An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Respir Crit Care Med

Selected Bibliography of Recent Research in COVID-19
Benjamin d. singer.
1 Department of Medicine, Division of Pulmonary and Critical Care Medicine,
2 Department of Biochemistry and Molecular Genetics,
3 Canning Thoracic Institute, and
4 Simpson Querrey Institute for Epigenetics, Northwestern University Feinberg School of Medicine, Chicago, Illinois;
Sanjay H. Chotirmall
5 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore;
6 Department of Respiratory and Critical Care Medicine, Tan Tock Seng Hospital, Singapore;
Lindsay M. Leither
7 Department of Medicine, Division of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah;
8 Department of Medicine, University of Utah School of Medicine, Salt Lake City, Utah;
Oliver W. Meldrum
Anthony m. joudi.
9 Critical Care Medicine Department, National Institutes of Health, Bethesda, Maryland; and
Samuel M. Brown
Başak Çoruh.
10 Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continued throughout 2021. Highly effective vaccines became widely available in 2021, and basic, translational, and clinical knowledge about COVID-19 expanded at a rapid pace. This bibliography cites and summarizes publications from American Thoracic Society journals and others centered on the year 2021 that inform our understanding of the pathophysiology, clinical manifestations, vaccines, and treatment advances in COVID-19 while considering broader effects on society, healthcare delivery, and medical education.
Chotirmall SH, Martinez FJ, Schumacker PT, Cooke CR, Seam N, Brochard L, et al. Life at the editorial “COVID frontline”. The American Thoracic Society Journal Family. Am J Respir Crit Care Med 2020;201:1457–1459.
Pathophysiology
The biomedical community’s grasp of the pathophysiology underlying the clinical spectrum of COVID-19, including basic, physiological, and environmental factors, grew in 2021.
National Institutes of Health. Clinical spectrum of SARS-CoV-2 infection. [accessed 29 Jan 2022]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ .
For example, we have gained new insights into the biology of the SARS-CoV-2 viral receptor, ACE2 (angiotensin-converting enzyme 2). Murine ACE2 does not efficiently bind to the spike protein of most SARS-CoV-2 variants. Accordingly, techniques are emerging to sensitize mice to SARS-CoV-2 infection by expressing human ACE2 via an adenovirus delivery system, although these mouse models remain limited to a mild phenotype.
Han K, Blair RV, Iwanaga N, Liu F, Russell-Lodrigue KE, Qin Z, et al. Lung expression of human angiotensin-converting enzyme 2 sensitizes the mouse to SARS-CoV-2 infection. Am J Respir Cell Mol Biol 2021;64:79–88.
Harker JA, Johansson C. Rapidly deployable mouse models of SARS-CoV-2 infection add flexibility to the COVID-19 toolbox. Am J Respir Cell Mol Biol 2021; 64:7–9.
In humans, infant nasal epithelial cells exhibit interferon-stimulated ACE2 expression similar to adult cells, arguing against a prior hypothesis that a lack of interferon-stimulated ACE2 expression provides a protective advantage in younger individuals.
Salka K, Abutaleb K, Chorvinsky E, Thiruvengadam G, Arroyo M, Gomez JL, et al. IFN stimulates ACE2 expression in pediatric airway epithelial cells. Am J Respir Cell Mol Biol 2021;64:515–518.
Yepsen EN, Harrod KS. Influenza antiviral subversion: now the host is in on the act. Am J Respir Cell Mol Biol 2021;65:1–3.
Patients with chronic obstructive pulmonary disease have increased expression of ACE2 and other genes encoding proteins that are predicted to mediate interactions between SARS-CoV-2 and human cells.
Agusti A, Sibila O, Casas-Recasens S, Mendoza N, Perea L, Lopez-Giraldo A, et al. Molecular interactions of SARS-CoV-2 in lung tissue of patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2021;18:1922–1924.
These interactions can be blocked, representing a potential therapeutic strategy. Madan and colleagues demonstrated that a recombinant fragment of surfactant protein D reduces viral infectivity and replication in vitro by inhibiting the binding of SARS-CoV-2 spike protein to ACE2 in clinical samples.
Madan T, Biswas B, Varghese PM, Subedi R, Pandit H, Idicula-Thomas S, et al. A recombinant fragment of human surfactant protein D binds spike protein and inhibits infectivity and replication of SARS-CoV-2 in clinical samples. Am J Respir Cell Mol Biol 2021;65:41–53.
Finally, despite multivariate analyses revealing no association of serum ACE or ACE2 concentrations with mortality in COVID-19 acute respiratory distress syndrome (ARDS), manipulating the enzymatic function of ACE2 may be a therapeutic strategy in ARDS because of COVID-19 or other etiologies.
Reindl-Schwaighofer R, Hodlmoser S, Eskandary F, Poglitsch M, Bonderman D, Strassl R, et al. ACE2 elevation in severe COVID-19. Am J Respir Crit Care Med 2021;203:1191–1196.
Gerard L, Lecocq M, Bouzin C, Hoton D, Schmit G, Pereira JP, et al. Increased angiotensin-converting enzyme 2 and loss of alveolar type II cells in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med 2021;204:1024–1034.
Collins SP, Chappell MC, Files DC. The renin-angiotensin-aldosterone system in COVID-19-related and non-COVID-19-related acute respiratory distress syndrome: not so different after all? Am J Respir Crit Care Med 2021;204:1007–1008.
Multiple studies have provided a mechanistic basis to explain the hypercoagulable state observed in patients with COVID-19.
Hernandez Cordero AI, Sin DD. Clotting in COVID-19: is it all in the genes? Am J Respir Cell Mol Biol 2021;64:647–649.
In human pulmonary microvascular endothelial cells, SARS-CoV-2 spike protein stimulates the expression of the prothrombotic molecule PAI-1 (plasminogen activator inhibitor 1).
Han M, Pandey D. ZMPSTE24 regulates SARS-CoV-2 spike protein-enhanced expression of endothelial PAI-1. Am J Respir Cell Mol Biol 2021;65:300–308.
Khan SS. The central role of PAI-1 in COVID-19: thrombosis and beyond. Am J Respir Cell Mol Biol 2021;65:238–240.
ZMPSTE24, an inhibitor of PAI-1, mediates ACE2 ectodomain shedding to generate SARS-CoV-2 decoy receptors. Its expression declines with age and inhalational injury, which may partially explain the thrombotic risk and poorer outcomes observed in older patients and those who smoke. KLF2 (Krüppel-like factor 2), which is downregulated in COVID-19 lung autopsy specimens, also inhibits PAI-1 while promoting the expression of antiviral genes and nitric oxide synthase in vitro .
Wu D, Lee TH, Huang RT, R DG, Schoettler N, Adegunsoye A, et al. SARS-CoV-2 infection is associated with reduced Kruppel-like factor 2 in human lung autopsy. Am J Respir Cell Mol Biol 2021;65:222–226.
Of note, patients with COVID-19 who experience anosmia demonstrate persistent elevations in nasal nitric oxide concentration up to 5 months after infection.
Hua-Huy T, Lorut C, Aubourg F, Morbieu C, Marey J, Texereau J, et al. Persistent nasal inflammation 5 months after acute anosmia in patients with COVID-19. Am J Respir Crit Care Med 2021;203:1319–1322.
Macrophages and damaged epithelial cells exert prothrombotic functions via the expression of plasminogen urokinase-localizing protein and tissue factor, respectively, together with PAI-2.
FitzGerald ES, Chen Y, Fitzgerald KA, Jamieson AM. Lung epithelial cell transcriptional regulation as a factor in COVID-19-associated coagulopathies. Am J Respir Cell Mol Biol 2021;64:687–697.
Elevated complement factors, specifically factor D, correlate with markers of endothelial injury and hypercoagulability, as well as poorer outcomes.
Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter JN, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol 2021;6:eabh2259.
Host immune responses and the microbiome are associated with tissue pathology and outcomes. A pathological reticuloendothelial response characterized by infiltration of reactive plasma cells, iron-laden macrophages, and MRP8 + mononuclear cells appears to be universal in severe and critical COVID-19. Notably, SARS-CoV-2 RNA and spike protein do not colocalize with these inflammatory changes.
Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med 2021;203:192–201.
Welte T. SARS-CoV-2-triggered immune reaction: for COVID-19, nothing is as old as yesterday's knowledge. Am J Respir Crit Care Med 2021;203:156.
Monocyte-derived CD68 + CD163 + macrophages adopt a damage response gene expression signature and profibrotic phenotype in severe SARS-CoV-2 pneumonia, potentially explaining the increased prevalence of fibroproliferative ARDS among patients with critical COVID-19.
Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021;184:6243–6261.e6227.
Lebreton G, Dorgham K, Quentric P, Combes A, Gorochov G, Schmidt M. Longitudinal cytokine profiling in patients with severe COVID-19 on extracorporeal membrane oxygenation and hemoadsorption. Am J Respir Crit Care Med 2021;203:1433–1435.
Analysis of the upper respiratory tract microbiome in patients with COVID-19 supports the associations among microbiome composition, pathogen burden, and viral immune response.
Althuwaybi A, Al Quaimi M, Krishnan A, Jones R, Pearson J, Ward C. Hospitalization outcomes for COVID-19 in patients with interstitial lung disease: a potential role for aerodigestive pathophysiology? Am J Respir Crit Care Med 2021;203:521–522.
McGinniss JE, Collman RG. The upper airway microbiome and lung injury in COVID-19. Am J Respir Crit Care Med 2021;204:1353–1355.
Before death, but not on admission, hospitalized patients with COVID-19 carry a higher proportion of potential copathogens in their oropharyngeal microbiome, particularly Enterococcus spp. and Candida spp., compared with those who recover.
Ren L, Wang Y, Zhong J, Li X, Xiao Y, Li J, et al. Dynamics of the upper respiratory tract microbiota and its association with mortality in COVID-19. Am J Respir Crit Care Med 2021;204:1379–1390.
Several studies have expanded our understanding of respiratory system physiology in severe or critical COVID-19. Before the initiation of noninvasive ventilation, patients with COVID-19 exhibit a lower overall work of breathing and higher dynamic respiratory system compliance than patients with other causes of respiratory failure, which may limit the degree of self-inflicted lung injury.
Tonelli R, Busani S, Tabbi L, Fantini R, Castaniere I, Biagioni E, et al. Inspiratory effort and lung mechanics in spontaneously breathing patients with acute respiratory failure due to COVID-19: a matched control study. Am J Respir Crit Care Med 2021;204:725–728.
In contrast, time spent with a Pa O 2 /F i O 2 < 100 mm Hg and respiratory rate >greater than 25 breaths per minute before intubation is independently associated with increased driving pressure and elastance after initiation of mechanical ventilation.
Tsolaki VS, Zakynthinos GE, Mantzarlis KD, Deskata KV, Papadonta ME, Gerovasileiou ES, et al. Driving pressure in COVID-19 acute respiratory distress syndrome is associated with respiratory distress duration before intubation. Am J Respir Crit Care Med 2021;204:478–481.
A recruitment-to-inflation ratio greater than 0.7, as determined by lung ultrasound, identifies patients with potential for alveolar recruitment.
Stevic N, Chatelain E, Dargent A, Argaud L, Cour M, Guerin C. Lung recruitability evaluated by recruitment-to-inflation ratio and lung ultrasound in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med 2021;203:1025–1027.
Plasma concentrations of the soluble form of the receptor for advanced glycation end-products, a marker of lung alveolar epithelial injury, are elevated in COVID-19 ARDS and correlate with Pa O 2 /F i O 2 , ventilatory ratio, shunt fraction, RALE (Radiographic Assessment of Lung Edema) score, and mortality.
Kapandji N, Yvin E, Devriese M, de Margerie-Mellon C, Moratelli G, Lemiale V, et al. Importance of lung epithelial injury in COVID-19-associated acute respiratory distress syndrome: value of plasma soluble receptor for advanced glycation end-products. Am J Respir Crit Care Med 2021;204:359–362.
Hence, the soluble form of the receptor for advanced glycation end products may serve as a physiologically relevant biomarker of the severity of lung injury in COVID-19 ARDS.
Environmental factors and exposures are important risk factors for COVID-19 diagnosis and severity. People living in neighborhoods with higher concentrations of air pollution have an increased risk of dying from COVID-19.
Stieb DM, Evans GJ, To TM, Lakey PSJ, Shiraiwa M, Hatzopoulou M, et al. Within-city variation in reactive oxygen species from fine particle air pollution and COVID-19. Am J Respir Crit Care Med 2021;204:168–177.
Kim H, Bell ML. Air pollution and COVID-19 mortality in New York City. Am J Respir Crit Care Med 2021;204:97–99.
Balmes JR. Stress is in the air: ambient reactive oxygen species and COVID-19. Am J Respir Crit Care Med 2021;204:118–120.
Data also demonstrate a dose-dependent correlation between alcohol consumption and the risk of developing ARDS during hospitalization for COVID-19.
Lassen MCH, Skaarup KG, Sengelov M, Iversen K, Ulrik CS, Jensen JUS, et al. Alcohol consumption and the risk of acute respiratory distress syndrome in COVID-19. Ann Am Thorac Soc 2021;18:1074–1076.
High viral loads, which were associated with increased disease severity and odds of requiring intubation or death in the era before Omicron (B.1.1.529), increased the transmission of viral particles into the surrounding environment.
Zacharioudakis IM, Prasad PJ, Zervou FN, Basu A, Inglima K, Weisenberg SA, et al. Association of SARS-CoV-2 genomic load with outcomes in patients with COVID-19. Ann Am Thorac Soc 2021;18:900–903.
Yang M, Li L, Huang T, Li S, Zhang M, Yang Y, et al. SARS-CoV-2 detected on environmental fomites for both asymptomatic and symptomatic patients with COVID-19. Am J Respir Crit Care Med 2021;203:374–378.
These transmission dynamics are important considerations when patients undergo aerosol-generating procedures.
Avari H, Hiebert RJ, Ryzynski AA, Levy A, Nardi J, Kanji-Jaffer H, et al. Quantitative assessment of viral dispersion associated with respiratory support devices in a simulated critical care environment. Am J Respir Crit Care Med 2021;203:1112–1118.
Dhand R. Mitigating viral dispersion during respiratory support procedures in the ICU. Am J Respir Crit Care Med 2021;203:1051–1053.
Clinical Manifestations
Reports describing mortality rates among patients receiving mechanical ventilation have exhibited high variability since the pandemic’s beginning.
Angriman F, Scales DC. Estimating the case fatality risk of COVID-19 among mechanically ventilated patients. Am J Respir Crit Care Med 2021;203:3–4.
In a meta-analysis, Lim and colleagues estimated overall mortality in this population to be 45% (95% confidence interval, 39–52%) with substantial between-study heterogeneity.
Lim ZJ, Subramaniam A, Ponnapa Reddy M, Blecher G, Kadam U, Afroz A, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med 2021;203:54–66.
Temporal heterogeneity is also relevant, as highlighted by a retrospective cohort study in the United Kingdom that detected associations between caseload peaks and the makeup of the population of patients admitted to an ICU.
Pilcher D, Durie M. Learning from the first wave of the pandemic in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021;203:532–534.
Doidge JC, Gould DW, Ferrando-Vivas P, Mouncey PR, Thomas K, Shankar-Hari M, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021;203:565–574.
Churpek and colleagues studied hospital-level variation in the mortality of critically ill patients with COVID-19; whereas 38% of patients died by Day 28, there was significant variation between hospitals that was largely attenuated after adjusting for acute physiology, socioeconomic status, and caseload/census.
Teja B, Wunsch H. Pinpointing the cause of variation in mortality in COVID-19. Am J Respir Crit Care Med 2021;204:381–382.
Churpek MM, Gupta S, Spicer AB, Parker WF, Fahrenbach J, Brenner SK, et al.; STOP-COVID Investigators. Hospital-level variation in death for critically ill patients with COVID-19. Am J Respir Crit Care Med 2021;204:403–411.
A retrospective cohort study of patients hospitalized with COVID-19 found that the most common causes of death were respiratory failure and septic shock with multiorgan dysfunction syndrome because of COVID-19 or a superinfecting pathogen, underscoring the paradigm that critical COVID-19 is best understood as severe community-acquired pneumonia.
Ketcham SW, Bolig TC, Molling DJ, Sjoding MW, Flanders SA, Prescott HC. Causes and circumstances of death among patients hospitalized with COVID-19: a retrospective cohort study. Ann Am Thorac Soc 2021;18:1076–1079.
Budinger GRS, Misharin AV, Ridge KM, Singer BD, Wunderink RG. Distinctive features of severe SARS-CoV-2 pneumonia. J Clin Invest 2021;131:e149412.
Nevertheless, important differences exist in comparing severe SARS-CoV-2 pneumonia with pneumonia owing to other pathogens, including influenza and other respiratory viruses.
Hardin CC. Influenza and COVID-19: times don’t get no better. Ann Am Thorac Soc 2021;18:586–587.
A retrospective study found that patients admitted to the ICU with severe SARS-CoV-2 pneumonia experienced longer durations of mechanical ventilation than patients with severe influenza and were at greater risk for in-hospital mortality.
Cobb NL, Sathe NA, Duan KI, Seitz KP, Thau MR, Sung CC, et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus Influenza. Ann Am Thorac Soc 2021;18:632–640.
Likewise, small observational cohort studies observed that patients with COVID-19 ARDS were similar to patients with non–COVID-19 ARDS except for experiencing longer durations of mechanical ventilation, exhibiting lower minute ventilation rates, and having lower blood concentrations of IL-6.
Sjoding MW, Admon AJ, Saha AK, Kay SG, Brown CA, Co I, et al. Comparing clinical features and outcomes in mechanically ventilated patients with COVID-19 and acute respiratory distress syndrome. Ann Am Thorac Soc 2021;18:1876–1885.
Bain W, Yang H, Shah FA, Suber T, Drohan C, Al-Yousif N, et al. COVID-19 versus non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc 2021;18:1202–1210.
In 2021, the literature expanded our understanding of short-term and long-term sequelae of COVID-19. An observational cohort study revealed short-term sequelae among survivors of COVID-19 who had been hospitalized, including a high prevalence of lung function deficits, functional impairment, and psychological symptoms.
Finney LJ, Doughty R, Lovage S, Spurr L, Mehta B, Kemp SV, et al. Lung function deficits and symptom burden in survivors of COVID-19 requiring mechanical ventilation. Ann Am Thorac Soc 2021;18:1740–1743.
Indeed, peritraumatic dissociative symptoms and symptoms related to anxiety and depression also appear to be common during hospitalization for COVID-19 and during the early postacute recovery period in patients, family members, and healthcare workers.
Derry HM, Lief L, Woubeshet N, Schenck EJ, Kakarala S, LaFond E, et al. Peritraumatic stress symptoms during early post-intensive care unit recovery. Ann Am Thorac Soc 2021;18:364–367.
McPeake J, Shaw M, MacTavish P, Blyth K, Devine H, Fleming G, et al. Long-term outcomes after severe COVID-19 infection: a multicenter cohort study of family member outcomes. Ann Am Thorac Soc 2021;18:2098–2101.
Benedict C, Partinen M, Bjorvatn B, Cedernaes J. Sleep in female healthcare workers during COVID-19: a cross-sectional survey study in Sweden during the flattening of the first wave of the pandemic. Ann Am Thorac Soc 2021;18:1418–1420.
Rare postacute manifestations of COVID-19 continue to be reported, including angioedema weeks after symptom onset.
Batarseh E, Kersten BP, Pinelo AC, Nadler JN, Schwartz SA. Angioedema in African American patients hospitalized for COVID-19. Am J Respir Crit Care Med 2020;202:1581–1584.
Chan ED, Majluf-Cruz A. Is the angioedema associated with COVID-19 a real entity, a mimic, or both? Am J Respir Crit Care Med 2021;203:645–646.
While still being defined, long COVID (formally postacute sequelae of SARS-CoV-2 infection [PASC]) refers to a constellation of signs and symptoms, including those attributable to neuromuscular, psychiatric, respiratory, cardiac, gastrointestinal, and other origins, that persist for weeks to months after acute COVID-19.
Gandotra S, Russell D. The long and the short of it: is “long COVID” more than slow resolution of the acute disease? Ann Am Thorac Soc 2021;18:948–950.
Investigators have reported PASC across the spectrum of acute COVID-19 severity.
Townsend L, Dowds J, O’Brien K, Sheill G, Dyer AH, O’Kelly B, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021;18:997–1003.
Cohort studies have observed respiratory symptoms, reduced functional capacity, and impaired lung function, particularly gas exchange abnormalities, in survivors of COVID-19 3–4 months after acute illness that may persist for at least 1 year.
Mendez R, Latorre A, Gonzalez-Jimenez P, Feced L, Bouzas L, Yepez K, et al. Reduced diffusion capacity in COVID-19 survivors. Ann Am Thorac Soc 2021;18:1253–1255.
Abdallah SJ, Voduc N, Corrales-Medina VF, McGuinty M, Pratt A, Chopra A, et al. Symptoms, pulmonary function, and functional capacity four months after COVID-19. Ann Am Thorac Soc 2021;18:1912–1917.
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747–758.
Among patients mechanically ventilated for COVID-19, most survivors exhibit abnormal respiratory physiology and imaging abnormalities 3 months after hospital discharge, with limited diffusion capacity, decreased lung volumes, and fibrosis characterizing the clinical syndrome.
van Gassel RJJ, Bels JLM, Raafs A, van Bussel BCT, van de Poll MCG, Simons SO, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med 2021;203:371–374.
As PASC is a syndrome associated with a novel pathogen, it may be years before we have robust, normative datasets regarding its course and outcomes.
Heesakkers H, van der Hoeven JG, Corsten S, Janssen I, Ewalds E, Simons KS, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 2022;327:559–565.
Numerous studies in 2021 evaluated diagnostics for SARS-CoV-2 and superinfection with other pathogens. Although some patients with SARS-CoV-2–induced respiratory failure will have negative PCR tests on samples obtained from their nasopharynx while testing positive on BAL samples, the accuracy of nasopharyngeal assessment remains approximately 90%.
Gao CA, Cuttica MJ, Malsin ES, Argento AC, Wunderink RG, Smith SB; NU COVID Investigators. Comparing nasopharyngeal and BAL SARS-CoV-2 assays in respiratory failure. Am J Respir Crit Care Med 2021;203:127–129.
Importantly, bronchoscopy performed using personal protective equipment for airborne infection isolation and aerosol-minimizing protocols can be accomplished in patients with severe SARS-CoV-2 pneumonia with a low infectious risk to bronchoscopists.
Centers for Disease Control and Prevention. Transmission-based precautions. [updated 2016 Jan 7; accessed 2022 Jan 20]. Available from: https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html#anchor_1564058235 .
Gao CA, Bailey JI, Walter JM, Coleman JM, Malsin ES, Argento AC, et al. Bronchoscopy on intubated patients with COVID-19 is associated with low infectious risk to operators. Ann Am Thorac Soc 2021;18:1243–1246.
Accordingly, investigators have used bronchoscopy and other lower respiratory tract sampling procedures to guide antibacterial pharmacotherapy in patients with severe SARS-CoV-2 pneumonia.
Kitsios GD, Morris A. Seek and ye shall find: COVID-19 and bacterial superinfection. Am J Respir Crit Care Med 2021;204:875–877.
Musher DM. Bacterial coinfection in COVID-19 and influenza pneumonia. Am J Respir Crit Care Med 2021;204:498–500.
Bronchoscopy within 48 hours of intubation identified that up to 21% of patients recently intubated for severe SARS-CoV-2 pneumonia have a bacterial superinfection. The same study found a higher rate of ensuing ventilator-associated pneumonia than reported in other ICU populations.
Pickens CO, Gao CA, Cuttica MJ, Smith SB, Pesce LL, Grant RA, et al.; NU COVID Investigators. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med 2021;204:921–932.
In a cohort study of patients with COVID-19 or influenza, sampling using primarily endotracheal aspirates detected an early superinfection rate of 10% in patients with critical COVID-19 and approximately 30% in patients with severe influenza.
Rouze A, Martin-Loeches I, Povoa P, Metzelard M, Du Cheyron D, Lambiotte F, et al.; coVAPid Study Group. Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: a European multicenter comparative cohort study. Am J Respir Crit Care Med 2021;204:546–556.
Although the literature supports an association between COVID-19 and invasive Aspergillus , evaluation of immunocompetent patients in French ICUs with severe SARS-CoV-2 pneumonia found a low rate of Aspergillus superinfection.
Baron A, Hachem M, Tran Van Nhieu J, Botterel F, Fourati S, Carteaux G, et al. Bronchoalveolar lavage in patients with COVID-19 with invasive mechanical ventilation for acute respiratory distress syndrome. Ann Am Thorac Soc 2021;18:723–726.
Wauters J, Lamoth F, Rijnders BJA, Calandra T. Invasive pulmonary aspergillosis goes viral again? Am J Respir Crit Care Med 2021;203:275–277.
Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 2021;203:307–317.
Importantly, a cohort study demonstrated that patients with severe SARS-CoV-2 pneumonia remain susceptible to nosocomial infection via traditional routes such as indwelling devices.
Adelman MW, Bhamidipati DR, Hernandez-Romieu AC, Babiker A, Woodworth MH, Robichaux C, et al.; Emory COVID-19 Quality and Clinical Research Collaborative members. Secondary bacterial pneumonias and bloodstream infections in patients hospitalized with COVID-19. Ann Am Thorac Soc 2021;18:1584–1587.
Preexisting comorbidities, such as hypertension, cardiovascular disease, diabetes mellitus, obstructive sleep apnea (OSA), and lung disease, are risk factors for severe COVID-19.
Perger E, Soranna D, Pengo M, Meriggi P, Lombardi C, Parati G. Sleep-disordered breathing among hospitalized patients with COVID-19. Am J Respir Crit Care Med 2021;203:239–241.
Paradoxically, several studies suggest that the course of COVID-19 is less severe among patients with asthma; indeed, exacerbations of asthma requiring hospitalization decreased during 2020.
Chan KF, Kwok WC, Ma TF, Hui CH, Tam TC, Wang JK, et al. Territory-wide study on hospital admissions for asthma exacerbations in the COVID-19 pandemic. Ann Am Thorac Soc 2021;18:1624–1633.
Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19. Prevalence and risk of severe disease. Am J Respir Crit Care Med 2021;203:893–905.
Beasley R, Hills T, Kearns N. Asthma and COVID-19: preconceptions about predisposition. Am J Respir Crit Care Med 2021;203:799–801.
Martinez FD. Asthma in the time of COVID-19. Am J Respir Crit Care Med 2021;203:785–786.
Likewise, patients with bronchiectasis experienced a significant reduction in exacerbations and hospitalizations when comparing 2019–2020 with baseline years.
Crichton ML, Shoemark A, Chalmers JD. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med 2021;204:857–859.
It is plausible that mitigation measures designed to decrease the spread of COVID-19, including self-imposed physical distancing, also decrease the transmission of other respiratory viruses that would otherwise contribute to exacerbations of chronic lung diseases.
Metersky ML. Fewer bronchiectasis exacerbations during the “lockdown” for COVID-19: can we convert knowledge into action? Am J Respir Crit Care Med 2021;204:759–760.
Singer BD. COVID-19 and the next influenza season. Sci Adv 2020;6:eabd0086.
Previous studies found increased mortality among patients with interstitial lung disease (ILD) and COVID-19. Survival rates differ between types of ILD, with higher mortality among patients with fibrotic ILD compared with other types of ILD.
Gallay L, Uzunhan Y, Borie R, Lazor R, Rigaud P, Marchand-Adam S, et al. Risk factors for mortality after COVID-19 in patients with preexisting interstitial lung disease. Am J Respir Crit Care Med 2021;203:245–249.
Obesity and low lung function independently increase the risk of COVID-19.
Bui DS, Cassim R, Russell MA, Doherty A, Lowe AJ, Agusti A, et al. Lung function levels influence the association between obesity and risk of COVID-19. Am J Respir Crit Care Med 2021;204:1106–1108.
Adults at high risk for OSA have an increased risk for poor outcomes from COVID-19, and loud snoring predicts slow clinical improvement and the need for hospitalization, supplemental oxygen, and intensive care.
Peker Y, Celik Y, Arbatli S, Isik SR, Balcan B, Karataş F, et al. Effect of high-risk obstructive sleep apnea on clinical outcomes in adults with coronavirus disease 2019: a multicenter, prospective, observational clinical trial. Ann Am Thorac Soc 2021;18:1548–1559.
Overadjustment bias can occur when evaluating the effect of OSA on outcomes, and further studies are needed to understand causal relationships.
Mulla ZD, Pathak IS. Sleep apnea and poor COVID-19 outcomes: beware of causal intermediates and colliders. Am J Respir Crit Care Med 2021;203:1325–1326.
Immunocompromised patients, including those with a history of hematopoietic stem cell transplantation, tend to exhibit prolonged viral shedding and associated high mortality.
Roedl K, Heidenreich S, Pfefferle S, Jarczak D, Urbanowicz TT, Norz D, et al. Viral dynamics of SARS-CoV-2 in critically ill allogeneic hematopoietic stem cell transplant recipients and immunocompetent patients with COVID-19. Am J Respir Crit Care Med 2021;203:242–245.
Finally, data continue to support that pregnancy is a risk factor for morbidity and mortality associated with COVID-19.
Chinn J, Sedighim S, Kirby KA, Hohmann S, Hameed AB, Jolley J, et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2120456.
Nevertheless, in a cohort of 32 critically ill pregnant women, all patients survived with no fetal deaths, and treatment outcomes were similar between pregnant and matched nonpregnant patients.
Easter SR, Gupta S, Brenner SK, Leaf DE. Outcomes of critically ill pregnant women with COVID-19 in the United States. Am J Respir Crit Care Med 2021;203:122–125.
Vaccination
Approaches to COVID-19 vaccine development have included multiple platforms ( Figure 1 ). BNT162b2 is an mRNA vaccine encoding a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein. In the landmark efficacy trial of BNT162b2, a two-dose regimen in subjects at least 16 years old demonstrated 95% protection against COVID-19 with safety profiles comparable to other vaccines.

