU) considerably alters patients' quality of life, often for extended periods, due to pruriginous skin lesions, impaired sleep, unexpected development of angioedema, and failure of conventional treatments in properly controlling signs and symptoms. Recent research focused on the development of new therapeutic agents with higher efficacy. Although the ...
Introduction. Chronic spontaneous (idiopathic) urticaria (CSU), defined as the occurrence of wheals, angioedema, or both for more than 6 weeks, affects 1-2% of the population. 1 It is more prevalent in women and represents an important burden that compromises patient's quality of life, interferes with routine daily activities, 2 and frequently is associated with psychiatric comorbidities ...
Chronic hives (chronic urticaria) are red, itchy skin welts that last more than six weeks. Many people have these welts every day for a year or longer. People with certain autoimmune diseases are more prone to chronic hives. But often, the cause of chronic hives is unknown. Antihistamines, steroids and immunosuppressants can soothe the hives.
The first patient has been dosed in Jasper Therapeutic's phase 1b/2a SPOTLIGHT clinical trial of subcutaneous briquilimab for the treatment of chronic inducible urticaria, the company announced ...
Urticaria is a common skin disorder also known as hives. It can present in two main forms: Chronic Spontaneous Urticaria (CSU) describes a condition where hives are present for most days of the week for at least six weeks. Acute Urticaria is a similar condition, but it presents for fewer than six weeks. Physical urticaria happens only when an ...
Chronic urticaria, also known as hives, are outbreaks of swollen, pale red bumps, patches, or welts on the skin that appear suddenly either as a result of allergies, or for other reasons. The main symptom is itchy rashes. Chronic urticaria occurs when histamine and other chemicals are released from under the skin's surface, causing the ...
Prolacta Bioscience is one company that aims to take the research long conducted on how human milk helps infants and make it work for adults who may be immunocompromised or otherwise could benefit ...
Urticaria is identified by the presence of pruritic wheals or hives, accompanied by angioedema (AE) in ~40% of cases. Up to 20% of patients present with isolated AE ( 1 - 3 ). The definition of wheals or hives involves the presence of polymorphic raised skin lesions that are rounded or irregular in shape, with a pale central region and ...
Lying on his bed 24 hours a day in complete darkness, Dan Harris's life is slipping away. His parents want money put into research to help the quarter of a million Australians with myalgic ...
An estimated 500,000 persons in the United States have chronic urticaria, with a prevalence of 0.23%. The disorder may occur at any age, but most affected patients are women, and patients of both ...
In 2023, researchers from UiT The Arctic University of Norway, the University Hospital of North Norway (UNN), and the Norwegian Institute of Public Health found that among more than 10,000 adults ...
Chronic urticaria is a mast cell-mediated condition characterized by the recurrent occurrence of urticaria and/or angioedema. ... of autologous serum skin test as a screen for autoantibodies to IgE and IgE receptors is an area of active research. A number of tools have been developed to assess disease ... Urticaria, Upper Arm DermNet New Zealand .
Aug. 10, 2022 — While research has revealed that children and adults hospitalized with COVID-19 are more susceptible to developing long COVID symptoms, a new study found that children infected ...
Epidemiology and Course of Urticaria. CSU is known to be the most common form of CU (66 to 93% of cases). Lifetime prevalence for urticaria is reported as 7.8-22.3%, with point prevalence being 0.5-1.0%. Approximately 4-33% of cases are reported to be physical urticaria and 1-7% of cases are cholinergic urticaria.