Vaccination against coronavirus disease 2019 (COVID-19). ( A ) Mechanisms of mRNA and viral vector vaccines. ( B ) Vaccine platforms in COVID-19. Examples of each category are listed. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–2615.
Vaccine efficacy waned through 6 months of follow-up; however, BNT162b2 remained highly efficacious in preventing hospitalization and death because of COVID-19.
Thomas SJ, Moreira ED, Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med 2021;385:1761–1773.
These results extended to 12- to 15-year-old recipients, who exhibited even greater immune responses than young adults.
Frenck RW, Jr., Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med 2021;385:239–250.
Most recently, randomized trials reported positive safety, immunogenicity, and efficacy signals, including protection against severe illness, of a two-dose BNT162b2 regimen in children ages 5–11 years as well as in adolescents.
Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al.; C4591007 Clinical Trial Group. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022;386:35-46.
Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al.; Overcoming Covid-19 Investigators. Effectiveness of BNT162b2 vaccine against critical COVID-19 in adolescents. N Engl J Med 2022;386:713–723.
A different mRNA vaccine, mRNA-1273, demonstrated 94% efficacy in preventing COVID-19, including severe disease, with no significant safety signals in a randomized controlled trial.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–416.
At more than 5 months of follow-up, mRNA-1273 continued to have preventive efficacy against symptomatic COVID-19 with additive protection against asymptomatic infection.
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al.; COVE Study Group. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774–1785.
Similar to BNT162b2, mRNA-1273 was found to be efficacious at preventing COVID-19 in healthy adolescents between 12 and 17 years of age with comparable immune responses to young adults.
Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med 2021;385:2241–2251.
Although rare and usually mild or moderate in severity, inflammatory heart disease has been reported after mRNA vaccination, mostly among young male recipients.
Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med 2021;385:1332–1334.
Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med 2021;385:2140–2149.
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–2139.
Alternative vaccine approaches, such as viral vectors and subunit proteins, provide similar protection against severe COVID-19 despite imparting less protection from symptomatic infection than mRNA vaccines.
Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, et al.; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021;384:2187–2201.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al.; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111.
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al.; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–1993.
Ad26.COV2.S is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding the SARS-CoV-2 spike protein. With a similar safety profile to other COVID-19 vaccines, a single dose protected against symptomatic and asymptomatic SARS-CoV-2 infection and was efficacious against severe disease, hospitalization, and death.
Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med 2021;384:1824–1835.
Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021;325:1535–1544.
A chimpanzee adenovirus-vector vaccine, ChAdOx1 nCoV-19 (AZD1222), demonstrated comparable immunogenicity across all age groups after a booster.
ChAdOx1 nCoV-19 was efficacious against symptomatic COVID-19, including the Alpha (B.1.1.7) variant; however, it did not impart protection against mild–moderate COVID-19 owing to Beta (B.1.351).
Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al.; COVID-19 Genomics UK consortium, AMPHEUS Project, Oxford COVID-19 Vaccine Trial Group. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021;397:1351–1362.
Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al.; NGS-SA Group, Wits-VIDA COVID Group. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1885–1898.
Concerns about the occurrence of a rare but clinically significant immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4 in recipients of the ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines intermittently interrupted administration in some countries.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101.
Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–2211.
Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021;384:1964–1965.
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine – United States, March-April 2021. MMWR Morb Mortal Wkly Rep 2021;70:680–684.
Other COVID-19 vaccines, including Gam-COVID-Vac (Sputnik V), NVX-CoV2373, SCB-2019, BBV152, and inactivated whole-virion CoronaVac, provide a broad vaccine portfolio going into 2022.
Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al.; 2019nCoV-501 Study Group. Efficacy of NVX-CoV2373 COVID-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1899–1909.
Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al.; 2019nCoV-302 Study Group. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med 2021;385:1172–1183.
Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021;397:682–694.
Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, et al.; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213–222.
Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021;326:35–45.
Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al.; COVAXIN Study Group. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021;398:2173–2184.
Heterologous vaccine schedules (“mix and match”) may provide superior protection.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al.; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671–681.
Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al.; Com-COV Study Group. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021;398:856–869.
Indeed, in people vaccinated with a two-dose CoronaVac series, heterologous vaccination with an mRNA vaccine (BNT162b2) led to increased titers of neutralizing antibodies compared with a third CoronaVac dose.
Mok CKP, Chen C, Yiu K, Chan TO, Lai KC, Ling KC, et al. A randomized clinical trial using CoronaVac or BNT162b2 vaccine as a third dose in adults vaccinated with two doses of CoronaVac. Am J Respir Crit Care Med 2022;205:844–847.
Substantial challenges remain, including waning immunity, inequitable vaccine distribution, vaccine hesitancy, and higher rates of breakthrough infection with increasingly transmissible strains capable of evading host immunity.
Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science 2021;374:1561–1562.
Razai MS, Oakeshott P, Esmail A, Wiysonge CS, Viswanath K, Mills MC. COVID-19 vaccine hesitancy: the five Cs to tackle behavioural and sociodemographic factors. J R Soc Med 2021;114:295–298.
Waning vaccine efficacy remains greatest in older individuals and higher-risk groups.
Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of COVID-19 vaccines over a 9-month period in North Carolina. N Engl J Med 2022;386:933–941.
Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med 2022;386:340–350.
Alpha, Beta, Gamma (P.1), Delta (B.1.617.2), Epsilon (B.1.427/B.1.429), and Omicron have challenged vaccine efficacy. Although vaccine efficacy against some variants is reduced, vaccines continue to provide strong protection against severe disease.
Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med 2021;384:1468–1470.
Concerns over waning immunity and the emergence of more transmissible variants prompted consideration of an additional (booster) vaccine beyond the initial series. In a report from the United States, the administration of a third BNT162b2 dose between 7.9 and 8.8 months after the two-dose regimen to 23 study participants suggested improved COVID-19 protection.
Falsey AR, Frenck RW, Jr., Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med 2021;385:1627–1629.
Large datasets from Israel in individuals at least 16 years of age confirmed that rates of COVID-19 and, in particular, severe illness, are substantially lower among those boosted with a third dose of BNT162b2.
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med 2021;385:1393–1400.
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Alroy-Preis S, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med 2021;385:2421–2430.
The importance of a booster has become more apparent during the emergence of Omicron, with 100-fold higher neutralization efficiency achieved after a third dose. Even with three vaccine doses, overall neutralization efficacy against Omicron remains lower than that observed against Delta.
Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med 2022;386:494–496.
Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med 2022;386:492–494.
Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med 2021;204:1274–1285.
Going forward, intranasal and pansarbecovirus vaccines currently in development may address many of these challenges.
Tan CW, Chia WN, Young BE, Zhu F, Lim BL, Sia WR, et al. Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N Engl J Med 2021;385:1401–1406.
Carmen JM, Shrivastava S, Lu Z, Anderson A, Morrison EB, Sankhala RS, et al. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spike-specific T cell responses. NPJ Vaccines 2021;6:151.
The primary message of COVID-19 therapeutics over the last year is the confirmation, with nuances related to the serological status, of the “two-phase” model proposed early in the pandemic. In general, it appears that virus-focused therapies are more effective in the early phase of illness, with host-focused therapies becoming more relevant later in the disease. Notwithstanding the methodological problems posed by substantial practice variation, the conceptual division into outpatient, inpatient, and intensive care continues to seem most useful for classifying therapies ( Table 1 ). Key priorities for therapeutics in the coming year include grappling with fluctuations in the standards of evidence motivated by pandemic urgency, conducting strategy trials to manage the accumulating independent evidence for multiple therapies, and tailoring trials to relevant physiological subtypes.
Therapeutics Granted Emergency Use Authorization by U.S. Food and Drug Administration for COVID-19 through 2021
Outpatient Therapy
The last year has witnessed transformational changes in outpatient care for COVID-19. Multiple neutralizing monoclonal antibody products have demonstrated efficacy for the treatment of early symptomatic disease, and some agents have demonstrated efficacy for preexposure (PROVENT [Prophylaxis Prevention] trial, {"type":"clinical-trial","attrs":{"text":"NCT04625725","term_id":"NCT04625725"}} NCT04625725 ) or postexposure prophylaxis.
O’Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al.; COVID-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med 2021;385:1184–1195.
Though this effect was not observed among patients treated in the emergency department with convalescent plasma, high-risk but less ill outpatients may have benefited from early administration of high-titer convalescent plasma.
Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al.; SIREN-C3PO Investigators. Early convalescent plasma for high-risk outpatients with COVID-19. N Engl J Med 2021;385:1951–1960.
Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, et al. Early outpatient treatment for COVID-19 with convalescent plasma. N Engl J Med 2022;386:1700–1711.
A trial ( {"type":"clinical-trial","attrs":{"text":"NCT04910269","term_id":"NCT04910269"}} NCT04910269 ) evaluating hyperimmune globulin among outpatients is ongoing. Given the expense, complexity of storage and administration, viral evolution limiting neutralization by established products, and ongoing constraints on supply, neutralizing monoclonal antibodies have generally been restricted to the highest-risk patients. Most available products have limited neutralization of the Omicron variant. Hence, these agents may need to be regularly updated and tested to cover new variants, similar to seasonal influenza vaccines. As we anticipate COVID-19 moving toward a more endemic state, a priority will be understanding how best to treat patients at the highest risk, particularly those with immune deficiency. It seems likely that a combination of passive immunity and complementary antiviral therapies will be useful in high-risk and immunocompromised patient populations.
The PINETREE (GS-US-540–9012) trial demonstrated a significant reduction in risk of hospitalization or death with a 3-day course of intravenous remdesivir in outpatients with an increased risk of severe COVID-19.
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al.; GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022;386:305–315.
Because remdesivir is a nucleoside analog, there may be better odds of it maintaining efficacy against new variants, although on-therapy resistance has been documented in an immunocompromised patient.
Gandhi S, Klein J, Robertson AJ, Pena-Hernandez MA, Lin MJ, Roychoudhury P, et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat Commun 2022;13:1547.
Through emergency use authorization, the U.S. Food and Drug Administration authorized the oral antivirals nirmatrelvir–ritonavir and molnupiravir for outpatient treatment of early COVID-19.
U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. 2021 [accessed 2022 Jan 20]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 .
U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. 2021 [accessed 2022 Jan 20]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain .
The pivotal trials for both agents ( {"type":"clinical-trial","attrs":{"text":"NCT04960202","term_id":"NCT04960202"}} NCT04960202 and {"type":"clinical-trial","attrs":{"text":"NCT04575597","term_id":"NCT04575597"}} NCT04575597 ) were stopped early for efficacy, although final data for molnupiravir suggested modest efficacy, whereas nirmatrelvir–ritonavir exhibited higher efficacy in the final analysis cohort. Although oral drugs are sometimes hailed as “game-changing” in the popular press, adherence may be low, particularly with nirmatrelvir–ritonavir, given its side effect profile and potential for drug–drug interactions.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al.; EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022;386:1397–1408.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al.; Group MO-OS. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022;386:509–520.
The antidepressant fluvoxamine has been studied as a treatment for COVID-19 because of its potential antiinflammatory properties. The TOGETHER trial in Brazil suggested a modest decrease in long emergency department stays or transfer to tertiary care hospitals for high-risk outpatients with COVID-19 treated with fluvoxamine (100 mg twice daily) compared with placebo; a small trial ( N = 153) also suggested possible efficacy for fluvoxamine, albeit using an idiosyncratic primary endpoint (dyspnea, hospitalization, or hypoxemia) and a different dosing regimen.
Reis G, Moreira-Silva EAD, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al.; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022;10:E36–E45.
Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020;324:2292–2300.
Despite considerable partisan fervor, the clinical data supporting ivermectin are limited. The ACTIV (Accelerating COVID-19 Therapeutic Interventions and Vaccines)-6 trial ( {"type":"clinical-trial","attrs":{"text":"NCT04885530","term_id":"NCT04885530"}} NCT04885530 ) is studying fluvoxamine as well as two dosing strategies for ivermectin in a pragmatic design.
Inpatient Therapy
For inpatients, remdesivir continues to be favored in the United States on the basis of findings of the ACTT (Adaptive COVID-19 Treatment Trial)-1 trial and less favored outside the United States because of a lack of mortality benefit in large pragmatic trials that were not designed to test time to recovery.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al.; ACTT-Study Group Members. Remdesivir for the treatment of COVID-19 – final report. N Engl J Med 2020;383:1813–1826.
Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al.; WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med 2021;384:497–511.
Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al.; DisCoVeRy Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis 2022;22:209–221.
Dexamethasone or an equivalent glucocorticoid is a cornerstone therapy in SARS-CoV-2 pneumonia, although it has not demonstrated benefit in a placebo-controlled trial.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al.; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693–704.
Targeted antiinflammatory therapies have been studied with mixed results. Inhibition of the IL-6 receptor with tocilizumab or sarilumab is a complex story, with positive readouts from two unblinded pragmatic trials. A large ( N = 4,116) trial of hospitalized patients found benefit of tocilizumab in patients requiring oxygen with evidence of systemic inflammation, and one moderately large ( N = 803) trial of critically ill patients also found benefit of tocilizumab.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637–1645.
Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al.; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021;384:1491–1502.
Nevertheless, two moderately sized (total N = 438 + 649 = 1,087) placebo-controlled trials showed no benefit of tocilizumab.
Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021;384:1503–1516.
Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med 2021;47:1258–1270.
The results of trials studying the JAK (Janus kinase) inhibitor baricitinib are somewhat clearer. ACTT-2 suggested a modest benefit for baricitinib without glucocorticoids, whereas ACTT-4 suggested no difference between dexamethasone and baricitinib, and trials with a background of glucocorticoids suggested efficacy for baricitinib (COV-BARRIER [A Study of Baricitinib (LY3009104) in Participants With COVID-19]) and tofacitinib (STOP-COVID [Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19]).
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al.; COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021;9:1407–1418.
Guimaraes PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al.; STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med 2021;385:406–415.
The efficacy signals were not large, and whether IL-6 receptor inhibition and JAK inhibition are interchangeable is unknown. Because the safety dataset for these agents in COVID-19 is not large, real-world pharmacovigilance will be important and will require methodological rigor to ensure that benefits continue to outweigh risks. Notably, nosocomial infections were more common in the placebo than in the tocilizumab groups in trials, although overall numbers of infections were low.
Although neutralizing monoclonal antibodies are not beneficial in unselected hospitalized patients, seronegative patients likely benefited from their administration, at least for the REGN-COV cocktail in the era before Omicron.
ACTIV-3/Therapeutics for Inpatients with COVID-19 Study Group. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis 2022;22:622–635.
Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, et al.; ACTIV-3/TICO Bamlanivimab Study Group. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann Intern Med 2022;175:234–243.
RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022;399:665–676.
The United Kingdom approved the REGN-COV cocktail for inpatients with a pragmatic approach to serological testing. Another unknown is whether the most important signal is viral load (perhaps sampled in blood as serum antigen), serostatus, or both. Whether antibody-dependent enhancement drives pathology in COVID-19 is controversial. Hence, it seems prudent not to administer neutralizing monoclonal antibodies to seropositive patients unless or until there is evidence to suggest that certain seropositive patients, such as those with high viral loads, are likely to benefit.
Because COVID-19 is associated with a prothrombotic state, several trials have studied anticoagulation. Full-dose heparinoid-based anticoagulation in hospitalized patients with COVID-19– related acute illness who did not require ICU care and did not have venous thromboembolism was beneficial on the basis of the primary efficacy endpoint in a multiplatform trial.
Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al.; ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med 2021;385:790–802.
Nevertheless, the interpretation is complex given the study design, with the primary effect being a decrease in progression from conventional oxygen to high-flow nasal oxygen. Smaller trials have provided confirmatory results, although inclusion criteria and beneficial outcomes differed somewhat from the multiplatform trial. The use of tissue plasminogen activator early in the pandemic was prominently discussed but had little clinical evidence supporting its administration.
Douin DJ, Shaefi S, Brenner SK, Gupta S, Park I, Wright FL, et al. Tissue plasminogen activator in critically ill adults with COVID-19. Ann Am Thorac Soc 2021;18:1917–1921.
There is growing interest in treatments for patients during the convalescent phase, especially the use of thromboprophylaxis, a topic being addressed by the ACTIV-4c trial ( {"type":"clinical-trial","attrs":{"text":"NCT04650087","term_id":"NCT04650087"}} NCT04650087 ), with encouraging early results from the Brazilian MICHELLE (Medically Ill Hospitalized Patients for COVID-19 THrombosis Extended ProphyLaxis With Rivaroxaban ThErapy) trial.
Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, et al.; MICHELLE Investigators. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 2022;399:50–59.
Critically Ill Patients
The most important intervention in critically ill patients is high-quality, multidisciplinary supportive care. Specific to COVID-19, the mainstays of therapy remain glucocorticoids and probably secondary immune modulation. A multiplatform trial of full-dose anticoagulation suggested probable harm among patients receiving life support at the time of randomization.
Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al.; REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med 2021;385:777–789.
On the totality of the evidence, it seems appropriate to stop full-dose anticoagulation and replace it with thromboprophylaxis for patients transferred to the ICU with organ failure.
The best ventilatory strategy before invasive mechanical ventilation remains an open question. The largest trial, RECOVERY-RS (Randomized Evaluation of COVID-19 Therapy–Respiratory Support), suggests that early noninvasive ventilation (NIV) may be superior to early high-flow nasal oxygen or conventional oxygen.
Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al.; RECOVERY-RS Collaborators. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA 2022;327:546–558.
Nevertheless, the results of the RECOVERY-RS trial do not mean that NIV should be used as salvage therapy for patients who otherwise require intubation and invasive mechanical ventilation. Other investigators have explored NIV use outside the ICU.
Bellani G, Grasselli G, Cecconi M, Antolini L, Borelli M, De Giacomi F, et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARd-COVID). Ann Am Thorac Soc 2021;18:1020–1026.
Despite an ongoing lack of clear evidence for efficacy from well-controlled trials, many centers have adopted extracorporeal membrane oxygenation as a centerpiece of their management for critical COVID-19.
Schmidt M, de Chambrun MP, Lebreton G, Hekimian G, Chommeloux J, Brechot N, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2021;203:1571–1573.
Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med 2021;204:34–43.
Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, et al. High in-hospital mortality rate in patients with COVID-19 receiving extracorporeal membrane oxygenation in Germany: a critical analysis. Am J Respir Crit Care Med 2021;204:991–994.
Patient positioning has also been of interest, focusing on the implementation of prone positioning, which had low uptake in the United States before the pandemic.
Klaiman T, Silvestri JA, Srinivasan T, Szymanski S, Tran T, Oredeko F, et al. Improving prone positioning for severe acute respiratory distress syndrome during the COVID-19 pandemic. An implementation-mapping approach. Ann Am Thorac Soc 2021;18:300–307.
Among patients not undergoing mechanical ventilation, prone positioning and various other positions to improve oxygenation have been proposed, including one on the basis of Rodin’s “The Thinker.”
Taylor SP, Bundy H, Smith WM, Skavroneck S, Taylor B, Kowalkowski MA. Awake prone positioning strategy for nonintubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc 2021;18:1360–1368.
Johnson SA, Horton DJ, Fuller MJ, Yee J, Aliyev N, Boltax JP, et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR). Ann Am Thorac Soc 2021;18:1424–1426.
Coppo A, Winterton D, Benini A, Monzani A, Aletti G, Cadore B, et al. Rodin’s Thinker: an alternative position in awake patients with COVID-19. Am J Respir Crit Care Med 2021;204:728–730.
Societal Effects
COVID-19 continues to cause widespread impacts on society, healthcare delivery, the conduct of research, and medical education. Striking racial and ethnic disparities noted early in the COVID-19 pandemic have persisted. In a retrospective cohort study, Gershengorn and colleagues found that Black race and Hispanic ethnicity increased the odds of COVID-19 diagnosis and hospitalization, a finding largely mediated by socioeconomic factors.
Gershengorn HB, Patel S, Shukla B, Warde PR, Bhatia M, Parekh D, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc 2021;18:1326–1334.
The prognostic accuracy of mortality prediction models was also examined through an equity lens. The SOFA (Sequential Organ Failure Assessment) score and LAPS2 (Laboratory-based Acute Physiology Score) underestimated in-hospital mortality for White patients and overestimated mortality for Black patients, indicating that overreliance on SOFA scores for triage could divert resources away from Black patients.
Ashana DC, Anesi GL, Liu VX, Escobar GJ, Chesley C, Eneanya ND, et al. Equitably allocating resources during crises: racial differences in mortality prediction models. Am J Respir Crit Care Med 2021;204:178–186.
Interestingly, an examination of the effect of four different allocation frameworks on disparities demonstrated no significant racial differences in allocation scores and no effect from the use of equity-focused modifications.
Ross-Driscoll K, Esper G, Kinlaw K, Lee YH, Morris AA, Murphy DJ, et al. Evaluating approaches to improve equity in critical care resource allocation in the COVID-19 pandemic. Am J Respir Crit Care Med 2021;204:1481–1484.
Crisis standard-of-care plans were proposed but plagued by unresolved ethical debates, underappreciated practical issues with scoring systems, and the fear of legal liability.
Oxman D. The crisis in crisis standards of care. Ann Am Thorac Soc 2021;18:1283–1284.
The medical community was urged to consider principles of distributive justice, including the use of racial or socioeconomic correction factors, training on ethics and equity, and a system of checks and balances through hospital triage and ethics committees.
Tukpah AM, Moll M, Gay E. COVID-19 racial and ethnic inequities in acute care and critical illness survivorship. Ann Am Thorac Soc 2021;18:23–25.
Ramnath VR, Lafree A, Staats K, Tomaszewski C. Promoting racial and health equity in COVID-19 by leveraging empathic interpreters, trained liaisons, and cross-institutional physician leadership. Ann Am Thorac Soc 2021;18:1262–1263.
Disruptions in the availability of personnel, equipment, and space continued through 2021. Alternative staffing from redeployment resulted in less supervision and lowered perceived quality and safety.
Hennus MP, Young JQ, Hennessy M, Friedman KA, de Vries B, Hoff RG, et al. Supervision, interprofessional collaboration, and patient safety in intensive care units during the COVID-19 pandemic. ATS Sch 2021;2:397–414.
Proposed strategies to maximize ventilator availability included using NIV and maximizing the existing supply of ventilators with operating room devices, supply from governments, and conversion of noninvasive devices to invasive ones.
Dar M, Swamy L, Gavin D, Theodore A. Mechanical-ventilation supply and options for the COVID-19 pandemic. Leveraging all available resources for a limited resource in a crisis. Ann Am Thorac Soc 2021;18:408–416.
With a scarcity of hospital beds and personnel, health systems also grappled with equity in resource allocation. Investigators used a simulated ventilator shortage to assess triage strategies for ventilator allocation and found that “color-blind” allocation exacerbated health inequities. Systematic triage algorithms saved more lives than a lottery system.
Bhavani SV, Luo Y, Miller WD, Sanchez-Pinto LN, Han X, Mao C, et al. Simulation of ventilator allocation in critically ill patients with COVID-19. Am J Respir Crit Care Med 2021;204:1224–1227.
Specific stakeholders in health care have been differentially affected by the pandemic. An observational cohort study of family members of severely ill patients with COVID-19 revealed high levels of anxiety, depression, and caregiver strain.
With the media focus primarily on physicians, there was a call for attention to nursing shortages.
Lynch J, Evans N, Ice E, Costa DK. Ignoring nurses: media coverage during the COVID-19 pandemic. Ann Am Thorac Soc 2021;18:1278–1282.
Many summer research programs, including those for high school, college, and medical students, remained virtual. Nevertheless, investigators discovered that program objectives could be accomplished remotely.
Hardie WD. Evaluation of a summer medical student research program during a pandemic. ATS Sch 2021;2:172–175.
Berr AL, Ridge KM, Hu JY-S. Pivoting to a remote-learning summer student program during the COVID-19 pandemic. ATS Sch 2021;2:521–534.
Early-career physician–scientists remained disadvantaged during 2021 because of pandemic effects on research productivity, access to mentors, professional development opportunities, funding, and threats to wellness.
Burden M, Flores SC, Schwartz DA. COVID-19: a time to reinvest in our scientists. Am J Respir Crit Care Med 2021;203:1190–1191.
Kliment and colleagues proposed interventions to address these challenges.
Kliment CR, Barbash IJ, Brenner JS, Chandra D, Courtright K, Gauthier MC, et al. COVID-19 and the early-career physician-scientist. Fostering resilience beyond the pandemic. ATS Sch 2020;2:19–28.
The pandemic has led to new approaches to clinical care that reflect requirements for physical distancing. Virtual focus groups supported qualitative research into these approaches.
Santhosh L, Rojas JC, Lyons PG. Zooming into focus groups: strategies for qualitative research in the era of social distancing. ATS Sch 2021;2:176–184.
In a qualitative study, clinicians and families reported that phone calls for routine updates and video calls for aligning clinician and family perspectives were somewhat effective communication tools.
Kennedy NR, Steinberg A, Arnold RM, Doshi AA, White DB, DeLair W, et al. Perspectives on telephone and video communication in the intensive care unit during COVID-19. Ann Am Thorac Soc 2021;18:838–847.
A national survey in the United Kingdom determined that virtual family visits decreased psychological distress for patients, reoriented those with delirium, and improved staff morale.
Rose L, Yu L, Casey J, Cook A, Metaxa V, Pattison N, et al. Communication and virtual visiting for families of patients in intensive care during the COVID-19 pandemic: a UK national survey. Ann Am Thorac Soc 2021;18:1685–1692.
Virtual peer support for ICU survivors was also valuable in promoting a greater understanding of illness management and improving quality of life.
Lassen-Greene CL, Nordness M, Kiehl A, Jones A, Jackson JC, Boncyk CS. Peer support group for intensive care unit survivors: perceptions on supportive recovery in the era of social distancing. Ann Am Thorac Soc 2021;18:177–182.
The COVID-19 pandemic has also provided opportunities to evaluate other processes of care.
Weissman GE. A bold first toe into the uncharted waters of evaluating proprietary clinical prediction models. Ann Am Thorac Soc 2021;18:1116–1117.
Investigators observed that the Epic Deterioration Index, a proprietary clinical prediction tool, possesses low sensitivity among patients with COVID-19, limiting its use as an early warning system.
Singh K, Valley TS, Tang S, Li BY, Kamran F, Sjoding MW, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc 2021;18:1129–1137.
A study comparing no face mask with surgical- or N95-type face masks found similar results on 6-minute-walk testing among patients undergoing evaluation for PASC.
Salles-Rojas A, Guzman-Valderrabano C, Madrid WA, Gonzalez-Molina A, Silva-Ceron M, Rodriguez-Hernandez C, et al. Masking the 6-minute walking test in the COVID-19 era. Ann Am Thorac Soc 2021;18:1070–1074.
Finally, in a United Kingdom-based ICU cohort in which the mean duration of mechanical ventilation was 19 days and 90% of patients received neuromuscular blockade, efforts to initiate rehabilitation were delayed by severe illness yet still resulted in increased mobility before ICU discharge.
McWilliams D, Weblin J, Hodson J, Veenith T, Whitehouse T, Snelson C. Rehabilitation levels in patients with COVID-19 admitted to intensive care requiring invasive ventilation. An observational study. Ann Am Thorac Soc 2021;18:122–129.
Graduate medical education, particularly for pulmonary and critical care trainees, has experienced continued disruption because of the pandemic.
Lenz PH. Adding to the COVID-19 educational script. ATS Sch 2021;2:497–499.
In a survey of interventional pulmonary fellows, over half of respondents reported deployment to care for patients with COVID-19, although most educational activities were retained.
Kalchiem-Dekel O, Schwalk AJ, Patel NM, Lin IH, Beattie JA, Husta BC, et al. COVID-19 impact on interventional pulmonology training. ATS Sch 2021;2:236–248.
A U.S. survey of pulmonary and critical care fellowship program directors identified educational gaps resulting from the pandemic, including pulmonary function test interpretation, elective bronchoscopy, and outpatient encounters.
Matta A, Adamson R, Hayes MM, Carmona H, Soffler MI, Benzaquen S, et al. Impact of the COVID-19 pandemic on US pulmonary and critical care medicine fellowship training. ATS Sch 2021;2:556–565.
Wahlster and colleagues’ global survey of critical care trainees and attendings revealed an overall negative impact of the pandemic on critical care training.
Wahlster S, Sharma M, Çoruh B, Town JA, Lewis A, Lobo SM, et al. A global survey of the effect of COVID-19 on critical care training. ATS Sch 2021;2:508–520.
The pandemic also affected trainee recruitment. Educators shared their experiences of pivoting to virtual recruitment interviews with proposals for minimizing cognitive load in this new format.
Chaisson NF, Ashton RW. Virtual interviews and their effect on cognitive load for graduate medical education applicants and programs. ATS Sch 2021;2:309–316.
A survey of both applicants and interviewers regarding the virtual interview process revealed benefits (reduction in travel costs and time required off-service) and identified new challenges, including a lack of training for virtual interviewing and a shift in attention to program websites.
Strumpf Z, Miller C, Livingston D, Shaman Z, Matta M. Virtual interviews: challenges and opportunities for pulmonary disease and critical care medicine fellowship programs. ATS Sch 2021;2:535–543.
In considering curricular changes, Brotherton and colleagues highlighted crucial topics for training frontline providers in low-to-middle-income countries during a pandemic, emphasizing the need for human resources.
Brotherton BJ, Mbugua E, Halestrap P, Lee BW. COVID-19 and the need for global critical care training. Why ventilators alone are not the answer. ATS Sch 2020;2:13–18.
A university health system deployed a tele-ICU system that benefited local clinicians as well as residents and fellows, who learned about critical care in resource-limited settings.
Leverone NA, Ramnath VR, Munce D, Raphelson JR, Ma J, Akuthota P, et al. Critical care education in a pandemic through tele-ICU. ATS Sch 2020;2:29–33.
Jackson and colleagues described their approach to a hybrid model of remote and in-person ultrasound teaching.
Jackson R, Brotherston D, Jain A, Doufle G, Piquette D, Goffi A. Teaching ultrasound at the point of care in times of social distancing. ATS Sch 2021;2:341–352.
Others found that remote simulation resulted in a trend toward higher comfort across technical and cognitive domains and improved competency in leading cardiac resuscitation.
Lin E, You AX, Wardi G. Comparison of in-person and telesimulation for critical care training during the COVID-19 pandemic. ATS Sch 2021;2:581–594.
Critical appraisal of the literature became even more important with the deluge of information on COVID-19, and the American Thoracic Society’s weekly COVID-19 Critical Care Training Forum provided a venue to address training and knowledge gaps.
Mazer AJ, Mazer MA, Meisenberg BR. Teaching deliberation and restraint in interpreting a tempest of COVID-19 “information.” ATS Sch 2021;2:163–167.
Cypro A, McGuire WC, Rolfsen M, Jones N, Shah NG, Cribbs SK, et al. An international virtual COVID-19 critical care training forum for healthcare workers. ATS Sch 2021;2:278–286.
As we enter 2022, the threat of novel mutations creating variants with increased transmissibility and virulence continues to strain healthcare systems, research teams, medical education, and society at large. The advent of highly effective vaccines has changed the course of the pandemic, yet questions remain about how to maximize the benefit and equitable distribution of these preventives as well as novel therapeutics. Undoubtedly, we will continue to face social strife in the face of contagion; however, as we concluded the “Update in COVID-19” a year ago, we can remain hopeful that “the dawn that always follows darkness is near.”
Chotirmall SH, Leither LM, Çoruh B, Chan LLY, Joudi AM, Brown SM, et al. Update in COVID-19 2020. Am J Respir Crit Care Med 2021;203:1462–1471.
Supported by the National Institutes of Health (NIH) awards R01HL149883, R01HL153122, P01HL154998, P01AG049665, and U19AI135964 (B.D.S.); the National Research Foundation Singapore under its COVID-19 Research Fund administered by the Singapore Ministry of Health’s National Medical Research Council (MOH-000409 [S.H.C.]); the Singapore Ministry of Health’s National Medical Research Council under its Clinician–Scientist Individual Research Grant (MOH-000141 [S.H.C.]); Clinician Scientist Award (MOH-000710 [S.H.C.]); the National Institutes of Health Clinical Center (N.S.); the Lee Kong Chian School of Medicine-Imperial College London Postdoctoral Fellowship (Grant#020458–00001 [O.W.M.]); LEARN Grant LEARN008 [O.W.M.]; NIH awards U01HL123018, OT2HL156812, and R01HL144624 (S.M.B.); and DoD award W81XWH-21–1-0050 (S.M.B.). The opinions expressed in this article are those of the authors and do not represent any position or policy of the National Institutes of Health, the U.S. Department of Health and Human Services, Department of Defense, or the U.S. government or any of their institutions.
Author Contributions: The following authors conceived and provided a first draft of each of the following sections: pathophysiology (O.W.M. and A.M.J.), clinical manifestations (B.D.S. and L.M.L.), vaccines (S.H.C. and B.Ç.), treatment (N.S. and S.M.B.), and societal effects (S.H.C. and B.Ç.). B.D.S., S.H.C., O.W.M., and B.Ç. conceived and drafted the figure and table. B.D.S. and S.H.C. conceived the overall project, coordinated the team, and planned and prepared the manuscript draft, including editing of all subsections, with assistance from all authors, who contributed to the editing of and approved the final submitted version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202202-0277UP on October 6, 2022
Author disclosures are available with the text of this article at www.atsjournals.org .
- Submit a Request
- BC COVID-19 Biobank Network
Data & Bibliography
Evidence and resources from institutions and agencies.
- A curated resource of publications and research findings on COVID-19 provided by the the World Health Organization
- A curated resource of publications and research findings on COVID-19 provided by the the European Centre for Disease Prevention and Control
- Data streams to track and analyze COVID-19 at the local, state, and national levels by the Centre for Disease Control, USA
- This is comprehensive, expert-curated source for publications and preprints related to either COVID-19 or the novel coronavirus SARS-CoV-2 provided by the National Institutes of Health, USA
- This is a curated literature hub from National Library of Medicine (USA) that provides a central access to 2019 novel Coronavirus articles in PubMed
- A curated resource of publications, studies, clinical, legal and other evidence on COVID10 from Public Health England, UK
- Curated collection of evidence reviews, data analysis and comments on the coronavirus pandemic provided by the Centre for Evidence-Based Medicine, UK
- A compilation of publications, AI-assisted clinical tools and genomic evidence curated by the China National Centre for Bioinfomation
- The site supports the COVID-19 response of the European Bioinformatics Institute and enables researchers to upload, access and analyse datasets relating to the COVID-19 genetics and protein structure
- Nextstrain provides a platfrom for real-time snapshots of evolving pathogen populations and interactive data visualizations fir virologists, epidemiologists, public health officials, and community scientists
- YouGov is an international research data and analytics group headquartered in London, UK. The site provides data on multiple aspect of the pandemic from the UK and many other countries worldwide
- Economy Data Tracker and Visualizations
- An initiative by Canadian scientists and volunteers from multiple disciplines aiming to support collaboration and research on COVID-19
- DataCelerate is a global cloud-based data sharing platform that allows for deidentified, anonymized pre-clinical and clinical data types to be requested, voluntarily shared and, in some instances, converted and harmonized to a uniform data model to facilitate its use
- A literature database and meta-analysis of risk factors for COVID-19 from published literature
- A compilation of reviews of multiple aspects of SARS-CoV-2 and COVID-19 curated by Johns Hopkins University
- A free resource of scholarly articles about the novel coronavirus and linked AI tools from the Semantic Scholar team at the Allen Institute for AI
- SARS-CoV-2 data and literature resources compiled by the University of Luxembourg, Grand Duchy of Luxembourgh
- Developed by McMaster University, Canada to support coordination of research activities and decision making with evidence-synthesis, technology assessment and guideline-development
- A collection of data, map and visualization resources and tutorials related to COVID-19 compiled by the University of Toronto Libraries, Canada
Bibliographical Resources from Journals and Publishers
- A curated resouce of practical guidance, online CPD courses, news, comments, and research from the BMJ
- COVID-19 publications from Cambridge University Press
- A resource of publications and data from the Chinese Medical Association Publishing House
- Publications and Reviews from the Cochrane
- Health and medical research on the novel coronavirus (SARS-CoV-2) and COVID-19 published by Elsevier
- Health and medical research on COVID-19 published by JAMA
- This resource compiles alls COVID-19 content from across The Lancet journals
- A collection of articles and other resources on the Coronavirus outbreak by the New England Journal of Medicine
- A collection of articles and other resources on the Coronavirus outbreak published by Oxford University Press
- Official PLOS blogs on the novel coronavirus (2019-nCoV) outbreak
- A collection of research evidence, analysis, and news coverage of COVID-19 and the coronavirus compiled by the Science journals
- A collection of research evidence and tools to facilitate research into COVID-19 and the coronavirus compiled by the Springer Nature
- Repository of research articles on COVID19 research and on its public health, legal, economic, societal and fiscal implications compiled by the Social Science Research Network
- A collection of research evidence into COVID-19 and the coronavirus compiled by Wiley
- COVID-19 SARS-CoV-2 preprints from medRxiv and bioRxiv
- AHA Communities
- Buy AHA Merchandise
- Cookies and Privacy Policy
In This Section
Aha and covid-19, government responses, public responses and human experiences, medical and scientific responses, global and historical perspectives, race and health, primary sources and teaching tools, collecting initiatives, explore the bibliography using zotero, a bibliography of historians' responses to covid-19.

As the novel coronavirus began to spread in the United States in early 2020, people across the country turned to historians for crucial insights regarding the history of epidemics and pandemics. From the Black Death to the 1918 flu to the AIDS epidemic, historians drew from a wealth of historical material to illustrate the range of human, governmental, societal, and scientific responses to massive disease outbreaks over time. As the COVID-19 crisis unfolded, historians were quick to assess the economic, political, and social fallout from the pandemic as it took its devastating toll on American life. The American Historical Association compiled a professionally vetted bibliography of historians’ responses to COVID-19, published from March 2020 to March 2021, as a resource for the public, teachers, and scholars seeking historical perspectives on the crisis and its local and global impacts.
The bibliography includes commentary and publications by historians in both scholarly and popular periodical literature; recorded lectures and webcasts; and digitized primary source materials from past epidemics and pandemics. In amassing these references, the AHA sought to provide a space where anyone, regardless of expertise, can find digital historical material relevant to the COVID-19 crisis. The bibliography may be especially useful for educators as a professionally vetted index of online resources.
Part of “Confronting a Pandemic: Historians and COVID-19,” the bibliography was funded by a National Endowment for the Humanities(NEH) CARES Grant. The AHA is grateful to the History of Science Society and the American Association for the History of Medicine for their many contributions to this bibliography project.
.jpg)
In the wake of COVID-19's disruption to daily life in the United States, the American Historical Association responded to the pandemic's impact by advocating for historians, emphasizing the importance of historical thinking in understanding the current crisis, and urging all institutions that employ historians to be flexible and humane in considering the needs of their employees and constituencies. This section includes AHA statements on the impact of COVID-19 on historians, as well as Perspectives on History articles on topics contextualizing the pandemic.
.jpg)
The entries in this section explore how diverse governments have responded to epidemic and pandemic disease outbreaks from the 14th century to the present. Most of the references focus on US governmental responses, ranging from the municipal to the federal level. The entries collectively consider the behavior of governments during the most critical moments of disease outbreaks.

The references featured here highlight people's lived experiences of epidemics and pandemics, past and present. The authors employ social and cultural analyses of disease outbreaks and their impacts on both individual people and larger social groups living within and beyond the United States.

The entries compiled here examine the medical community's efforts to cure and contain infectious diseases that have reached epidemic and pandemic proportions, beginning with the sixth century. The authors showcase the contributions of individuals and institutions in the fight against diseases outbreaks from The Plague to COVID-19.
The references in this section consider the challenges posed by massive disease outbreaks to the discipline of history and to global inequalities over time. The authors employ broader disciplinary and global perspectives in assessing the large-scale impacts of epidemics and pandemics that transcend regions and time periods.

This section addresses current and historical public health crises within the context of xenophobia, racism, and racial inequity. The authors address the Black Lives Matter movement, Indigenous peoples' experiences of epidemics, and how epidemics have spurred prejudice against marginalized populations.

This section includes primary sources, maps, and timelines related to past epidemics that are suitable for research or classroom use.
.jpg)
AHA Statement Regarding Historians and COVID-19
The etiology of the novel coronavirus is at once scientific and historical. In a statement endorsed by several peer organizations, the AHA emphasizes the importance of historical thinking in understanding the current crisis and urges all institutions that employ historians to be flexible and humane in considering the needs of their employees and constituencies.
The AHA’s Bibliography of Historians’ Responses to COVID-19 Zotero Library allows users to sort and filter entries using more than 145 topics.
NEH Cares Act Support
This project is supported by the national endowment for the humanities, using funding from the cares act. any views, findings, conclusions, or recommendations expressed in this forum do not necessarily represent those of the national endowment for the humanities..
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 107, Issue 2
- Bibliography of published COVID-19 in children literature
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-9178-8985 Philippa Anna Stilwell 1 ,
- Alasdair P. S. Munro 2 , 3 ,
- Emre Basatemur 4 ,
- Nishanthi Talawila Da Camara 5 ,
- http://orcid.org/0000-0003-3440-3142 Rachel Harwood 6 , 7 ,
- http://orcid.org/0000-0001-9334-5144 Damian Roland 8 , 9
- 1 Department of Community Paediatrics , Evelina London Children's Hospital , London , UK
- 2 NIHR Southampton Clinical Research Facility and NIHR Southampton Biomedical Research Centre , University Hospital Southampton NHS Foundation Trust , Southampton , UK
- 3 Faculty of Medicine and Institute for Life Sciences , University of Southampton , Southampton , Hampshire , UK
- 4 UCL Great Ormond Street Institute of Child Health Population Policy and Practice , London , UK
- 5 Research and Quality Improvement , RCPCH , London , UK
- 6 Department of Paediatric Surgery , Alder Hey Children's NHS Foundation Trust , Liverpool , UK
- 7 Department of Cellular and Molecular Physiology , University of Liverpool , Liverpool , UK
- 8 Department of Health Sciences , University of Leicester , Leicester , UK
- 9 Paediatric Emergency Medicine Leicester Academic (PEMLA) Group , University Hospitals of Leicester NHS Trust , Leicester , UK
- Correspondence to Alasdair P. S. Munro, National Institute of Health Research Southampton Clinical Research Facility, University Hospital Southampton NHS Foundation Trust, Southampton SO16 6YD, UK; A.Munro{at}soton.ac.uk
Background The COVID-19 pandemic is the biggest worldwide health challenge in this century. Research concerning the role of children in the spread of SARS-CoV-2, and investigating the clinical effects of infection in children, has been vital. This paper describes the publication trend for pertinent scientific literature relating to COVID-19 in children during the first 6 months of the pandemic.
Methods A comprehensive search of preprint and published literature was conducted daily across four databases (PubMed, Scopus, Ovid-Embase and MedRXiv) between 1 January 2020 and 30 June 2020. Titles and abstracts were screened against predefined inclusion and exclusion criteria.
Findings Over the study period, a total of 45 453 papers were retrieved, of which 476 met our inclusion criteria. The cumulative number of children described in included publications totalled (at most) 41 396. The median number of children per paper was 6 (IQR 1–33). Nearly one-third of papers (30.2%) reported on a single child, and a further 28.3% reported on between 1 and 9 children. Half of all the publications originated from Asia.
Interpretation Our prospective bibliographic analysis of paediatric COVID-19 publications demonstrated a steady increase in the number of papers over time. Understanding and policy evolved with new information that was gathered over the course of the study period. However, over half of publications were individual case reports or small case series, which may have had a limited contribution to advancement of knowledge. During a pandemic, literature should be interpreted with great caution, and clinical/policy decisions should be continually reviewed in light of emerging evidence.
- data collection
- epidemiology
Data availability statement
Data are available on reasonable request. Data available on request.
This article is made freely available for personal use in accordance with BMJ’s website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
https://doi.org/10.1136/archdischild-2021-321751
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is already known?
There was an urgent need for rapid dissemination of evidence during the early phases of the COVID-19 pandemic. However, the quality and timing of availability of evidence has not previously been described.
What this study adds?
This paper describes the dynamic changes in global volume of literature relating to COVID-19 in children and young people published over the first 6 months of the pandemic. Two-thirds of all papers published described just nine patients or fewer.
Introduction
The COVID-19 pandemic is the biggest worldwide emerging health challenge in this century. This has created a huge need for research, from basic science to all phases of pharmacological studies and qualitative health science evaluation. While COVID-19 disease has been reported in children and young people (CYP) of all ages, including at birth, 1–3 most confirmed cases have been in adults.
Infection with SARS-CoV-2 has taken a milder course in children than in adults: most infected children have presented with mild symptoms or have been asymptomatic. 4–7 However, numerous publications have warned of unintended consequences to children in the form of adverse life events, poor access to education and widening inequality. 8 The balance between the direct and indirect effects of the disease on children highlights the challenge of interpreting scientific certainty in complex systems and its impact on national and local public health decision making.
As the COVID-19 pandemic unfolded, the urgency of the need for evidence to inform policy making and practice came to the fore. As a result, an expert COVID-19 literature in children group was brought together, with membership from clinicians, the Royal College of Paediatrics and Child Health (RCPCH) and Don’t Forget the Bubbles (DFTB) (a not-for-profit educational website). 9 The aim of this was to be able conduct a daily rapid and dynamic review process, balancing methodological rigour with the need to quickly synthesise and produce clinically useful output for clinicians on the front line.
This paper describes the publication trends of all the pertinent scientific literature relating to COVID-19 in children in the first 6 months of the global pandemic.
Data sources and search strategy
A comprehensive search of preprint and published literature was conducted daily across four databases between 1 January 2020 and 30 June 2020. After this point, the decision was made to move to weekly searches and a more selective review process due to the volume of publications and the less acute need for rapid dissemination.
The search strategy, developed in PubMed, Scopus, Ovid-Embase and, manually, on MedRxiv, consisted of keywords and Medical Subject Headings (MeSH) terms related to COVID-19. 10 Synonyms, alternate spellings, abbreviations and historical terms were incorporated.
Study types
Observational studies including case reports, case series, cross-sectional studies, intervention studies (including randomised controlled trials) and cohort studies.
Inclusion and exclusion criteria
Studies that described either epidemiological data, clinical features, vertical transmission, neonatal outcomes, predictors of severity, or prognosis and complications of SARS-CoV-2 in CYP aged between 0 and 18 years old were deemed eligible. Only publications in English were included. Papers where adult and paediatric data were combined, and paediatric data could not be extracted, were excluded. Systematic reviews, other review articles, letters/communications that did not provide clinical data and studies with irredeemable methodological flaws as determined by consensus of at least two clinical academics were excluded.
Study screening and quality assessment: all references identified by searches were exported to endnote, and duplicates were removed. One reviewer screened titles and abstracts against the inclusion/exclusion criteria (NTDC). All potentially relevant articles were sent for full text review by one of a panel of 45 independent reviewers, who confirmed that inclusion criteria were met. We were unable to formally assess methodological quality and risk of bias due to feasibility constraints on the large volume of articles and the rapid nature of the review process. Quality and bias were informally, qualitatively assessed by the study reviewers and a clinical academic for written reviews.
Data extraction: 45 independent reviewers extracted key data from studies that met the inclusion criteria into a data extraction tool. This captured information about each paper’s date of publication (representing either the date of first online publication in a peer-reviewed journal or in some cases the date of publication in a preprint repository), number of children described and country of origin. In addition, each paper was categorised into one of the three main themes: epidemiology (Epi), clinical studies (Clin) and neonatal (Neo). Subsequently, further subcategories were added and applied both prospectively and retrospectively as the evidence base broadened (Epi: disease burden, Epi: transmission, Clin: clinical features, Clin: comorbidities, Clin: paediatric multisystem inflammatory syndrome – temporally associated with SARS-CoV-2 (PIMS-TS) and Clin: therapeutics).
All papers that met the inclusion criteria and deemed potentially relevant were summarised and published on the DFTB website. 9 A narrative synthesis of all the summaries was published on the RCPCH website, 11 updated on a weekly. To quantify the utility of the evidence and review output, data on the respective websites was collected over time, including Altmetric scores (an automatically calculated weighted count of all the attention a research output has received).
Following early concerns of the same patients appearing in multiple reports, 12 all papers originating from China were further assessed for potential duplicate reporting of participants. Data were extracted regarding the institution(s) from which patients were recruited, the start and end dates for recruitment, the age range of included participants and any other limitations to the inclusion criteria (eg, studies limited to children admitted to intensive care). For each publication from China, the number of other papers with overlapping inclusion criteria and recruitment site(s) were determined. Papers for which data were missing regarding recruitment site or recruitment period were excluded from this analysis, as were papers reporting data at a regional or national level.
Bibliography
Between 1 January 2020 and 30 June 2020, a total of 45 453 papers were retrieved by the search, of which 476 satisfied our inclusion criteria ( figure 1 and online supplemental file 1 ). The median number of search hits per week was 906 (IQR: 211–3345) and peaked at 5178 during the week commencing 18 May. The median number of papers meeting criteria for inclusion per week was 18 (IQR: 3–29). The number of search hits remained stable at around 4000 per week until the end of the study period, whereas the number of included papers declined as time went on, falling to 15 during the last week of June.
Supplemental material
Table 1 summarises the distribution of papers included by study size (number of children included), continent and category. The cumulative number of children described in all publications totalled 41 396; however, there is likely to be substantial overlap of patients across publications.
- View inline
Distribution of papers by study size, paper theme and geographical location
The median number of children per paper was 6 (IQR 1–33). Nearly one-third of papers (30.2%) reported on a single child only, and a further 28.3% reported on between one and nine children (ie, 58% of studies included reported on nine or fewer children). Figure 2 shows the number of children reported on, in included studies, over time. The largest category of publications was classified under the theme ‘clinical features’, with a total of 190 (39.9%) publications, followed by 131 (27.5%) for ‘neonates’.
- Download figure
- Open in new tab
- Download powerpoint
Graph to show number of search hits and included papers, by fortnight, between f1 January 2020 and 28 June 2020 (with stacked bars demonstrating theme/category breakdown of papers). Clin, clinical studies; Epi, epidemiology; PIMS-TS, paediatric multisystem inflammatory syndrome – temporally associated with SARS-CoV-2.
Scatter plot demonstrating number of children reported on in included literature, over time.
Number of publications from each continent by fortnight.
World map showing number of included publications by country.
The number of publications by continent varied widely ( table 1 ), with half of the publications from Asia (50.0%) and just under a third from Europe (29.6%). One hundred and eighty-one publications (38.0%) originated from China. The distribution of publications from different regions broadly followed the path of the pandemic, peaking in Asia first, followed by Europe and subsequently North America ( figure 3 and figure 4 ). PIMS-TS papers peaked in May and June and were published almost exclusively from Europe and North America. Despite publications peaking in Asia first, only two publications regarding PIMS-TS came from Asia. 13 14
Of the 181 publications from China, data were available for 142 (78.5%) regarding potential duplicate reporting of participants. Among the 142 studies with data available, 103 (72.5%) were found to have overlapping inclusion criteria and recruitment site(s) with at least one other paper ( table 2 ). The median number of overlapping publications per paper was 2 (IQR 0–5, range 0–26). Among papers reporting data from Hubei Province (n=75), where SARS-CoV-2 was first reported, the median number of overlapping publications per paper was 5 (IQR 2–16). There were 23 studies reporting patients recruited from Wuhan Children’s Hospital.
Potential duplicate reporting among Chinese publications (n=142)
Our prospective bibliographic analysis of COVID-19 publications relevant to children demonstrated a steady increase in the number of papers meeting the inclusion criteria over time, before peaking in May 2020 and tailing off in June. The rate of publication closely mirrored the trends in numbers of cases during the first phase of the pandemic in the northern hemisphere, following 1 month behind. While the number of included papers fell, the number of search hits continued to increase, reflecting fewer original research articles in proportion to review articles/guidelines/opinion articles.
Critically, the numbers of children included in studies was very small, with one-third of studies reporting on a single child. This is notable given seroprevalence studies have shown up to 4% of children within some of the worst affected European countries may have been infected. 15 This almost certainly reflects under testing of cases of COVID-19 in children due to their milder disease phenotype. 16 While large observational studies require resources and time to deliver, the huge numbers of case studies make a significant proportion of COVID-19 research of limited applicability to clinical practice, due to the inherent bias and unrepresentative nature of n=1 studies.
No interventional studies were reported. There was an extreme paucity of articles regarding therapeutics in children, which were all descriptive in nature and included no clinical trials. Children are commonly excluded from clinical trials of novel therapeutics until they have been conducted in adults, however given a number of trials such as RECOVERY, 17 were predominantly tested repurposed pharmaceuticals that have a long safety record in children, delays in recruitment of children into high-quality trials only results in random assortments of therapies being given outside of a research context, without the safety provision or oversight of a formal clinical trial. It would be recommended to consider enrolling children in clinical trials earlier and to encourage flexible, rapidly deployable descriptive and interventional studies that can be rolled out using existing research networks and, where possible, generate large and generalisable clinical cohort information.
The importance of research networks and collectives becomes apparent; organisations such as the International Severe Acute Respiratory and Infection Consortium (ISARIC) demonstrated how collaboration can generate generalisable information relatively quickly. ISARIC-4C 18 provided invaluable clinical data at the point of publication, but in the event of future pandemics, consideration should be given to publishing the basic data ‘real-time’.
A significant proportion of large studies were prepublished on medRxiv, a preprint server that allows papers to be made available prior to peer-review and publication in journals. This enabled information to be made available rapidly and often preceded journal publication which, during a period of relative data paucity made an impact on information availability.
Geographical spread of publications was uneven but appeared to follow the path of the pandemic. The absence of some clinical phenotypes, such as PIMS-TS, being reported in Asia, even subsequent to cases being described in Europe and North America, remains unexplained and warrants further investigation.
Analysis of papers from China suggested that there was likely to be substantial overlap of patients between some publications. Duplicate reporting falsely inflates the apparent size of the evidence base and has the potential to introduce bias when data across publications is synthesised in systematic reviews and meta-analyses. However, while overlap in inclusion criteria and recruitment site(s) can suggest the potential for duplicate reporting, the true extent of multiple counting cannot be ascertained without individual patient data. This highlights the need for formal, centralised data collection systems.
The prospective nature of this work was only possible due to the generosity of volunteer reviewers. Being able to access continuous, up-to-date information was important for frontline clinicians, who could access summaries directly online but also for policy makers, addressing questions such as when to open schools, which clinical groups to shield and how to manage COVID-19 and PIMS-TS most effectively.
The acute need for rapid, real-time evidence synthesis is reflected in the utilisation statistics for the DFTB and RCPCH web pages, including their use in international policy documents. The DFTB COVID-19 In Children evidence review page 9 went live in March 2020 and was accessed 128 492 times between then and 30 June 2020. As of 30 June, it had an Altmetric score of 3139, has been cited numerous times in academic publications and referenced in 10 policy documents including from the WHO. 19 The RCPCH research evidence summaries page 11 was accessed 38 094 times between 9 April and 30 June, advised numerous policy outputs and was cited by, for example, UK Research and Innovation. 20
While the large volumes of research produced provided benefits for sharing information quickly with a wider audience, judicious caution was used when considering the conclusions made by individual papers. We found cross-over publications where data from the same children had been reused.
Strengths and limitations
To our knowledge, this is the only comprehensive bibliographic overview of all the literature published on COVID-19 in children during the first 6 months of the pandemic. In addition, through the establishment of an expert review network, we were able to identify and formally review a select number of papers that would be most relevant to healthcare professionals working directly with children, organisations supporting clinicians, CYP and their families and policy makers. We have displayed how the research output, globally, evolved over the pandemic.
A decision was made, early in the programme, to exclude data that had not been published in traditional academic literature, due to limitations in formal search strategies and language translation. As a result, when certain organisations or governments published pertinent data that had not been peer reviewed, the data were not included in this report. This is worthy of consideration in the event of future pandemics, as some data were made publicly available by state publications in native languages significantly earlier than when it became available in the formal academic literature, 15 and many countries published relevant documents in native language only. In addition, many national reports were published in PDF format only, impeding web searches. HTML publications would improve accessibility and dissemination of these important data. Systematic reviews and meta-analyses were not included given that the process in place was a rolling systematic review of the literature.
Considering the volume of search hits and limited resource, each paper was only reviewed by one independent reviewer. However, as all written reviews were subsequently screened and edited by clinical academics, we do not believe this to have significantly impacted on the papers sent for review and ultimately included in the evidence summaries that were displayed.
While children have been spared the worst of the clinical impact of COVID-19, evidence regarding features of the disease and its transmission within this population group is essential for healthcare workers, children, families, teachers, as well as local, regional and national policy makers. An expert group was formed between clinicians, RCPCH and DFTB to conduct daily rapid searches of key databases, identifying all published literature relating to CYP and selecting the most pertinent papers to be reviewed, summarised and made available to all, with a significant impact on international policy. Over the first 6 months of the pandemic, 476 papers were identified, describing (at most) 41 396 CYP affected by COVID-19. The number of relevant papers declined as the pandemic progressed, and it is noteworthy that just under one-third of the papers reported on a single child.
Ethics statements
Patient consent for publication.
Not required.
Acknowledgments
All the reviewers who contributed to the review process: Alison Boast, Grace Leo, Dani Hall, Dan Yeoh, Melody Redman, Sarah Sloan, Tricia Barlow, Anne Bean, Maeve Kelleher, Victoria Dachtler, Irnthu Premadeva, Daniel Hawcutt, Lilian Nyirongo, Esther Alderson, Tessa Davis, Sunil Bhopal, Aimee Donald, Sarah Blackstock, Alice Armitage, Anne-Lise Goddings, Lyda Jadresic, Maham Zaman, Celia Avigdor, David Beverley, Hilary Smith, Vivienne van Someren, Brendan Harrington, Louise Tina Day, Sarah Hall, Alastair Falconer, Sarah Lee, Noel Murphy, Chris Lamming, Carlos de Sousa, Mike Hall, Nandhini Prakash, Maggie Fitzpatrick. Robert Scott Jupp, Bhupinder Sandhu, Rob Primhak and Richard Morton. The Children and Young People’s Transformation Team at National Health Service (NHS) England and NHS Improvement. The Research and Evidence Team at the Royal College of Paediatrics and Child Health for managing the literature searches and the Senior Officers for their advice and feedback. Tessa Davis from Don’t Forget the Bubbles for support with data analysis.
- Hong L , et al
- Kang Q , et al
- Yuan W , et al
- World Health Organisation
- Bellino S ,
- Rota MC , et al
- Davies NG ,
- Crawley E ,
- Feder G , et al
- Goldstein H
- ↵ Covid-19 search protocol and inclusion criteria . Available: https://www.rcpch.ac.uk/sites/default/files/2020-05/rcpch_covid-19_search_strategy.pdf [Accessed 9 Dec 2020 ].
- ↵ Covid-19 – research evidence summaries , 2020 . Available: https://www.rcpch.ac.uk/resources/covid-19-research-evidence-summaries [Accessed 9 Dec 2020 ].
- Bauchner H ,
- Balasubramanian S ,
- Nagendran TM ,
- Ramachandran B , et al
- Schnapp A ,
- Abulhija H ,
- Maly A , et al
- Pérez-Gómez B ,
- Pastor-Barriuso R , et al
- Waterfield T ,
- Moore R , et al
- ↵ Recovery: randomised evaluation of Covid-19 therapy . Available: https://www.recoverytrial.net/ [Accessed 5th Jan 2020 ].
- Docherty Annemarie B ,
- Harrison Ewen M ,
- Green Christopher A
- World Health Organisation (WHO)
- UK Research and Innovation (URKI).
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
PAS and APSM are joint first authors.
Twitter @RachelHarwood10, @damian_roland
Contributors PAS contributed to the conception and design, interpretation of data, drafted the initial draft, revised the draft and approved the final version to be published. AM contributed to the conception and design, acquisition and interpretation of data, revised the draft and approved the final version to be published. PAS and AM contributed equally as joint first authors. EB contributed to the design, analyses/interpretation of data, revised the draft and approved of the final version to be published. NTDC contributed to the conception and design, acquisition and analyses of data, revised the draft and approved of the final version to be published. RH contributed to the design, revised the draft and approved of the final version to be published. DR contributed to the conception and design, interpretation of data, revised the draft and approved of the final version to be published.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Advertisement

- Previous Article
- Next Article
1. INTRODUCTION
2. background, 5. discussion, 6. conclusions, author contributions, competing interests, funding information, data availability, covid-19 publications: database coverage, citations, readers, tweets, news, facebook walls, reddit posts.
Handling Editor: Ludo Waltman
- Cite Icon Cite
- Open the PDF for in another window
- Permissions
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Search Site
Kayvan Kousha , Mike Thelwall; COVID-19 publications: Database coverage, citations, readers, tweets, news, Facebook walls, Reddit posts. Quantitative Science Studies 2020; 1 (3): 1068–1091. doi: https://doi.org/10.1162/qss_a_00066
Download citation file:
- Ris (Zotero)
- Reference Manager
The COVID-19 pandemic requires a fast response from researchers to help address biological, medical, and public health issues to minimize its impact. In this rapidly evolving context, scholars, professionals, and the public may need to identify important new studies quickly. In response, this paper assesses the coverage of scholarly databases and impact indicators during March 21, 2020 to April 18, 2020. The rapidly increasing volume of research is particularly accessible through Dimensions, and less through Scopus, the Web of Science, and PubMed. Google Scholar’s results included many false matches. A few COVID-19 papers from the 21,395 in Dimensions were already highly cited, with substantial news and social media attention. For this topic, in contrast to previous studies, there seems to be a high degree of convergence between articles shared in the social web and citation counts, at least in the short term. In particular, articles that are extensively tweeted on the day first indexed are likely to be highly read and relatively highly cited 3 weeks later. Researchers needing wide scope literature searches (rather than health-focused PubMed or medRxiv searches) should start with Dimensions (or Google Scholar) and can use tweet and Mendeley reader counts as indicators of likely importance.
The international scientific effort to mitigate COVID-19 is unprecedented in scale and rapidity. For instance, PubMed added related publications daily between January 17 and April 18, 2020 1 ( Figure 1 ), reaching over 300 in a single day. This effort is in response to the lethality and rapid spread of the disease, as well as the major economic and social consequences of COVID-19 lockdowns. As part of the response, researchers, professionals, and the public may need to consult the scientific literature for the latest findings. Although this is normal for science, standard literature search methods may be ineffective in a rapid publishing environment. Traditional citation indexes may not be fast enough, especially given that they do not index most preprints, and citation counts may not help point to important studies. The more inclusive online citation indexes of sites such as Google Scholar and Dimensions.ai seem like suitable alternatives because they index both the traditional scholarly literature and documents not published in journals, including preprints ( Herzog, Hook, & Konkiel, 2020 ; Kousha & Thelwall, 2019a ). There are initiatives to help various communities with curated collections of COVID-19 documents, such as published biomedical documents from PubMed Central ( PMC, 2020 ), preprints from medRxiv and bioRxiv ( medRxiv, 2020 ), and a data mining collection ( Allen Institute, 2020 ; Colavizza, Costas, et al., 2020 ), but none are complete. It is therefore important to assess the COVID-19 coverage and growth of scholarly publication indexes, as well as the value of citation counts for new COVID-19 research.
![bibliography for covid 19 research Daily additions of COVID-19 publications to PubMed (January 17 to April 18). Query used ((((((("COVID-19") OR "Novel coronavirus") OR "2019-nCoV") OR "SARS-CoV-2") OR "coronavirus 2") OR "Coronavirus disease 2019") OR "Corona virus disease 2019") AND ("2019/12/01"[Date - Publication] : "3000"[Date - Publication]).](https://mitp.silverchair-cdn.com/mitp/content_public/journal/qss/1/3/10.1162_qss_a_00066/2/m_00066f01c.png?Expires=1716290373&Signature=IA0OCuQJGTrd4A5xIBkadxP5jF-J4MgBoxC~HjBWRraG4TfiY5N-fgGK9mkAVWppUaKqGtr-ttepB9scQZSYBR1~BOlkgEcwX0gl-GjYfTHOSA9k0wY6ccWiIcj6atahGyt1t73S1OGASw4fnBEL7wbc0XSjgVg8kfmvO6fRBWds-StXlF2usdAhFa6TdEmG-nZKe6yGrZVOvf1yipxEsVdxTvGL5o77EC66ZLkWjr92v3I8BXFcfdErxrTc2wR3xHKmK2S2JOC3IVbBX7w833eJ7asbbTVgTBZmuJOoc1tWKz8T-9ZkfanpMgnp1GY9nZpvqPCe4i-XDdp5wccxtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Daily additions of COVID-19 publications to PubMed (January 17 to April 18). Query used ((((((("COVID-19") OR "Novel coronavirus") OR "2019-nCoV") OR "SARS-CoV-2") OR "coronavirus 2") OR "Coronavirus disease 2019") OR "Corona virus disease 2019") AND ("2019/12/01"[Date - Publication] : "3000"[Date - Publication]).
In parallel with scholarly needs for literature, the public, professionals, and policy-makers also need to access current COVID-19 research to inform their decision-making, such as whether to recommend wearing protective masks. This may be in addition to, or to clarify, World Health Organization guidelines ( WHO, 2020 ). They may therefore share relevant academic research in the social web (e.g., Merchant & Lurie, 2020 ), generating interest that may picked up by alternative indicators (altmetrics). Thus, altmetrics may be useful in helping the public to identify the most relevant research or may help point researchers to topics considered important by the public. It would therefore be helpful to assess whether altmetrics can perform this role. In particular, because altmetrics can reflect both academic and nonacademic interests ( Mohammadi, Barahmand, & Thelwall, 2019 ; Mohammadi, Thelwall, et al., 2018 ), it is not clear whether they will essentially be early indicators of citation impact or whether they reflect societal or other impacts for COVID-19. Altmetrics have already been shown useful to identify the spread of a misleading COVID-19 paper that was subsequently withdrawn ( Ioannidis, 2020 ).
Which scholarly databases index the most COVID-19 publications (extending: Torres-Salinas, 2020 )?
Which COVID-19 documents have become highly cited or highly discussed?
Do altmetrics and early citation counts reflect similar types of COVID-19 impact?
Can any altmetrics serve as early indicators of future citation impact for COVID-19 documents?
The novel coronavirus SARS-CoV-2, which causes COVID-19, was first recorded in Wuhan City, China in December 2019. Quickly disseminating scientific results about COVID-19 is vital to allow the rapid exploitation of successful clinical results ( Song & Karako, 2020 ). The importance of scientific publishing to respond to infectious disease outbreaks has been emphasized by many bibliometric studies of previous cases ( Rethlefsen & Livinski, 2013 ), such as SARS ( Kostoff & Morse, 2011 ; Tian & Zheng, 2015 ), H7N9 influenza ( Tian & Zheng, 2015 ), HIV/AIDS ( Pouris & Pouris, 2011 ), Ebola ( Pouris & Ho, 2016 ), and Zika ( Delwiche, 2018 ).
One recent study using Dimensions, Scopus, Web of Science (WoS), and the LitCovid ( Chen, Allot, & Lu, 2020 ) curated list has investigated the daily growth of COVID-19 related publications in citation databases and digital libraries from January 1 to April 7, finding that Dimensions had the best coverage (9,435 publications) compared to WoS (718) and Scopus (1,568). The weekly growth of PubMed was about 1,000 publications and the PubMed Central (1,398), medRxiv (989), and SSRN (608) repositories had the best coverage of open access COVID-19 publications ( Torres-Salinas, 2020 ). Google Scholar was not assessed, and all evidence was extracted from Dimensions, so the counts for other repositories may not be complete.
2.1. Dimensions Citations
Dimensions.ai ( Herzog et al., 2020 ) is an online scholarly database that operates similarly to Google Scholar, in the sense of indexing documents using public information from the Web, but has an Applications Programming Interface (API) that supports automatic downloading for all query matches. It indexes most documents in Scopus ( Thelwall, 2018b ), although not for all fields ( Orduña-Malea & Delgado-López-Cózar, 2018 ). It seems to have substantial coverage of preprint servers, such as arXiv, and so probably has much larger coverage overall, especially for recently published papers. Its coverage seems to be higher than Scopus and WoS, comparable to CrossRef but lower than Google Scholar and Microsoft Academic ( Harzing, 2019 ). In line with this, citation counts for papers in Dimensions can be expected to be slightly higher than for Scopus and WoS but substantially higher for newer documents.
2.2. Altmetrics: Mendeley Readers
Counts of readers from the social reference sharing site Mendeley form the most extensively researched and understood altmetric. A nontrivial minority of researchers (about 5%) used Mendeley by 2014 according to one survey, with disciplinary differences ( Van Noorden, 2014 ). People typically register documents in Mendeley when they have read them or intend to read them ( Mohammadi, Thelwall, & Kousha, 2016 ), so it is reasonable to regard Mendeley counts as an indicator of readership. According to self-reports in the site, users are predominantly academics and postgraduate students, with a few undergraduates, librarians, and people in nonacademic occupations ( Mohammadi, Thelwall, et al., 2015 ). Thus, Mendeley is an indicator of predominantly academic readership, with an element of student readership. One difference is that nonarticle publications in journals, such as editorials and news items, are relatively more likely to be registered in Mendeley than to be cited ( Zahedi & Haustein, 2018 ). Mendeley reader counts can help with the early identification of highly cited documents ( Zahedi, Costas, & Wouters, 2017 ).
A range of studies have investigated the relationship between Mendeley reader counts and citation counts, finding moderate or strong positive correlations ( Costas, Zahedi, & Wouters, 2015 ). Correlations between mature citation counts and Mendeley reader counts are strong and positive in almost all narrow fields in Scopus ( Thelwall, 2017a ), supporting their use as a citation impact type of indicator. Although the two types of data seem to be close to interchangeable for sets of mature articles (although they can differ sharply for individual education-oriented papers: Thelwall, 2017c ), the advantage of Mendeley reader counts is that they appear and are useful a year before citation counts ( Thelwall, 2017b ). They may even be common enough to be used for scientometric purposes by the publication month of the publishing journal. Moreover, as early Mendeley reader counts correlate positively with later citation counts ( Thelwall, 2018a ), Mendeley reader counts are early academic impact indicators. They should therefore be a better academic impact indicator than citation counts for fast-moving issues, such as COVID-19.
2.3. Altmetrics: Tweeters, Facebook Walls
Twitter is potentially a source of societal attention evidence ( Holmberg & Vainio, 2018 ; Priem, Taraborelli, et al., 2010 ). More articles have nonzero tweet counts than nonzero scores on any other altmetric, other than Mendeley ( Costas et al., 2015 ; Thelwall, Haustein, et al., 2013 ). As Twitter is a news-oriented social media platform, articles can expect to get a substantial proportion of their tweets in the week of publication, so tweets are visible long before citations ( Ortega, 2018a , b ).
Tweeter counts (counting the number of tweeters rather than the number of tweets) are problematic to interpret. About half of people that tweet academic research are not academics ( Mohammadi et al., 2018 ), and tweets typically contain just article titles or brief summaries ( Thelwall, Tsou, et al., 2013 ; Robinson-García, Costas, et al., 2017 ), serving as publicity rather than evidence of impact. Many academic tweets are also created by bots ( Haustein, Bowman, et al., 2016 ; Robinson-García, Costas, et al., 2017 ). Together with often close to zero correlations with citation counts ( Costas et al., 2015 ; Haustein, Larivière, et al., 2014 ; Thelwall, Haustein, et al., 2013 ), there is insufficient evidence to claim that tweeter counts are indicators of either academic or societal impact. Nevertheless, they may have some value for health-related research, where there is more public interest in academic research ( Haustein, Larivière, et al., 2014 ; Mohammadi, Gregory, et al., 2020 ). Editorials and news articles are relatively more likely to be tweeted than cited ( Haustein, Costas, & Larivière, 2015 ), reflecting the news orientation of Twitter.
Facebook wall posts function like tweeter counts except that they are rarer ( Costas et al., 2015 ; Thelwall, Haustein, et al., 2013 ). As most of Facebook is private and Altmetric.com obtains its Facebook wall counts only from public pages, this altmetric probably reflects a tiny fraction of all Facebook posts and may be oriented to organizational uses of Facebook (including journals) rather than typical users; few posts are directly from academics ( Mohammadi et al., 2019 ).
2.4. Altmetrics: News and Reddit
Altmetric.com harvests citations from online free news websites and the news-oriented site Reddit. Altmetrics from both are relatively rare and have very low correlations with citation counts ( Costas et al., 2015 ; Thelwall, Haustein, et al., 2013 ). Nevertheless, health-related topics are newsworthy ( Clark & Illman, 2006 ; Kousha & Thelwall, 2019b ), including for infectious diseases (e.g., SARS: Lewison, 2008 ), so they may be useful for COVID-19.
The research design is in three parts. First, to assess the relative coverage of scholarly databases, the main candidates were queried daily from March 21, 2020 to record the number of COVID-19 documents indexed. Second, lists of documents matching a set of COVID-19 queries were downloaded from Dimensions.ai and altmetrics for these were gathered from Mendeley ( Gunn, 2014 ) and Altmetric.com ( Adie & Roe, 2013 ; Robinson-García, Torres-Salinas, et al., 2014 ) daily and the individual scores and documents compared. Third, a March 24 data set was created to track a set of documents indexed on the same day.
3.1. Scholarly Database Indexing of COVID-19 Publications
To assess the indexing of COVID-19-related publications, the two mainstream scholarly databases, Scopus and WoS, were queried as well as other major academic sources that may index relevant documents. After testing with the original and current names of the virus and disease and “Corona virus disease 2019” and “Coronavirus disease 2019”, the core queries used to identify relevant documents were as shown in Table 1 . The queries are designed to be as inclusive as possible for the database in terms of document type and part of the document searched: full text, if available in the database, otherwise all metadata fields (e.g., title, abstract, keywords). The queries are not comprehensive but are high precision, unless stated, and should include the most recent research focusing on the issue, assuming that it includes the current official disease description.
The combined queries did not work in Google Scholar, giving false matches. The results for Google Scholar seemed to be substantially inflated by its web search component indexing advertisements or warnings in webpages alongside articles irrelevant to the disease, so its results are not reported. To illustrate the existence of these false matches, a search for “COVID-19” in Google Scholar with a date range specified as 1990–2000 (i.e., 20 years before the name was coined) on April 21, 2020 returned an estimated 5,010 matches 2 . Each incorrect Google Scholar match reported snippets not from the paper, such as, “PEDIATRICS COVID-19 COLLECTION We are fast-tracking and publishing the latest research and articles related to COVID-19 for free.” The exact COVID-19 coverage of Google Scholar is difficult to assess because it is not possible to download and check all matches in the absence of a Google Scholar API to download large sets of publication records. Because of these issues, no results are reported for Google Scholar.
3.2. Document and Altmetric Comparison Data Sets
search publications for "COVID-19" where year >= 2019
search publications for "Novel coronavirus" where year >= 2019
search publications for "2019-nCoV" where year >= 2019
search publications for "SARS-CoV-2" where year >= 2019
search publications for "coronavirus 2" where year >= 2019
search publications for "Coronavirus disease 2019" where year >= 2019
search publications for "Corona virus disease 2019" where year >= 2019
The resulting 21,395 publications were mainly open access (53%; 75% for the March 24 set—see later) and predominantly from health-related specialties ( Table 2 ).
Counting 1/ n for a paper with n subject codes.
The data sets analyzed include substantial numbers of papers from preprint planforms, including medRxiv, SSRN, arXiv, bioRxiv, ChemRxiv, and Research Square ( Table 3 , as in Torres-Salinas, 2020 ), as well as books and more traditional journals ( Table 3 ).
Although most documents were classified as Articles by Dimensions, this type includes medRxiv preprints and diverse types of document published in journals, such as notes, short communications, editorials, and commentaries ( Table 4 ). As many editorials seemed to discuss the impact of COVID-19 on the journal or field, this added fewer citable documents to the Article class. The surprising number of books and book chapters (13% overall) seems to be primarily due to pre-COVID-19 discussions about coronaviruses, matching the query “Coronavirus 2”. The low number of conference proceedings may be due to conference cancellations, or the inability of most conferences to respond to the COVID-19 timescale.
Because the Dimensions type Article includes documents that would not be classed as standard journal articles in scientometric analyses, the 295 Dimensions “Articles” from March 24 were visited to classify them by type. Only 106 of these seemed to be standard journal articles. The rest were mainly editorials, letters (called letters , letters to the editor , or correspondence ; one detailed letter was classed as an article), or news stories. In some cases, documents were called “article” by the publishing journal but were clearly news stories published in a news-focused magazine/journal. The reduced set of 106 journal articles from March 24, 2020 was used for follow-up correlation tests.
After Webometric Analyst had downloaded a complete set of records each day, the Mendeley API was used to identify the number of Mendeley readers for each document, again using Webometric Analyst. It queries by DOI and by title/author/year and combines nonoverlapping results for the most complete reader count. This follows best practice ( Zahedi, Haustein, & Bowman, 2014 ). Webometric Analyst was also used to identify counts of citations in Twitter, Facebook, Reddit, and online news outlets to these documents, as identified by DOI queries to Altmetric.com . This data provider seems to have the most comprehensive coverage of Twitter, the largest of the sources ( Ortega, 2018a ). Twitter and Facebook are logical choices to investigate because they seem to be the social media sources that most cite academic research ( Costas et al., 2015 ; Thelwall, Haustein, et al., 2013 ). Reddit and news may give a news perspective, although Reddit is a multipurpose site ( Ovadia, 2015 ; Stoddard, 2015 ) and the news sources harvested by Altmetric.com presumably exclude some major paywalled press sources.
There were some gaps in the data collection due to documents not being returned by a query on one day when they had been returned on a previous day. This produced missing citation and altmetric scores, affecting the analysis. To avoid this issue, these missing values were replaced with approximate values by linear interpolation (when scores were available for previous and subsequent dates), linear extrapolation (when at least two previous but no subsequent scores were available), or constant values (when only one previous value was available).
3.3. Analysis
The coverage of the different sources was evaluated by comparing (on a graph) the number of query matches over time. This is not a fair comparison because the queries are not equivalent, a researcher may use other queries, and the sources index with different levels of comprehensiveness. For example, a source that indexed the full text of documents would get more and probably less relevant hits than a source indexing the title and abstract, even if they had the same coverage.
To assess the types of document generating the most impact for each source, the top 5 for each indicator was extracted to give a manageable set. A comparison of the relative ranks of these documents for the different indicators was used to guide the evaluation of the relative importance of the document characteristics, along with the document age (younger documents would tend to have lower scores in less rapidly evolving indicators). This focus on the highest scoring documents seems reasonable because they are likely to be the most influential or important, even though different trends may apply to more average documents.
To compare the average accumulation speed and scores of COVID-19 documents, a base set was chosen, consisting of documents first indexed in Dimensions on March 24, 2020. This was the date from the first week with the most new documents (excluding the first day). These documents form a set that are likely to have been published on or shortly before March 24, 2020. The altmetric and citation scores for this set were compared over time to assess their evolution and relative magnitude. Averages were calculated with geometric means (with a +1 offset: Fairclough & Thelwall, 2015 ) rather than arithmetic means due to the highly skewed nature of citations ( de Solla Price, 1976 ; Wallace, Larivière, & Gingras, 2009 ) and altmetrics ( Thelwall & Wilson, 2016 ; Yu, Xu, et al., 2017 ). The scores of this set were then compared using Spearman correlations to assess the extent to which they may reflect similar types of impact ( Sud & Thelwall, 2014 ). Because altmetrics other than Mendeley tend to have very weak correlations with citation counts ( Costas et al., 2015 ; Haustein et al., 2014 ; Thelwall, Haustein, et al., 2013 ), high correlations are not expected. Correlations were used rather than regression because this is the standard technique for altmetrics and in this case matches the hypothesis’ use case: sorting documents matching COVID-19 queries using altmetrics or citations.
Field normalization was not used for either analysis because (a) the papers cover a relatively narrow topic (COVID-19) even though they span many subject areas and (b) it is impractical to field normalize the values because this would require daily updates of the whole of Dimensions, Altmetric.com , and Mendeley for the world reference sets.
4.1. Coverage of Scholarly Databases
Based on the estimated number of manual search results returned by the sources queried, it seems that Dimensions has substantially wider coverage of COVID-19 publications than all other sources or finds more because of its full text indexing rather than just searching metadata ( Figure 2 ). Google Scholar probably indexes at least as many documents as Dimensions, although this could not be checked because of the number of false matches it returned (a graph including Google Scholar is in version 1 of this paper at https://arxiv.org/abs/2004.10400v1 ).

The daily number of hits for COVID-19 queries (see Table 1 ) from a range of scholarly sources (March 22 to April 18).
Google Scholar and Dimensions index both publisher records and other online publications (preprint archives for Dimensions, wider web sources for Google Scholar). As Dimensions seems able to identify COVID-19 publications more quickly or more widely than WoS and Scopus, academics studying the area should consider Dimensions (or Google Scholar, if the false matches are not a concern) if more specialist databases, such as PubMed, are not adequate. This argument does not take into account the importance of the documents, however, and it is possible that the key publications are quickly peer reviewed, published, and indexed by Scopus and WoS. The Dimensions results include editorials, news, and letters, and may include recent documents not about the disease but that mention it for background information in their full text.
Overlaps Between Dimension, Scopus and WoS
The extent of overlaps between the COVID-19 query results for Dimensions, Scopus, and WoS were estimated on April 19, 2020 to assess whether they were indexing the same publications. To obtain a relevant set of COVID-19 publications, only publications from 2019–2020 with the terms "COVID" OR "coronavirus" OR "2019-nCoV" OR "SARS-CoV-2" OR "Corona" in their titles were selected. Publications with DOIs were matched between the three databases to assess the percentage overlap between them ( Table 5 ). Few of the Dimensions publications were also in Scopus (23.3%) or WoS (11.8%). Two-fifths (40.4%) of the Scopus publications were in WoS and four-fifths (81.9%) of WoS publications were in Scopus. Google Scholar could not be compared without a comprehensive list of search matches.
Publications in Dimensions but neither WoS nor Scopus were investigated to identify the document types uniquely found by Dimensions. The Dimensions-only publications were almost all from 2020 (95%), as were the publications that were also in Scopus or WoS (99%). The biggest single source was preprint archives (39% of the documents unique to Dimensions): medRxiv, SSRN, Research Square, bioRxiv, chemRxiv, and JMIR Preprints. An additional 2% were other nonjournal publications (e.g., book chapters). The remainder were either in journals not indexed by WoS and Scopus or in journals indexed more slowly by WoS and Scopus. For example, Dimensions had indexed 58 Nature articles that Scopus and WoS had not yet recorded (although they included 19 others in Nature ), and neither Scopus nor WoS had indexed Medical Gas Research or Chinese Journal of Internal Medicine .
In terms of citations found by the three databases for the matching publications, Dimensions citation counts for all its matching COVID-19 publications were 4.9 and 2.8 times as numerous as WoS and Scopus, suggesting that for recently published or in-press articles, Dimensions had faster citation indexing than WoS and Scopus or from faster sources, such as preprint archives. This could be important when scholars want to consider early citation impact evidence for identifying relevant COVID-19 publications or for the impact assessment of published articles.
4.2. Most Cited Papers
Other factors being equal, the most cited papers are likely to be at the core of humanity’s early response to COVID-19 and the most mentioned papers illustrate the public perception of the most relevant research. The age, type, publication venue, and titles of these documents may therefore give insights into important early scientific contributions to the disease. Lower ranked documents are likely to have a different character, however, so the results should not be used as proxies for all COVID-19 research.
The documents with the most Mendeley readers and Dimensions citations tended to be similar and to provide primary clinical and epidemiological evidence about COVID-19 ( Table 6 ). Shorter publication formats and analyses are more evident in the social web and news sources, representing a partially different type of document. The social web and news articles also seemed to give information that might be particularly useful, as public health information for the vast majority of the planet’s population that had not yet caught COVID-19 by 18 April 2020. These include studies on facemasks, the stability of the virus on surfaces, and pregnancy risks.
NEJM : New England Journal of Medicine; JAMA : Journal of the American Medical Association.
None of the five documents most cited on Reddit were also in the top five for the other sources, although they seem to cover similar topics ( Table 7 ). The paper about Malayan pangolins is the exception for not covering the primary characteristics of the disease or public health issues. This may be an artifact of the relatively low numbers of Reddit citations.
Although the top five articles for Dimensions were published in 2020, by March 21 they had all been cited at least 200 times in Dimensions ( Figure 3 ), perhaps mainly by preprints, letters, and short-form fast-publishing formats, such as brief communications, (academic) news, and case reports. All five documents exhibit a reasonably steady rate of increase. The simultaneous jumps in the lines presumably reflect weekly large-scale database refreshing for Dimensions, although there were also smaller daily changes.

The cumulative number of Dimensions citations for the five most cited COVID-19 documents.
The top five Mendeley documents also started March 21 with a high number of readers, but almost five times more than the number of Dimensions citations ( Figure 4 ). There was a similar pattern of steadily increasing numbers of Mendeley readers with periodic interruptions. In this case the interruptions resulted in temporary decreases in the numbers of Mendeley readers. This could be due to two factors. Either the database consolidates weekly, such as by merging duplicates, or its search is somehow weakened periodically so that the free text search (which is submitted in parallel with the DOI search) matches fewer documents. It is not possible to check which is correct from the data because Mendeley reports reader counts, not the identities of these readers.

The cumulative number of Mendeley readers for the five most read COVID-19 documents.
Twitter ( Figure 5 ) has a very different pattern to Dimensions and Mendeley. First, some of the documents are much younger, published during the date range analyzed. Second, the number of tweeters achieves close to its maximum when first found by Dimensions, although this is not necessarily the original publication date.

The cumulative number of Tweeters for the five most tweeted COVID-19 documents.
Facebook has a similar growth pattern to Twitter, except that there is a period of increasing interest for the proximal origin paper ( Figure 6 ), which has a more moderate growth on Twitter. An (apparently speculative) news story about CERN scientists that was popular on Facebook did not get traction on Twitter and seems unlikely to become highly cited or read.

The cumulative number of Facebook wall posts for the five most walled COVID-19 documents.
The top news-cited articles were all covered by at least 400 news sources by the end of the period ( Figure 7 ). Perhaps surprisingly, given that news is very time-dependant, all the sources experienced significant increases in the number of citing sources. Altmetric.com is constantly expanding its coverage of news sources (which is possible, but seems unlikely), it is slow to update its news coverage, or news stories about COVID-19 are prepared to cite old articles, perhaps for a more in-depth commentary or as background context for new articles.

The number of news citations for the five most News-cited COVID-19 documents.
There were relatively few citations from Reddit, despite its use as a news source and many academic themes (subreddits) within the site ( Figure 8 ). Perhaps reflecting its news status, older articles do not seem to increase their Reddit citation counts.

The cumulative number of Reddit posts for the five most posted COVID-19 documents.
4.3. A Comparison Between Average Scores for Different Sources
March 24, 2020 was selected for a time series analysis because this date in the first week had the most new articles (349) found by Dimensions. For documents first found by Dimensions on March 24, 2020 and matching the COVID-19 queries, the average score was highest for Twitter and already above 1 on the start day ( Figure 9 ). Average tweeter counts then increased slowly after the first few days. In contrast, average Mendeley reader counts for these 349 articles started close to zero and increased rapidly, except for weekly Mendeley indexing adjustments. Mendeley overtook Twitter after a week.

Daily average (geometric mean) citations by source for documents first found by Dimensions on March 24, 2020 ( n = 349).
The low initial value for Dimensions citations and the high initial average number of tweets are unsurprising, given that citations take time to accrue due to publication delays (even for preprints), but articles can be tweeted as soon as they are published. As Twitter is a news source and authors/editors/publishers/current awareness browsers might tweet to announce a publication, high initial tweet counts are to be expected. Mendeley users can also add papers to their libraries as soon as they are published, but the slow growth might represent researchers and students saving the articles to read on the day of publication and then adding them to Mendeley after reading them. The figures for Mendeley are likely to also include people that found the articles through literature searches rather than current awareness, adding them to Mendeley when read, found, or cited in a paper (Mendeley automatically builds reference lists).
The average citation counts for the remaining three sources were all much lower than for Mendeley and Twitter ( Figure 10 ). Although Facebook and Reddit both displayed a similar growth pattern to Twitter (rapid initially, then slow), both News citations and Dimensions citations increased steadily. The low number of Dimensions citations is unsurprising, given publication delays, and the small but nontrivial increase for Dimensions suggests that many authors of COVID-19 papers quickly found current research and added it to their papers, then published them as preprints. The constant growth for news sources is unexpected, given that news is supposed to be current. Possible explanations for this (in addition to those discussed above) are delays in the production of slower news sources (e.g., magazine-type articles), delays in writing university press releases, and articles being discussed in the press after passing peer review or after being formally published in early view or an online volume.

Daily average (geometric mean) citations by source for documents first found by Dimensions on March 24, 2020, excluding Mendeley and Twitter ( n = 349).
4.4. Overlaps in Citation Counts Between Sources
Spearman correlation tests reveal the extent to which the same documents that are cited by one source are also cited by another source, together with the extent that they are cited. By April 18, 2020, correlations between Dimensions citations and altmetrics for documents first found by Dimensions on March 24 were strong, except for Reddit ( Table 7 ). Because most (229; 66%) documents were uncited by April 18, the correlation mainly confirms that, except for Reddit, news stories, publishing authors, and users of the different platforms tended to select the same documents for attention. The altmetrics also correlated moderately or strongly with each other, except for Reddit, in agreement with this conclusion. Thus, for the narrow topic of COVID-19, there seems to be a researcher-news-social media consensus about the most important topics, at least in the (very) short term.
The correlations ( Table 8 ) do not take into account field differences or document type differences. The relatively high correlations could be at least partially due to ignoring contributions of low relevance to COVID-19, such as book chapters mentioning the possibility of a coronavirus 2, editorials, letters, and subject areas making relatively peripheral contributions to immediate needs.
Statistically significant at p = 0.001.
The positive correlations might be influenced by a mix of publication venues and document types. The 349 documents included 239 (68.5%) papers in journals, 67 (19.2%) papers in preprint archives, and 27 (7.7%) magazine articles, with 16 (4.6%) not assigned to a publication venue by Dimensions (e.g., book chapters, reports). In terms of rank order, for all five sources, on average, journal articles were more highly ranked than the other types and preprints were more highly, or equally ranked with, magazine articles. The average ranks were Journals (D: 158; M: 136; T: 154; F: 168; N: 165; R: 167); preprints (D: 198; M: 258; T: 195; F: 191; N: 186; R: 191.5); magazines (D: 235; M: 258; T: 280; F: 191; N: 221; R: 191.5). The magazines ( Alcoholism & Drug Abuse Weekly ; Focus on Catalysts ) included news stories about the societal side-effects of the disease rather than research about the disease (e.g., “China refineries reduce operating rates”). Preprints presumably attract less attention because they have not been peer reviewed. In addition, the documents in journals included letters and news stories, which may also have lower relevance to COVID-19 research and many received little attention from any source (e.g., the uncited news article “Seven days in medicine: 11–17 March 2020”, in the BMJ , with 27 readers and two tweets). Thus, both altmetrics and citations seem to focus on contributions of types that are more core to COVID-19 as a medical and public health research issue.
The influence of nonarticle document types on the correlations were tested by filtering out all nonarticles. After manually removing documents that were not journal articles (mainly editorials, news, and letters), there were 106 standard journal articles (including reviews). Nevertheless, the correlations did not substantially change ( Table 9 ). Some of the removed editorials had been cited, read, and shared, explaining the similar positive correlations.
*Statistically significant at p = 0.05; **Statistically significant at p = 0.01; ***Statistically significant at p = 0.001.
The two most common Dimensions subject codes for the March 24 set were 1117 Public Health and Health Services ( n = 78) and 1103 Clinical Sciences ( n = 32). Except for Reddit (correlations close to 0), the pairwise correlations change little if the set is restricted to only subject categories 1117 or 1103, with or without excluding nonarticle types. For example, the lowest correlation between Twitter and Dimensions for any of these four restricted sets is .638 (category 1103 with all document types, n = 32). Thus, except for Reddit, the strong positive correlations between indicators do not seem to be due to field differences in the data set.
Focusing on the smallest data set mentioned above, 1103 Clinical Sciences, journal articles only ( n = 19), one of the reasons for the strong positive correlations is that three of the articles were in national journals ( Korean Journal of Radiology , Chung-Hua Wai Ko Tsa Chih , Chinese Journal of Gastrointestinal Surgery ) and the rest were in international journals or a prestigious national journal that is effectively international ( JAMA ). The three national articles collectively had one citation, three tweets, no mentions in the other altmetrics, and two of the three lowest Mendeley reader counts. This is consistent with lower quality or impact national research being less cited and less read, which is unsurprising. It is more surprising that national research is less tweeted than international research, which has not previously been found by altmetrics studies. In this case, two articles were not in English and this, combined with Twitter not being used in China, might be the explanation.
The top article for all metrics in the 1103 Clinical Sciences journal articles only set (32 citations, 604 readers, 1504 tweeters, three Facebook walls, 31 news stories, one Reddit) was the research letter (classified here as an article) “Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State” from JAMA (published March 19, 2020, but picked up by Dimensions on March 23/24). This seems similar to the Annals of Palliative Medicine article, “Risk factors associated with disease progression in a cohort of [17] patients infected with the 2019 novel coronavirus” from March 22, 2020, which had low scores on all metrics (zero citations, 92 (third fewest) Mendeley readers, one tweet, zero on the others). The first article was in a more prestigious journal and concerned patients from the United States, whereas the second article was more detailed (e.g., pictures, full article, more words, statistical analysis, more references) and was about patients from Nanchang, China. This, combined with the previous three cases, suggests that the regional bias of Twitter (a natural side-effect of news focusing on local issues) coincides with the US/UK or Western domination of more prestigious medical journals. This might not have been visible previously in altmetric studies for the medical domain because the current data set presumably has a higher proportion of Chinese articles than normal, given China’s earlier research into the disease. This is a speculative conclusion, however, and may not be correct. Not all research from China or in nonprestigious sources was ignored in academia. The second most cited article (in an apparently nonprestigious international journal) was, “Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study” from the World Journal of Pediatrics (seven citations, 347 readers (second highest), only 32 tweets, zero others). This article’s focus on children may have been relatively unique, and therefore particularly valuable for researchers.
Also for the same set of 19 Clinical Sciences articles from March 24, there seemed to be a tendency for articles attracting more attention to be more central to COVID-19. Ignoring the four articles discussed above, the remaining uncited article, also with low social media scores, was “Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease,” from Academic Radiology , which focuses on the radiology dimension. Another relatively specialist and universally low scoring article was, “COVID-19 – what should anaethesiologists and intensivists know about it?” from Anaesthesiology Intensive Therapy (1 citation, 218 readers, 18 tweets, 0 others).
4.5. Early Altmetrics and Later Citation Counts
Ideally, an indicator would help researchers and policy-makers to identify important articles when they are first published, without having to wait for enough citations. To check for early evidence of later citation impact, the indicators were correlated with Dimensions citation counts on April 18, representing longer term citation counts (this is a weak proxy, because decades are sometimes used for long-term citations in other contexts, such as Stegehuis, Litvak, & Waltman, 2015 ).
On the day that a document is first findable in Dimensions, its tweeter count is the best indicator of likely long-term citation impact ( Figure 11 ). Twitter users seem to be able to notice documents approximately on the date of first publication for their potential importance to COVID-19. After this date, the tweeter count does not increase much and its correlation with longer term Dimensions citations is stable. After about 3 weeks, Mendeley reader counts take over as a marginally better indicator of longer term citation impact. It is not clear whether the same would be true for more mature citation counts, however, such as after a year. It is possible that early Dimensions citations (and Mendeley readers) reflect more temporary interest and are themselves highly influenced by the news or social sharing on Twitter, for example. The most cited sets of five papers analyzed above suggest that highly recognized papers are particularly important for the disease, however. As above, this correlation ignores field differences and document type differences, although document differences seem to have little effect ( Tables 8 and 9 ).

Spearman correlations between altmetrics and Dimensions citation counts from April 18 for COVID-19 documents first found by Dimensions on March 24, 2020 ( n = 349). Correlations with Dimensions citation counts on the same dates are also reported for context.
The results are limited by the range of factors mentioned in Section 3 . In particular, the coverage figures for the sources are not directly comparable due to the different scopes of the queries. In addition, the count data has not been field-normalized, so the coverage comparisons do not reveal disciplinary differences. The correlations may also be exaggerated by not taking into account disciplinary differences. The results may show different patterns for earlier or later time periods. The properties of the scholarly databases and Altmetric.com ’s strategies may evolve over time, rendering the results obsolete. They may also not be applicable for later stages of COVID-19 research or for future epidemics or pandemics.
The COVID-19 query results comparison confirms the previous finding that COVID-related academic publications are appearing rapidly ( Torres-Salinas, 2020 ). In addition, it confirms that Dimensions finds many publications not in Scopus and WoS but that Scopus indexes nearly all relevant publications found in the WoS core collection with the Conference Proceedings Citation Index. Presumably the difference would be smaller if other parts of WoS were included, such as the Book Citation Index, although the core collection includes the Emerging Sources Citation Index ( Clarivate, 2020 ).
The results are not directly comparable to studies from before COVID-19 due to the unprecedented speed and volume of publishing on the topic. For example, Dimensions citation counts accrue more rapidly than previously reported for any topic. For comparison, the Scopus citations of 12 subject categories (full journal articles only) were a maximum of 0.12 in the month of publication, whereas the COVID-19 mixed set averaged almost double this after 3 weeks. The results are also qualitatively different in some respects. Although correlation tests have previously found tweeter counts to have little value as a scholarly impact indicator due to very low correlation with citation counts, typically close to 0 or even negative ( Costas et al., 2015 ; Haustein et al., 2014 ; Thelwall, Haustein, et al., 2013 ), the current study has found tweet counts to be reasonable academic impact indicators and the best early impact indicator for the first 3 weeks. This may be partly due to the set of articles here covering multiple disciplines, but the results for the top-cited documents suggest that altmetrics are effective at pointing to the documents that are most central to COVID-19 as a medical and public health issue. Thus, the unprecedented threat of COVID-19 seems to have led to an unprecedentedly high and focused level of societal and academic attention being given to the most relevant research. There was some support for this from the correlation analysis for March 24, 2020 documents. This correlation analysis also suggested that the high correlation may also be at least partly due to an international issue: a relatively high amount of publishing from China not in prestigious journals coupled with greater interest in research concerning patients in Twitter-using countries (particularly the United States), and that research sometimes being published in more prestigious journals.
The confirmed rapid increase in COVID-19 academic publications is encouraging in terms of the academic community rapidly reacting to the need for relevant research and commentaries. The importance of short-form and quick contributions (viewpoints, correspondence, brief reports) is also evident in the highly cited papers, as is the importance of academic research for practical public health issues. Dimensions seems to be the most comprehensive database to find relevant literature, although Google Scholar might have wider coverage and be useful to those that do not mind its false matches.
Despite the apparent high medical and public health value of some academic papers, the huge number of publications returned by a relevant search will presumably make the most important publications more difficult to find. This should not be a problem for medical researchers trained to use MeSH queries effectively, but might be problematic for other researchers, end users, and the public, who may find bewilderingly many matches for their queries. The altmetric results suggest that altmetrics may be helpful for researchers needed to quickly identify the most useful new documents from the large number published daily. Altmetric counts may help to distinguish between core primary research and other contributions, such as editorial commentaries with narrower disciplinary or professional relevance (e.g., radiographers). Perhaps ironically, given that a core original goal for altmetrics was to develop indicators of societal impact that were different from scholarly impact indicators ( Priem et al., 2010 ), their greatest value (as early impact indicators) seems be occurring when the two concepts are most closely converging.
Kayvan Kousha: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing. Mike Thelwall: Conceptualization, Data curation, Investigation, Methodology, Software, Visualization, Writing—original draft, Writing—review & editing.
The authors have no competing interests.
This research was not funded.
The processed data used to produce the graphs are available in the supplementary material ( https://doi.org/10.6084/m9.figshare.12301475 ).
https://www.nlm.nih.gov/pubs/techbull/nd08/nd08_pm_new_date_field.html
https://scholar.google.co.uk/scholar?q=covid-19&hl=en&as_sdt=0%2C5&as_ylo=1990&as_yhi=2000
Mendeley does not report the scope of its search feature https://www.mendeley.com/guides/web/02-paper-search
https://connect.biorxiv.org/relate/content/181
https://clinicaltrials.gov/ct2/results?cond=COVID-19
Author notes
Email alerts, related articles, affiliations.
- Online ISSN 2641-3337
A product of The MIT Press
Mit press direct.
- About MIT Press Direct
Information
- Accessibility
- For Authors
- For Customers
- For Librarians
- Direct to Open
- Open Access
- Media Inquiries
- Rights and Permissions
- For Advertisers
- About the MIT Press
- The MIT Press Reader
- MIT Press Blog
- Seasonal Catalogs
- MIT Press Home
- Give to the MIT Press
- Direct Service Desk
- Terms of Use
- Privacy Statement
- Crossref Member
- COUNTER Member
- The MIT Press colophon is registered in the U.S. Patent and Trademark Office
This Feature Is Available To Subscribers Only
Sign In or Create an Account

Supporting Research: A COVID-19 Citation Database

My normal week is satisfyingly hectic: offering trainings to colleagues at the Smithsonian’s National Zoological Park (NZP), hopping on the Metro, providing reference support at the National Museum of Natural History ’s (NMNH) main library, retrieving references for Mineral Sciences staff, and on Fridays, traveling 160 miles (round trip) to Smithsonian’s Conservation Biology Institute (SCBI) in Front Royal, VA. Add on committee meetings, literature searches, Association of Zoos & Aquarium bibliographies, and long-term projects, and it seems there is never enough time in a work week. And I love it.
Since being home, I have been just as busy, but in a much different way. I still meet with colleagues (virtually), provide reference support, and run literature searches, but I’ve also been able to complete my to-do list and make real progress on long-term projects. I was already an old hand at teleworking, which has served me well since mid-May, when I joined the Smithsonian’s COVID-19 Reopening Task Force (RTF) on the kind recommendation of an SI colleague.
But what does a zoo librarian know about public health? I’m glad you asked.
Before coming to the Smithsonian, I:
- Biology, Environmental Science, Kinesiology, Microbiology, and Zoology
- ran comprehensive literature reviews and occupational health systematic reviews while stationed at federal libraries
- sat ex officio on a university’s Institutional Animal Care and Use Committee (IACUC)
- created EndNote libraries with 50,000+ unique citations on subjects ranging from occupation lifting and pregnancy to the carcinogenic properties of asphalt sealant
To assist the important work of my colleagues on the RTF, I did what I know best…created a citation database. I chose to build it in Zotero, an open source citation manager, which I extensively use to provide research support for SI colleagues and external organizations.
My goal for the COVID-19 citation database is simple: a one-stop repository of all SI accessible scholarly citations and curated (select) newspaper articles, video recordings, and websites. Reducing redundant efforts and increasing efficiency is the best way I know to provide support to my patrons, whether at NZP, NMNH, or SI in general.
- Main search box
- Tag search box
- Articles with full-text retrieved ( SI staff only )
- Source Types
- Digital Object Identifiers (DOI) – permalinks to article full-text PDFs on publisher’s sites
- Articles that have had their full-text PDFs retrieved ( SI staff only )
- Articles that require publisher site access for full-text retrieval (possible paywall)
- Articles that require interlibrary loan requests for full-text retrieval ( SI staff only )
Methodology:
To create the baseline for literature, I utilized the National Library of Medicine’s Medical Subject Headings (MeSH) Browser , as well as the search string used by Battelle for the OCLC / Institute of Museum and Library Services Reopening Archives, Libraries and Museums (OCLC/IMLS REALM) literature review , and created the following search string:
(“COVID-19” OR “severe acute respiratory syndrome coronavirus 2” OR “2019-nCoV” OR “SARS-CoV-2” OR coronavir* OR hcov)
I did not use any other search terms, in order to retrieve the maximum number of potentially relevant citations.
I retrieved results in Web of Science Core Collection, PubMed, Elsevier’s Scopus, and Google Scholar. To identify burgeoning research, business reactions, and government assessments, I created a Google News Alert.
Maintenance:
Citation databases do not always export full records (even if the option is selected). In order to create complete records, I deduplicated nearly 40,000 results, making sure that (when possible) each citation had at least a title, author(s), date of publication, publication title, volume, issue, pages, and an abstract. Since COVID-19 literature has almost totally been written in 2020, most citations include DOIs. Additionally, many citations appear in databases during the pre-print stage, meaning they are often incomplete, so it is important to deduplicate/merge across multiple databases to create complete references.
A database of this size requires a lot of discipline and can take over one’s life. I have created schedules for recurring searches (1) and maintenance (2) so I can have time to respond to patron requests.
I have since added more sources, and in addition to the aforementioned databases, I also run recurring searches in Cochrane Library, ProQuest Coronavirus Database and Publicly Available Content Databases, SciELO, and Zoological Record.
To date, I have reviewed 167,250 citations, adding an average of 950 new citations per day.
The online version of the Zotero database has two search boxes (including the ability to search just the tags). Since the online version does not reflect saved search folders created in the desktop version, I have created numerous keyword folders and manually update them every few days. Examples of delimiters include Source Types, Locations, Pathology, and Cleaning.
Users are able to highlight specific articles or even an entire folder and export the results as a bibliography with abstracts, making skimming to locate relevant sources much easier (CTRL-F is your friend).
Points of Interest:
I run searches every business day and base the frequency of searching on the refresh rate of a database (PubMed is run daily; ProQuest is run every few days). Each database has limits on the number of citations that can be exported (e.g., Web of Science – 500 at a time) or per day (ProQuest – 10,000). ProQuest includes keywords as notes, so I have set up automated searches so I can quickly find and delete these data hogs (that, coincidentally, just mirror information in the citation proper).
Print journalism is added regardless of the author’s perceived or overt bias. Once the COVID-19 pandemic has passed, I hope the database will serve SI’s historians well.
Going Live:
Since the database’s initial release , I have used it to create reports on mask and social distancing compliance for the NZP and the Steven F. Udvar-Hazy Center; face mask efficacy based on type (e.g., N95, KN95, cloth, etc.); air monitoring in office spaces; surface wipe sampling of different types of materials (e.g., steel, plastic, wood); temporal patterns in viral loads; and contact tracing.
Because COVID-19 research is relatively new, I also run pertinent searches on ancillary topics as they pertain to previous outbreaks (e.g., sanitization of surfaces for the elimination of viruses).
I have also created derivative databases for other groups. Currently, I’m providing reference and citation database training and support to the SI group tasked with creating risk assessment models.
If scientia potentia est , then surely convenient access to orderly data is just as important. Supporting researchers is one of the things Smithsonian Libraries does best. If my efforts make the work of my SI colleagues any easier, I’ve done my job.
Categories: Collection Highlights Natural and Physical Sciences Research
- National Museum of Natural History Library
- National Zoological Park Library
- Science Research
One Comment
This is awesome, Stephen!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Covid-19 Pandemic Resources and Research
- Start Your Research Here
- Find Articles
- Government Resources
- Health Statistics
- Cite Sources

Ask a Librarian
- Make an Appointment
Online Citation Manuals
- AP Stylebook (Associated Press) This link opens in a new window Features the updated version of the Associated Press (AP) Stylebook and Briefing on Media Law used by journalists. The stylebook provides grammar, punctuation, usage, and journalistic writing guidelines. The online version includes Topical Guides, Ask the Editor, and the AP Blog. NOTE: Permits only ten simultaneous users. Please log off when finished.
- Chicago Manual of Style Online (The University of Chicago) This link opens in a new window Features formatting and documentation rules for the 16th (2010) and 17th (2017) editions of the Chicago Manual of Style. This site includes the Citation Quick Guide, Chicago Style Q & A, and related resources.
- MLA Handbook Plus (Modern Language Association) (VALE) This link opens in a new window Provides access to the online version of the 9th edition of the MLA Handbook. The site includes practice exercises, tutorials, and the MLA Guide to Undergraduate Research in Literature and MLA Guide to Digital Literacy .
Citation Style Web Sites
The following Websites provide formatting and documentation rules for various citation styles.
- APA Formatting and Style Guide (6th Edition): The OWL at Purdue
- APA Formatting and Style Guide (7th Edition): The OWL at Purdue
- APA Style (6th Edition): Frequently Asked Questions
- APA Style (7th Edition): Style and Grammar Guidelines
- APA Style Blog
- Ask the MLA
- Associated Press Stylebook: Ask the Editor
- Chicago Manual of Style 17th Edition: The OWL at Purdue
- Chicago Manual of Style Online
- Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers
- Formatting in Sociology (ASA Style): The OWL at Purdue
- MLA Formatting and Style Guide: The OWL at Purdue
- MLA Style Center: Writing Resources from the Modern Language Association of America
- Resources for Documenting Sources in the Disciplines: The OWL at Purdue
- Scientific Style and Format Online, 8th Edition
- Turabian Manual for Writers of Research Papers, Theses, and Dissertations
Citation Style Brochures
The following brochure provides information regarding documenting sources in various citation styles, including APA, MLA, Chicago style, and more.
- Resources for Documenting Sources: Citation Style Websites and Manuals
Citation Style Guides Gallery
ACS Style Guide: Effective Communication of Scientific Information
Service Desk (First Floor) Non-circulating
AMA Handbook of Business Writing
Electronic Resource
AMA Manual of Style (10th Edition)
(American Medical Association) Service Desk (First Floor) Non-circulating
AMA Manual of Style (11th Edition)
(American Medical Association) Electronic Resource
APA Style Guide to Electronic References, Sixth Edition
Electronic Resource Due to Federal Copyright Law, users may print one copy for their personal use only.

American Sociological Association Style Guide (7th Edition)
American sociological association style guide.
The Associated Press Stylebook 2020
The Associated Press Stylebook 2022-2024
The Bluebook: A Uniform System of Citation
The Chicago Manual of Style, 17th Edition
A Manual for Writers of Research Papers, Theses, and Dissertations (Turabian manual)
Reference Collection (Second Floor)
MLA Handbook
(8th Edition) Service Desk (First Floor) Non-circulating
MLA Handbook (9th Edition)
Publication Manual of the American Psychological Association (APA)
(6th Edition) Service Desk (First Floor) Non-circulating
(7th Edition) Service Desk (First Floor) Non-circulating
Scientific Style and Format: The CSE Manual for Authors, Editors, and Publishers (Council of Science Editors)
Academic integrity at kean university.
Kean University's Academic Integrity Policy explains the importance placed on intellectual honesty, personal ethics and values for all members of the Kean academic community.
Plagiarism is one type of academic integrity violation, and it has very serious consequences. In order to avoid committing plagiarism, the work presented by an individual must truly represent the work of that person, and such work must acknowledge the source of any words, ideas, data, images, music, etc. that have been incorporated into a paper, presentation, Web site, multimedia project, and so on.
Citations to the original source material must accompany word-for-word quotes enclosed in quotation marks, block quotations, and paraphrased material (ideas that you have put into your own words).
While you gather source material for your research projects, make sure that you keep track of the information you will need in order to acknowledge your sources.

EndNote Online

EndNote Online will help you to organize your source information and then to create bibliographies in your required citation styles (such as APA or MLA).
Find out more about EndNote Online, including how to sign up for your own free account
- << Previous: Health Statistics
- Last Updated: Apr 19, 2024 2:53 PM
- URL: https://libguides.kean.edu/Covid-19

COVID-19 Research Articles Downloadable Database
March 19, 2020
Updated January 12, 2024
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
Important announcement:
The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
If you have any questions, concerns, or problems accessing the WHO COVID-19 Database please email the CDC Library for assistance.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
Below are options to download the archive of COVID-19 research articles. You can search the database of citations by author, keyword (in title, author, abstract, subject headings fields), journal, or abstract when available. DOI, PMID, and URL links are included when available.
This database was last updated on October 9, 2020 .
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
Excel download:
- Articles from August until October 9 2020 [XLS – 29 MB]
- Articles from December 2019 through July 2020 [XLS – 45 MB]
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide.
- October 8 in Excel [XLS – 1 MB]
- October 7 in Excel [XLS – 1 MB]
- October 6 in Excel [XLS – 1 MB]
- Note the main Excel file can also be sorted by date added.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download:
- Part 1 [ZIP – 38 MB]
- Part 2 [ZIP – 43 MB]
- October 8 in citation management software format [RIS – 2 MB]
- October 7 in citation management software format [RIS – 2 MB]
- October 6 in citation management software format [RIS – 2 MB]
- Note the main RIS file can also be sorted by date added.
The COVID-19 pandemic is a rapidly changing situation. Some of the research included above is preliminary. Materials listed in this database are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
To access the full text, click on the DOI, PMID, or URL links. While most publishers are making their COVID-19 content Open Access, some articles are accessible only to those with a CDC user id and password. Find a library near you that may be able to help you get access to articles by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
CDC users can use EndNote’s Find Full Text feature to attach the full text PDFs within their EndNote Library. CDC users, please email Martha Knuth for an EndNote file of all citations. Once you have your EndNote file downloaded, to get the full-text of journal articles listed in the search results you can do the following steps:
- First, try using EndNote’s “Find Full-Text” feature to attach full-text articles to your EndNote Library.
- Next, check for full-text availability, via the E-Journals list, at: http://sfxhosted.exlibrisgroup.com/cdc/az .
- If you can’t find full-text online, you can request articles via DocExpress, at: https://docexpress.cdc.gov/illiad/
The following databases were searched from Dec. 2019-Oct. 9 2020 for articles related to COVID-19: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, and LitCovid. Selected grey literature sources were searched as well, including the WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints).
Detailed search strings with synonyms used for COVID-19 are below.
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF – 135 KB]
Note on preprints: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
To receive the COVID-19 Science Update, please enter your email address to subscribe today.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Research + Activism Bibliography
A UC Santa Barbara English Dept. Project
Bibliography Menu (show/hide)
Methods & Theories of Research Activism (show/hide)
Causes & issues (show/hide), movements, actions, events, & initiatives (show/hide), social & other groups (show/hide), in or related to higher education (show/hide), in or related to lower education (show/hide), in or related to other organizations (show/hide), in or related to disciplines & professions (show/hide), in or related to geopolitical regions, nations, states, cities, borders, etc. (show/hide), publications by type & media (show/hide), searchable version of bibliography (and developer resources), simple search (on this website ).
Advanced Search (on Zotero site )
National Nurses United Covid-19 Bibliography
This is an annotated bibliography of some of the relevant scientific literature on SARS-CoV-2 and Covid-19. The nursing profession is rooted in science and the protection of nurses should also reflect both emerging scientific research and the precautionary principle. The precautionary principle asserts that we should not wait for scientific proof of harm before taking action to protect people’s health. Protections for nurses and other health care workers should be proactive and preventive, based on the precautionary principle, rather than reactive to Covid-19. This bibliography is categorized into four main sections, from asymptomatic and aerosol transmission, personal protective equipment (PPE), to children and Covid-19.
Asymptomatic and presymptomatic SARS-CoV-2 transmission
Asymptomatic and presymptomatic individuals– meaning people who may not be aware that they are infected because they don’t yet have any symptoms-- transmit SARS-CoV-2 virus efficiently. Both symptomatic and asymptomatic individuals infected with SARS-CoV-2 have similar viral loads, which is associated with increased disease severity and mortality. Asymptomatic cases play a significant role in Covid-19 transmission; approximately half of all transmission is from asymptomatic cases. Being asymptomatic does not necessarily mean that no damage has occurred in someone’s body. Several studies have reported lung and cardiac damage among asymptomatic individuals infected with Covid-19. The long-term health implications of asymptomatic infection are still not fully known.
1. Yanes-Lane et al., “Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis,” PLOS ONE, November 2020, link .
- This study conducted a systematic review and meta-analysis on the proportion of asymptomatic infection among Covid-19 positive individuals and their transmission potential.
- Researchers found that the proportion of asymptomatic Covid-19 infections at time of testing ranged from 20 to 75%. Meta-analysis estimated a secondary attack rate of 18.8% in contacts of asymptomatic cases.
2. Abkarian et al., “Speech can produce jet-like transport relevant to asymptomatic spreading of virus,” Proceedings of the National Academy of Sciences, September 2020, link .
- This study examined and visualized airflows during breathing and speaking, with a high-speed camera to capture the movement of aerosols.
- Researchers found that normal conversations can create a turbulent, jet-like airflow that can transport exhaled breath over 2 meters (6.5 feet) in front of the speaker, potentially further, within 30 seconds. The results of this study are relevant to asymptomatic spread of SARS-CoV-2 as transmission can occur in the absence of a cough. It also highlights the importance of the proximity to, and time spent with an asymptomatic speaker, especially in indoor settings.
3. Buitrago-Garcia et al, “Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis,” PLOS Medicine, September 2020, link .
- Literature review and meta-analysis of papers on asymptomatic/presymptomatic transmission published through June 10.
- 20% of people with SARS-CoV-2 infections remain asymptomatic during follow-up “but biases in study designs limit the certainty of this estimate.”
4. Sugano et al., “Cluster of SARS-CoV-2 infections linked to music clubs in Osaka, Japan: asymptomatically infected persons can transmit the virus as soon as 2 days after infection,” J of Infectious Diseases, August 2020, link .
- Report on a case cluster linked to music clubs in Japan with 108 cases.
- The index case was a health care worker who was exposed at work on Feb 14 and attended a club on Feb 15 and developed symptoms on Feb 24.
- The index case transmitted the virus while symptomatic. Within the cluster, all cases transmitted the virus within 2 to 3 days of exposure.
5. Corcorran et al., “Prolonged persistence of PCR-detectable virus during an outbreak of SARS-CoV-2 in an inpatient geriatric psychiatry unit in King County, Washington,” American Journal of Infection Control, August 2020, link .
- This study described key characteristics, interventions, and outcomes of a SARS-CoV-2 outbreak within an inpatient geriatric psychiatry unit at the University of Washington Medical Center – Northwest.
- Researchers identified 10 patients and 7 staff members with SARS-CoV-2 infection; 30% of patients remained asymptomatic over the course of infection. The median duration of PCR positivity was 25.5 days among symptomatic patients and 22.0 days among asymptomatic patients. Cycle threshold values (viral load) was similar between symptomatic and asymptomatic patients.
6. Lee et al., “Clinical Course and Molecular Viral Shedding Among Asymptomatic and Symptomatic Patients With SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea,” JAMA Internal Medicine, August 2020, link .
- Cohort study of patients from South Korea (n=303), including 110 who were asymptomatic at time of isolation (19.1% went on to develop symptoms during isolation).
- Upper and lower respiratory tract samples were taken, and viral load was measured.
- Asymptomatic patients had very similar viral loads to symptomatic patients.
7. Long et al., “Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections,” Nature Medicine, June 2020, link .
- Researchers compared Covid-19 antibody responses of 37 asymptomatic patients and 37 symptomatic patients in China. They found that asymptomatic group had a significantly longer duration of viral shedding than the symptomatic group. In addition, IgG levels and neutralizing antibodies diminished significantly within 2 to 3 months after infection. No antibodies were detected 8 weeks after recovery in 40% of asymptomatic group and 12.9% of symptomatic group.
8. Widders et al., “SARS-CoV-2: The viral shedding vs infectivity dilemma," Infection, Disease & Health, May 2020, link .
- This study evaluated the evidence around viral shedding and infectivity of SARS-CoV-2.
- Researchers found that the percentage of asymptomatic SARS-CoV-2 infections range from 1% to 78%. They also found that immunosuppression and disease severity appear to prolong the duration of viral shedding; though, the correlation between duration of shedding and infectivity is unclear.
9. Kimball et al., “Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility — King County, Washington, March 2020,” Morbidity and Mortality Weekly Report (MMWR) 2020;69:377–381, link .
- This study examined infections in a long-term care skilled nursing facility in Washington.
- Researchers found that 56.5% of residents were infected and infectious but asymptomatic at the time of testing. They also note that symptom-based screening of nursing home residents might fail to identify all SARS-CoV-2 infections.
Evidence of SARS-CoV-2 aerosol transmission
SARS-CoV-2, the virus that causes Covid-19 disease, is transmitted through infectious aerosols that are emitted when infected individuals breathe, vocalize, cough, or sneeze. Numerous studies have found that SARS-CoV-2 can survive and remain infectious for at least 16 hours suspended in aerosols and travel more than 26 feet. Researchers have also recovered viable SARS-CoV-2 in the air from hospital rooms with Covid-19 patients, collected at nearly 16 feet away, in the absence of a cough or aerosol generating procedure. Multiple outbreak investigations have concluded that aerosol transmission is the only possible explanation.
1. Zhou et al., “Breath-, air- and surface-borne SARS-CoV-2 in hospitals,” Journal of Aerosol Science, October 2020, link .
- Exhaled breath condensate (EBC) samples were collected from 13 recruited patients. Nine were recovering Covid-19 patients and four had influenza symptoms and repeatedly tested negative for SARS-CoV-2 using throat swabs.
- 318 swab samples were collected from surfaces associated with the Covid-19 patients and medical staff, and from many other surfaces inside four hospitals in Wuhan.
- 44 air samples were collected from the corridors, hospital waste storage rooms, ICU rooms, toilets, medical preparation rooms, clinical observation rooms, and general wards.
- Researchers detected SARS-CoV-2 in exhaled breath (22.2%), air samples (6.8%), and surface swabs (3.1%) collected from hospitals of Wuhan using both RT-PCR and digital PCR. Two recovering Covid-19 patients, in Wuhan hospitals, ready for hospital discharge were emitting SARS-CoV-2 RNA, about (7.35–7.77) x 104 viruses per hour estimated by the method described, via breathing.
- RT-PCR diagnosis using throat swab specimens had a failure rate of more than 22% in safely discharging Covid-19 patients who were otherwise still exhaling the SARS-CoV-2 by a rate of estimated ~1400 RNA copies per minute into the air.
- Direct surface contact might not represent a major transmission route, and lower positive rate of air sample (6.8%) was likely due to natural ventilation (1.6–3.3 m/s) and regular disinfection practices.
2. Riddell et al., “The effect of temperature on persistence of SARS-CoV-2 on common surfaces,” Virology Journal, October 2020, link .
- This study measured the survival rates of infectious SARS-CoV-2 virus, suspended in a standard ASTM E2197 matrix, on several common surface types. All experiments were carried out in the dark, to negate any effects of UV light.
- Inoculated surfaces were incubated at 20 °C (68 °F ~room temp), 30 °C and 40 °C and sampled at various time points.
- SARS-CoV-2 survived longer at lower temperatures; lasted longer on smooth surfaces than porous surfaces such as cotton.
- SARS-CoV-2 lasted 10 days longer than influenza on some surfaces
- While the primary spread of SARS-CoV-2 appears to be via aerosols and respiratory droplets, fomites may also be an important contributor in transmission of the virus.
3. Miller et al., “Transmission of SARS‐CoV‐2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event,” Indoor Air, September 2020, link .
- Detailed investigation of the Skagit County choir outbreak from WA early in outbreak.
- 1 index case led to 53 cases identified from 61 individuals in attendance (33 confirmed via testing, 20 probable based on timing and symptoms).
- No spatial pattern was identified in the distribution of cases. The heater was on (with MERV11 filter and unknown outdoor air proportion) at beginning of rehearsal but probably turned off with so many people in the room because it was 45 deg F outside.
- The index case did not participate in moving chairs or handling snacks so fomite transmission unlikely. No common points of contact were identified (e.g., bathroom handle only common for about 6 other people).
- No one was located within 3 m in front of the index case; therefore, droplet transmission did not occur. The paper goes through other details to very clearly rule out droplet transmission. This virus was transmitted via aerosols/airborne.
4. Lednicky et al., “Viable SARS-CoV-2 in the air of a hospital room with Covid-19 patients,” International Journal of Infectious Diseases, September 2020, link .
- Researchers recovered viable (infectious) SARS-CoV-2 virus in the air from a hospital room with 1 Covid-19 patient and a 2nd patient who had previously tested positive for Covid-19 but tested negative prior to the study. The air was collected 2 to 4.8 meters (6.5 to 15.7 feet) away from the patients.
- Airborne virus was detected in the absence of health-care aerosol-generating procedures.
- The virus strain detected in the aerosols matched the virus strain isolated from a patient with acute Covid-19.
5. de Man et al., “Outbreak of Coronavirus Disease 2019 (COVID-19) in a Nursing Home Associated with Aerosol Transmission as a Result of Inadequate Ventilation,” Clinical Infectious Diseases, August 2020, link .
- Paper reports on the investigation of a Covid-19 outbreak in a Dutch nursing home where 81% of residents and 50% of health care workers in 1 ward were diagnosed with Covid-19 but tests of all residents and workers in 6 other wards were negative.
- Direct transmission cannot be ruled out, but the rate of transmission was extremely rapid. Investigators found that the outbreak ward’s ventilation system had been recently renovated and a CO2 controlled energy-efficient system was installed.
- That meant that indoor air was only refreshed when the CO2 level exceeded 1000 ppm. Otherwise, indoor air was recirculated.
- The other 6 wards (with no infections) were ventilated with outdoor air, indicating that aerosol transmission via the ventilation system was likely at play in the outbreak
6. Tang et al., “Aerosol transmission of SARS-CoV-2? Evidence, prevention and control,” Environment International, August 2020, link .
- Literature review reporting on the overwhelming evidence for aerosol transmission. Notes the need for airborne precautions for health care workers.
7. Ma et al., “Covid-19 patients in earlier stages exhaled millions of SARS-CoV-2 per hour,” Clinical Infectious Diseases, August 2020, link .
- Researchers collected exhaled breath condensate from 57 Covid-19 patients, 4 hospitalized non-Covid-19 patients, and 15 healthy individuals in Beijing. Exhaled breath samples had the highest positive rate (26.9%); Covid-19 patients emitted millions of SARS-CoV-2 particles into the air per hour.
- Air samples and surface swabs were also collected from quarantine hotels and hospitals. Toilet room air and surface swab samples were most likely to be positive, followed by hospital floor, other surfaces, patient touching surfaces, and medical touching surfaces.
8. Monroe et al., “Incidence and Persistence of Viral Shedding in Covid-19 Post-acute Patients with Negativized Pharyngeal Swab: A Systematic Review,” Frontiers in Medicine, August 2020, link .
- Literature review examining 147 studies measuring viral shedding in patients.
- Researchers found variation in length of time patients shed SARS-CoV-2 virus in different body fluids. The fecal viral positivity duration was (median 19 days) longer than respiratory tract viral positivity (median 14 days).
9. Santarpia et al., “Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care,” Scientific Reports, July 2020, link .
- Researchers collected air and surface samples to examine viral shedding from isolated Covid-19 patients. Significant environmental contamination was found in bedrails, toilets, ventilation grates, window ledges and hallways.
- SARS-CoV-2 was found in air samples taken greater than 6 feet from the patients.
- SARS-CoV-2 was found in air samples worn by sampling personnel, even in the absence of cough.
10. Santarpia et al., “The Infectious Nature of Patient-Generated SARS-CoV-2 Aerosol,”medRxiv, July 2020, link .
- This study looked at the presence and viral replication of SARS-CoV-2 in aerosol samples around 6 patients admitted into mixed acuity wards in April 2020. Samples were collected greater than 6 feet from patients, beyond the foot of the bed.
- SARS-CoV-2 RNA was found in respired aerosols <5 µm around all 6 patients. When placed in cell cultures, aerosol samples <1 µm in diameter replicated.
- Researchers note that the study shows that some aerosol particles smaller than 5µm produced through normal breathing, vocalization, and coughing can contain infectious SARS-CoV-2.
11. Fears et al., “Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions,” Emerging Infectious Diseases, June 2020, link .
- This study looked at the viability of SARS-CoV-2 in suspended aerosols and found that SARS-CoV-2 remained infectious after 16 hours suspended in aerosols. This further reinforces airborne/aerosol transmission of SARS-2.
- The authors state: “Our approach of quantitative measurement of infectivity of viral airborne efficiency complemented by qualitative assessment of virion morphology leads us to conclude that SARS-CoV-2 is viable as an airborne pathogen.”
12. Kasloff, Samantha B. et al., “Stability of SARS-CoV-2 on Critical Personal Protective Equipment,” medRxiv, June 2020, link .
- Studied how long SARS-CoV-2 virus can survive on different surfaces in the healthcare environment, including nitrile medical examination gloves, reinforced chemical resistant gloves, N-95 and N-100 particulate respirator masks, Tyvek coveralls, plastic from face shields, heavy cotton, and stainless steel.
- Coupons of each type of material were inoculated with SARS-CoV-2 virus along with compounds meant to mimic the organic components of virus-containing fluid typically shed by patients. Coupons were then dried and maintained at ambient temperature and humidity (35-40%).
- Plastic (from face shield) up to 21 days
- N-95 respirator up to 21 days, with significant quantity of virus recovered after 14 days
- N-100 respirator up to 21 days
- Tyvek up to 14 days
- Stainless steel up to 14 days
- Nitrile gloves up to 7 days
- Chemical resistant gloves up to 4 days
- Cotton up to 24 hours
- This study starkly underlines the risks of reusing single-use N-95 respirators and other single-use PPE. And it underlines the importance of effective cleaning protocols for powered air-purifying respirators (PAPRs), elastomeric respirators, and other PPE designed for reuse.
13. Chia, Po Ying et al., “Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients,” Nature Communications, May 2020, link .
- This study examined surface and air contamination in airborne infection isolation rooms of patients with confirmed Covid-19 infections in Singapore.
- They found that 56.7% of the rooms had at least one environmental surface contaminated, with 18.5% of the toilet seats and toilet flush button being contaminated.
- High touch surface contamination was shown in ten (66.7%) out of 15 patients in the first week of illness, and three (20%) beyond the first week of illness (p = 0.010).
- Air sampling of two Covid-19 patients (both day 5 of symptoms) detected SARS-CoV-2 PCR positive particles of sizes >4 µm and 1-4 µm. In a single subject at day 9 of symptoms, no SARS-CoV-2 PCR-positive particles were detected.
14. van Doremalen et al., “Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1,” New England Journal of Medicine, April 2020, link .
- This study examined how long SARS-CoV-2 can survive in aerosols suspended in the air and on surfaces of different types (metal, plastic, cardboard).
- They found that SARS-CoV-2 can survive up to three hours in aerosols, four hours on copper, 24 hours on cardboard, 2-3 days on plastic and stainless steel.
- The authors conclude, “Our results indicate that aerosol and fomite transmission of [SARS-CoV-2] is plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days.” This study was conducted by NIH and CDC scientists in addition to UCLA and Princeton.
15. Leung, Nancy H. L. et al. “Respiratory virus shedding in exhaled breath and efficacy of face masks,” Nature Medicine, April 2020, link .
- This study examined viral presence and load in exhaled breath of patients with lab-confirmed influenza, seasonal coronaviruses, or rhinovirus.
- Found viral presence in exhaled breath, even without cough, for all types of viruses in both droplet (>5 micron) and aerosol (<5 micron) particles.
16. Chin, Alex W H et al., “Stability of SARS-CoV-2 in different environmental conditions,” The Lancet Microbe, April 2020, link .
- This study examined the ability of SARS-CoV-2 to survive outside the human body in different environmental conditions.
- They found that SARS-CoV-2 can survive outside the human body for up to 14 days at 39 degrees Fahrenheit, 7 days at 72 degrees Fahrenheit and remains infectious in both situations.
- Printing and tissue papers- up to 3 hours
- Wood and cloth- up to 2 days
- Glass and banknote- up to 4 days
- Stainless steel and plastic- up to 7 days
- Surgical mask- detectable level of infectious virus found after 7 days on outer layer of mask
- Household bleach (1:49)
- Household bleach (1:99)
- Ethanol (70%)
- Povidone-iodine (7.5%)
- Chloroxylenol (0.05%)
- Chlorhexidine (0.05%)
- Benzalkonium chloride (0.1%)
17. Wölfel, Roman, et al., “Virological assessment of hospitalized patients with Covid-2019,” Nature, April 2020, link .
- This study examined viral loads and isolates for patients hospitalized with Covid-19. The majority of patients in this study presented with upper respiratory tract symptoms. Viral loads from upper respiratory tract samples were extremely high (more than 1000 times higher than SARS). Live virus was isolated from upper respiratory tract tissues.
- Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said, “The findings [of this study] confirm that Covid-19 is spread simply through breathing, even without coughing… They also challenge the idea that contact with contaminated surfaces is a primary means of spread,” (emphasis added). http://www.cidrap.umn.edu/news-perspective/2020/03/study-highlights-ease-spread-covid-19-viruses.
18. Guo, Zhen-Dong et al., “Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020,” Emerging Infectious Diseases, April 2020, link .
- This study looked at environmental contamination in an ICU and a general ward in hospital in China where patients with Covid-19 were placed.
- They found SARS-CoV-2 on many surfaces in patient rooms and on units, including doorknobs, bedrails, patient masks, computer mouse, keyboards, etc.
- Many positive results on floors not just in patient room but throughout the unit. 50% of the samples from the soles of healthcare workers’ shoes were positive.
- They also measured SARS-CoV-2 in air samples and found several air samples positive in addition to finding that the samples from the air outlets were positive for virus.
- Underlines nurses’ need for PPE!
19. Chin, Alex W H et al. “Stability of SARS-CoV-2 in different environmental conditions,” The Lancet Microbe, April 2020, link .
20. Bourouiba, Lydia, “Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of Covid-19,” JAMA, March 2020, link .
- This paper reported on what is known about disease transmission via respiratory droplets created by human exhalations, sneezes, and coughs.
- Droplet transmission was originally defined in 1897, large and small droplets defined in 1930s. This model of infectious disease transmission hasn’t been updated since. And yet, the CDC and WHO maintain use of this paradigm despite more recent research.
- More recent research over the past few decades performed with instrumentation that better measures particle sizes and movement has determined that human exhalations, coughs, and sneezes (the things that supposedly create large droplets under old model) are actually made of multiphase turbulent gas clouds (a puff) that entrains ambient air and traps and carries clusters of particles of a wide range of sizes.
- This includes viral particles in people who are sick.
- Pathogen-carrying gas clouds emitted when people breath, cough, and sneeze can travel up to 23-27 feet.
Scientific evidence about SARS-CoV-2 underlines the need for multiple measures, including optimal PPE, to prevent infections among nurses and other health care workers
Nurses and health care workers, like all workers, have the right to a safe and healthful workplace. Protection of nurses and other health care workers is a fundamental part of limiting the spread of Covid-19. Studies have shown that SARS-CoV-2 is transmitted via infectious aerosols emitted when people who are infected breathe, speak, cough, sneeze, sing, or have aerosol-generating procedures performed. These aerosols range from very small to large and can travel long distances (up to about 27 feet) and stay suspended in the air. Multiple studies have also shown that Covid-19 infections occurred with less than 15 minutes of exposure. Thus, health care employers must implement the fullest protections, including screening all patients, isolating both possible and confirmed Covid-19 cases in negative pressure rooms, providing optimal PPE, and safe staffing, in order to ensure that health care workers maintain their right to a safe and healthy workplace. To see more information about NNU’s standards for health care infection control during Covid-19, visit https://www.nationalnursesunited.org/covid-19.
Covid-19 infections can occur with exposures less than 15 minutes and/or beyond 6 feet in distance.
1. Karan et al., “The Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission from Patients with Undiagnosed Coronavirus Disease 2019 (COVID-19) to Roommates in a Large Academic Medical Center,” Clinical Infectious Diseases, June 2021, link .
- This study analyzed all adult patients hospitalized at Brigham and Women’s Hospital in Massachusetts, between 1 September 2020 and 15 April 2021, to assess SARS-CoV-2 transmission between patients in shared rooms. During the study period, all patients were tested for SARS-CoV-2 via PCR on admission; symptomatic patients were tested twice 12 hours apart and isolated in single rooms until the second test returned negative; asymptomatic patients were tested once and managed with standard precautions while the test was pending.
- Roommates were considered exposed if they shared a room for ≥15 minutes with an index patient during their infectious window, defined as 48 hours prior to symptom onset (or positive test in the absence of symptoms) until isolation.
- Exposed roommates were tested if they remained hospitalized; discharged patients were contacted by phone whenever possible and offered testing.
- Roommates who were not tested between 3 and 14 days following exposure were excluded from the study.
- PCR-positive patients were excluded on the basis of serial high cycle thresholds and/or prior history of SARS-CoV-2 infection.
- Median time-to-positivity was 5 days post-exposure (range 2–10); 33% initially tested negative before testing positive.
- Only 1 index case and 2 roommates had been fully vaccinated at the time of exposure; one vaccinated roommate was infected by an unvaccinated index case.
- The median interval from hospital admission until positive test among infected roommates was 9.5 days (IQR 7.8–12).
- Ten exposed roommates tested positive after discharge (median 4.5 days post-discharge, IQR 2–7.75).
- Patients were >6 feet apart with closed curtains in between; only 3 out of 12 infected roommates were exposed to aerosol-generating procedures.
- The authors noted, “Our study underscores the importance of isolating and testing all patients exposed to roommates with SARS-CoV-2, including those who have been discharged. The majority of exposed roommates tested positive after discharge, hence in the absence of active follow-up of exposed patients many nosocomial transmission events will be missed.”
2. de Oliveira et al., “Evolution of spray and aerosol from respiratory releases: theoretical estimates for insight on viral transmission,” Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, January 2021, link .
- This paper provides a description of and exploration into the physics of aerosol and droplet emission, evaporation, and settling. It considers the important dynamics of composition (respiratory droplets are not just pure water but contain proteins and salts that impact evaporation rates) in the context of relative humidity and gravity-induced settling.
- They found that “The time-of-flight to reach 2m (6.5 feet) is only a few seconds resulting in a viral dose above the minimum required for infection, implying that physical distancing in the absence of ventilation is not sufficient to provide safety for long exposure times.”
3. Mack et al., “Implementation and Evolution of Mitigation Measures, Testing, and Contact Tracing in the National Football League, August 9–November 21, 2020,” MMWR, January 2021, link .
- “Subsequent contact tracing identified multiple instances of transmission that likely occurred during <15 minutes of cumulative interaction within 1.8 meters (6 feet).” Some instances of transmission occurred with interactions less than 5 minutes.
- These findings led the National Football League to change their definition of “high risk contact” to go beyond the CDC’s definition of 15 minutes within 6 feet.
4. Kwon et al., “Evidence of Long-Distance Droplet Transmission of SARS-CoV-2 by Direct Air Flow in a Restaurant in Korea,” Journal of Korean Medical Science, November 2020, link .
- This study investigated a restaurant outbreak in South Korea, where the index case infected two other people after 5 minutes of exposure from a distance of 6.5 meters (21 feet) and 4.8 meters (15 feet).
- Researchers collected data from credit card records, closed-circuit television (CCTV) footage, cell phone location data as well as interviews. Nasopharyngeal specimens of cases and close contacts were also collected and tested using RT-PCR. The authors concluded that transmission can occur at a distance greater than 6 feet if there is direct air flow from an infected person.
5. Pringle et al., “Covid-19 in a Correctional Facility Employee Following Multiple Brief Exposures to Persons with Covid-19 – Vermont, July–August 2020,” MMWR Early Release, October 2020, link .
- Correctional officer contracted SARS-CoV-2 after multiple brief encounters with six asymptomatic incarcerated individuals. Each interaction lasted about 1 minute, totaling 17 minutes of exposure over an 8-hour shift.
- Infected inmates did not wear masks for some of the interactions, while the officer wore a microfiber cloth mask, gown, goggles and gloves, and maintained 6 feet the entire time.
- The correctional officer had no known contact with anyone else with Covid-19 and coronavirus cases were low in his home county and in the rest of the correctional facility at the time, leading researchers to conclude that his exposure most likely came from the brief encounters.
People infected with Covid-19 can remain infectious beyond 10 days after symptom onset.
6. Truong et al., “Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity,” medRxiv, March 2021, link .
- This study describes three patients with acute lymphoblastic leukemia who were persistently positive for SARS-CoV-2 by PCR. Specimens from all 3 patients were collected and used to detect SARS-CoV-2 RNA over the course of 6 months. Whole-genome sequencing and serological studies were performed to measure viral evolution and evidence of immune escape.
- Patient 1 is a previously healthy female under 5 years of age. Patient 2 is in the 20–25-year age range who was previously diagnosed with B-cell all six months prior to his SARS-CoV-2 positive test. Patient 3 is under 5 years of age who was diagnosed with high-risk B-cell all seven months prior to presentation to the ED with fever and confirmed positive for SARS-CoV-2 upon admission.
- Viral load in patient 1 was highest at day 0 and then gradually declined and was last reliably detected on day 46.
- Viral load in patient 2 remained consistent though day 172; SARS-CoV-2 was recovered by viral culture for up to day 144.
- Viral load in patient 3 also remained consistent through day 162 despite weak seroconversion on day 83, and finally tested negative on day 196.
7. Yan et al., “Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis, Frontiers of Public Health, March 2021, link .
- Respiratory tract specimens: 17.5 days pooled mean viral shedding time
- Upper respiratory tract specimens: 17.5 days pooled mean viral shedding time
- Stool specimens: 30.3 days pooled mean viral shedding time
8. Arnaout et al., “The Limit of Detection Matters: The Case for Benchmarking Severe Acute Respiratory Syndrome Coronavirus 2 Testing,” Clinical Infectious Diseases, February 2021, link .
- Study looked at 4774 patients’ viral loads and test limit of detection. “The diagnostic priorities in the COVID-19 pandemic are to robustly identify 3 populations: the infected, the infectious, and the susceptible. Our study addresses the first of these. Specifically, it illustrates the clinical and epidemiologic impact of assay LoD [limit of detection] on SAR-CoV-2 diagnosis and the challenges of interpreting and comparing molecular assay results across various platforms. First, viral loads vary widely among infected individuals, from individuals with extremely high viral loads (potential “superspreaders”) who presumably would be picked up by even the least sensitive assays, to those whose viral loads are near, at, or even below the LoD of many assays. Therefore, a substantial fraction of infected patients will be missed by less sensitive assays. Concerningly, some of these missed patients are, have been, or will become infectious, and such misses will undermine public health efforts and put patients and their contacts at risk. This must give pause in the rush to approve additional testing options and increase testing capacity and emphasizes the importance of defining infectivity as a function of viral load and other factors (e.g., time of exposure), which remains a critical unknown in this pandemic. The relative ability of different sampling techniques to obtain specimens with the highest viral loads may also substantially impact detection rate.”
9. Baang et al., “Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient,” The Journal of Infectious Diseases, January 2021, link .
- This study demonstrated ongoing replication of infectious SARS-CoV-2 for at least 119 days from an immunocompromised patient through viral cultures and sequencing analysis. “This case highlights challenges in managing immunocompromised hosts, who may act as persistent shedders and sources of transmission.”
10. Aydillo et al., “Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer,” New England Journal of Medicine,” December 2020, link .
- Researchers used cell cultures to detect viable virus in serially collected nasopharyngeal and sputum samples obtained from 20 immunocompromised patients who had Covid-19. Live virus was isolated in Vero cells and genetic variants were identified by whole-genome sequencing.
- Of the 20 patients, 15 were receiving active treatment or chemotherapy. 11 had severe Covid-19.
- A total of 78 samples were collected from the 20 patients. Viral RNA was detected for up to 78 days after symptom onset (interquartile range, 24 to 64 days). Viable virus was detected in 10 of 14 nasopharyngeal samples that were available from the first day of lab testing. Follow-up samples obtained from 5 patients grew virus in culture for 8, 17, 25, 26, and 61 days after symptom onset.
11. Li et al., “Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with Covid-19,” Emerging Microbes & Infections, December 2020, link .
- Researchers monitored the clinical characteristics and virological features of 38 patients with Covid-19 (long-term carriers) who recovered from the acute disease, but still shed viral RNA for over 3 months.
- The median carrying history of the long-term carriers was 92 days after the first admission, and the longest carrying history was 118 days.
- Infectious SARS-CoV-2 was isolated from the sputum, where high level viral RNA was found. All nine full-length genomes of samples obtained in March–April 2020 matched early viral clades circulating in January–February 2020, suggesting that these patients persistently carried SARS-CoV-2 and were not re-infected. IgM and IgG antibodies and neutralizing-antibody profiles were similar between long-term carriers and recovered patients with similar disease courses.
12. Avanzato et al., “Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer,” Cell, November 2020, link .
- Case study of a 71-year-old immunocompromised patient who was infected with Covid-19 for at least 105 days, and infectious for at least 70 days. The patient remained asymptomatic throughout the course of the infection.
- “Given that immunocompromised individuals could have prolonged shedding and may not have typical symptoms of Covid-19, symptom-based strategies for testing and discontinuing transmission-based precautions, as recommended by the CDC, may fail to detect whether certain individuals are shedding infectious virus.”
13. Fontana et al., “Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature,” Cambridge University Press, October 2020, link .
- Researchers analyzed 77 studies on SARS-CoV-2. All studies reported PCR-based assessments of viral shedding and 12 also included viral culture data.
- Among 28 studies, the overall pooled median duration of RNA shedding from respiratory sources was 18.4 days.
- When stratified by disease severity, the pooled median duration of viral RNA shedding from respiratory sources was 19.8 days among severely ill patients and 17.2 days in mild-to-moderate illness.
- Viable virus was isolated by culture from 6 to 20 days relative to symptom onset.
14. Jeong et al., “Viable SARS-CoV-2 in various specimens from Covid-19 patients,” Clinical Microbiology and Infection, July 2020, link .
- Researchers collected nasopharyngeal swabs, saliva, urine and stool samples from 5 Covid-19 patients. Samples were tested by PCR; positive samples were subjected to virus isolation in Vero cells. Urine and stool samples were used to intranasally inoculate ferrets and evaluated the virus titres in nasal washes on 2-, 4-, 6- and 8-days post infection.
- All samples were positive by PCR. Viable virus was recovered from nasopharyngeal swabs and saliva samples. Viable virus was also recovered from ferrets’ nasal washes.
- Authors conclude: “Viable SARS-CoV-2 virus was demonstrated in saliva, urine, and stool from COVID19 patients up until days 11 to 15 of the clinical course. This result suggests that viable SARS-CoV-2 can be secreted in various clinical samples as well as respiratory specimens.” In particular, note the timeframe here – patients may be infectious for longer than the 10 days the CDC says.
Optimal PPE, including, a powered air-purifying respirator, coveralls that are impervious to viral penetration, head and shoe coverings, and medical grade gloves, is important to protect nurses and other health care workers from exposure to SARS-CoV-2. At minimum, nurses and health care workers should have an N95 respirator, isolation gown, eye protection, and medical grade gloves when caring for a confirmed or possible Covid-19 patient.
15. Barros et al., “Effectiveness of Elastomeric Half-Mask Respirators vs N95 Filtering Facepiece Respirators During Simulated Resuscitation. A Nonrandomized Controlled Trial,” JAMA Network Open, March 2021, link .
- Nonrandomized controlled trial of clinicians and HCWs working on Covid-19 units at the University of Virginia. Participants had either been assigned an FFR (filtering facepiece respirator, N95) or an EHMR (elastomeric half-mask respirator), fit-tested. The final analysis included 100 participants.
- 0% of participants wearing EHMRs detected Bitrex
- 28.1% of participants wearing N95 FFRs detected Bitrex, at median 69 seconds (interquartile range 42 to 107 seconds). There was no association between type of N95 model (5 models were reported) and failure.
- “Our results suggest that FFR fit during CPR is poor and that EMHRs provide superior fit, confirming previous research. We chose to evaluate fit during CPR because it is highly aerosolizing, physically strenuous, and has been associated with occupational transmission. Strengths of our study include our real-world design and prospective data collection. Limitations include the lack of blinding or randomization and the use of participant-reported detection. The data suggest that the EMHR is more effective at preventing aerosol inhalation during strenuous clinical work and should be considered for preventing Covid-19 transmission.”
16. Baffoe-Bonnie et al., “Filtration evaluation of expired elastomeric P-100 filter cartridges after months of real-world use during the COVID-19 pandemic,” Infection Control & Hospital Epidemiology, March 2021, link .
- Cleaning protocol was to wipe down the outer casing of the filters with pre-moistened quarternary ammonium/isopropyl alcohol wipes between patients. Up to 10 times per day.
- At the end of a shift, the wiped filter pair was disassembled from the mask, which underwent further cleaning.
- Users reported no observed changes in breathability. No external damage to the filters was reported, except for wear and tear of cartridge label.
- Filtration efficiencies of elastomeric filters used for 5 months (respiratory therapist) and 6 months (progressive care unit), as well as the control (unused filters), were mostly >95% across particle sizes. The filtration efficiency of filters used for 6 months (intensive care unit) was 90-95% but not statistically significantly different from the control filters.
- “We caution that these findings may not be applicable to nonexpired filters, but intuitively suspect that the duration of use could be longer.”
17. Jung et al., “Fit-failure rate associated with simulated reuse and extended use of N95 respirators assessed by a quantitative fit test,” Infection Control & Hospital Epidemiology, January 2021, link .
- (reuse) repeat 1-hour donnings: all participants passed fit test at baseline. 60% participants failed fit test after 2 consecutive 1-hour donnings. 70% failed after 3 consecutive 1-hour donnings. 90% failed after 4 consecutive 1-hour donnings.
- (extended use) repeat 3-hour donning: all participants passed at baseline. 50% of participants failed fit test after the first 3-hour donning.70% of participants failed fit test after the second 3-hour donning.
- “These data suggest that the extended use or reuse of N95 respirators due to the shortage of N95 respirators and other practical issues should be done with caution, especially in high-risk settings such as those involving aerosol-generating procedures. Therefore, more short-term use or other strategies such as powered air-purifying respirators (PAPR) should be considered in these circumstances.”
- Study was performed with infection control practitioners- “The activity of healthcare workers caring for patients with COVID-19 may be greater than that of infection practitioners and thus associated with higher rates of fit failure.”
- Only 10 female Asian subjects. Only 1 model of N95
18. NIOSH – Filtering Facepiece Respirators with an Exhalation Valve: Measurements of Filtration Efficiency to Evaluate Their Potential for Source Control, December 2020, link .
- “The findings in this report are based on tests of 13 FFR models from 10 different manufacturers. These findings show that FFRs with an exhalation valve provide respiratory protection to the wearer and can also reduce particle emissions to levels similar to or better than those provided by surgical masks, procedure masks, or cloth face coverings.”
19. Khonyongwa et al., “Incidence and outcomes of healthcare-associated COVID-19 infections: significance of delayed diagnosis and correlation with staff absence,” The Journal of Hospital Infection, October 2020, link .
- Hospital in London identified hospital-acquired vs community-acquired Covid-19 in patients, from March 1 to April 18. Hospital-acquired Covid-19 was defined as: alternative etiology for symptoms upon admission, development of Covid symptoms more than 14 days after admission, or positive RNA respiratory sample more than 14 days after admission.
- All patients with symptoms matching Covid-19 were tested, all patients reviewed daily for symptoms. Patients were separated between Covid and non-Covid wards depending on test at admission, though sometimes when wards were full, “cohort” areas separated by curtains were established. Separate area for donning and doffing PPE was established for each ward. Staff wore FFP3 masks “as appropriate” (not defined).
- 7.1% of Covid patients admitted were classified as hospital-acquired and 3.7% as indeterminate (not positive on admission but before 14 days). Hospital-acquired Covid population was more likely to be more than 65 years, less likely to be Black Asian and Minority Ethnic (BAME), diabetes, CKD, malignancy, and others listed in the paper. There were no differences in outcomes.
- Self-reported staff sickness significantly correlated with weekly incidence of hospital acquired Covid cases.
- “Our results also suggest that 34.5% of all HA-COVID-19 and late indeterminate infections could be traced back to cases where the acquisition was from a community case, but an RNA based diagnosis could not be made within 48h of admission.”
20. Glasbey et al., “Elective Cancer Surgery in COVID-19–Free Surgical Pathways During the SARS-CoV-2 Pandemic: An International, Multicenter, Comparative Cohort Study,” Journal of Clinical Oncology, October 2020, link .
- International multicenter study of adults undergoing elective surgery for solid cancer types in areas impacted by the Covid-19 pandemic.
- Each patient was classified as undergoing surgery in Covid-free surgical pathway or with no defined pathway. Covid-free surgical pathway was if complete segregation in all 3 areas (OR, critical care, and inpatient ward) away from patients with Covid. Only patients without a positive test or CT scan at time of surgery were included in study. A total of 9,171 patients from 445 hospitals were included in the study.
- Patients who had surgery when OR, critical care, and inpatient units were separated between Covid and non-Covid patients had lower rates of complications, lower rates of postop SARS-CoV-2 infection, and lower 30-day mortality rates than patients care for in mixed ORs/critical care/inpatient units.
21. Liu et al., “Protecting Healthcare Workers Amid the Covid-19 Crisis: A Safety Protocol in Wuhan,” Frontiers in Public Health, October 2020, link .
- This study outlines successful safety measures that were adopted in an orthopedic department in Wuhan, China, in which none of the HCWs or their families contracted SARS-CoV-2.
- safety protection classification, including infection control measures and PPE including N95 respirator, eye protection, gown, gloves, and shoe and head coverings for patient care.
- reasonable working hours, including health care workers in quarantine area were limited to 3-hour shifts,
- ward protection, including splitting up the ward into three distinct sections for Covid quarantine, buffer, and clean zones with multiple measures to stop cross-contamination from occurring,
- operating room protection, and
- rest area protection
22. Ambrosch et al., “Effect of a strict hygiene bundle for the prevention of nosocomial transmission of SARS-CoV-2 in the hospital: a practical approach from the field,” Journal of Infection and Public Health, October 2020, link .
- Paper reports on a study conducted retrospectively at a hospital in Bavaria, Germany, March 1 to June 10 (905 bed hospital).
- Patients were tested for SARS-CoV-2 based on respiratory symptoms, fever, and other symptoms (including taste and smell disturbances) or contact with a Covid-19 case. If one of the criteria was identified, the patient was classified as potentially infectious and separated in an isolation area. All possible and confirmed Covid patients were placed in a separate isolation area and each patient was isolated in a single room or cohorted. Staff wore FFP2 respirators, safety goggles, protective gowns and gloves. Visitors were not allowed. Universal masking was introduced March 26. Patients were daily evaluated for symptoms and all suspected patients were transferred to the isolation ward for testing.
- Nosocomial infections defined as positive PCR test more than 6 days after hospitalization. 10 nosocomial cases were identified out of 5081 patients not considered suspected/confirmed at admission.
- After introducing daily screening of all inpatients and universal masking, nosocomial rate decreased almost 80%. It should be noted that the hospital only identified 5 nosocomial Covid cases before the introduction of these additional measures. The study did not screen for asymptomatic nosocomial cases
23. Maltezou et al., “Hospital factors associated with SARS-CoV-2 infection among healthcare personnel in Greece,” The Journal of Hospital Infection, October 2020, link .
- Report on Covid-19 infections among health care providers in 224 hospitals in Greece (126 are public, the rest are private or military). 13 tertiary care hospitals and 1 pediatric were designated as Covid-19 referral hospitals, 3 more were added later in the epidemic (total of 17 Covid hospitals).
- Confirmed patients requiring hospitalization were transferred to a Covid referral hospital. Health care providers caring for suspected or confirmed Covid patients wore FFP2 respirators, gloves, goggles or face shield, and gown resistant to fluid penetration. FFP3 was recommended for aerosol-generating procedures. Surgical masks were used during shortages.
- Health care providers who were exposed were tested upon development of symptoms though sometimes asymptomatic exposed HCPs were also tested. Health care providers included all staff in hospitals regardless of patient contact.
- From Feb 26 to May 3, 2020: 1,287 Covid-19 patients were hospitalized, and 158 health care providers were infected in public hospitals. Health care providers were statistically significantly more likely to be infected in non-Covid hospitals. Covid hospitals had significantly higher staffing, significantly more Covid patients.
24. Park et al., “Mass screening of healthcare personnel for SARS-CoV-2 in the Northern Emirates,” Journal of Hospital Infection, October 2020, link .
- Hospital in UAE invited healthcare personnel (HCP) to screen for SARS-CoV-2 three times between April 2 and May 14, regardless of symptoms. HCP were also tested if they developed suspicious symptoms or had close contact with Covid patients. Contact tracing followed a confirmed case in HCP.
- All staff wore masks from March 12, no visitors starting March 24, all patients admitted to negative pressure rooms first after PCR test for SARS-CoV-2 from April 13. Covid patients were transferred to Covid hospital immediately. Patient-facing staff wore N95s, face shields, gowns, and gloves until patients had 2 consecutive negative Covid tests.
- Screening found few cases among the non-support staff- indicating these infection control measures were effective.
- 98% of the cases identified were among support staff, who were much more likely to share accommodation and commuter vehicles. “As the travel ban and lockdown had been imposed since late March 2020 in the UAE, a high incidence of COVID-19 in support staff might be related to share accommodation and crowded commuter vehicles…. Most migrant workers in Gulf countries are confined to small rooms which are shared up to a dozen workers.”
25. Piapan et al., “Covid-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy,” The Journal of Hospital Infection, August 2020, link .
- Article describes health care worker infections in a region of Italy where health care workers accounted for 16.2% of all Covid infections in this province.
- Index patient without respiratory symptoms
- 2 patients not suspected to be Covid+ at first
- Community-acquired infection in HCW led to coworker infections
- Identifies (without significant detail) that asymptomatic cases and not recognizing Covid-positive quickly patients upon admission and health care worker meetings (which reportedly occurred with limited/no PPE) as key places of transmission in their hospitals.
26. Zhan et al., “Lesson learned from China regarding use of personal protective equipment,” American Journal of Infection Control, August 2020, link .
- Hospitals in Hubei Province adopted World Health Organization (WHO) PPE protocols for Ebola. The article details PPE and protocols around entering and exiting locked designated Covid-19 units. As a result of the level-3 protection protocols combined with admitting patients to only Covid-19 designated hospitals, the number of health care workers infected declined significantly.
27. Bhaskar and Arun, “SARS-CoV-2 Infection Among Community Health Workers in India Before and After Use of Face Shields,” JAMA, August 2020, link .
- Community health workers in India conducting home visits to follow up with asymptomatic family contacts of COVID+ patients
- 2 workers developed symptoms, prompting testing of all workers (62), 19% were positive
- No workers develop symptoms
- (note that the paper states that no workers tested positive, but they only were referred for testing if developed symptoms vs the first phase where all were tested)
- Face shields may have some added benefit, though unclear of what type (face shields are NOT respiratory protection, but could help reduce contact with eyes/mucous membranes/contamination of mask)
28. Tong et al., “Surveillance of SARS-CoV-2 infection among frontline health care workers in Wuhan during COVID-19 outbreak,” Immunity, Inflammation and Disease, August 2020, link .
- 222 health care workers traveled to Wuhan to support outbreak response
- 14-day quarantine upon return, throat swabs taken on day 3 and day 14 of quarantine, CT scans on day 14, antibody tests also conducted. All tests negative.
- Detailed donning and doffing PPE procedures/training
- PPE: N95 respirator, coverall gown, goggle/face shield, and gloves
- 4-6 hour shifts in contaminated area
- Psychological and nutritional support
29. Oksanen et al., “Healthcare workers’ high COVID-19 infection rate: the source of infections and potential for respirators and surgical masks to reduce occupational infections,” medRxiv, August 2020, link .
- Study reporting on healthcare worker exposures and infections at hospital in Finland
- 866 healthcare workers (majority nurses) filled out questionnaire on PPE use, exposure, etc. All participants with symptoms were tested- 4.7% were positive.
- 13 infections were confirmed from workplace exposure, 9 likely. 80% of exposure from patients happened in normal or cohorted ward. Lots of other data in the paper.
- Of HCW infections confirmed to result from workplace exposures, 0% reported wearing an FFP2/3 (N95 equivalent) respirator at time of exposure vs the remainder reported wearing a surgical mask (69.2%) or no mask (30.8%) at time of exposure.
- Same for HCW infections likely to result from workplace exposure, 0% reported wearing an FFP2/3 (N95 equivalent) respirator at time of exposure vs the remainder reported wearing a surgical mask (55.6%) or no mask (44.4%) at time of exposure.
30. Zhu and Zong, “Why did so few healthcare workers in China get COVID-19 infection,” QJM An International Journal of Medicine, August 2020, link .
- This article is a summary of the main tenets of the response in China that protected health care workers and led to few health care worker infections.
- universal masking of HCWs in healthcare settings at early stage.
- quick steps to secure supply of PPE and remedy shortage.
- higher standard of protection: “The main difference is that we used fluid-resistant protective clothing (coverall) with long sleeve and conjoined cap rather than uncapped isolation garment, as well as use respirators (i.e., N95 or European Union standard FFP2) rather than medical surgical masks, in wards dedicated for Covid-19 patients. A respirator, double rubber gloves, eye protection (i.e., goggles or a face shield), coverall and shoe covers were the standard equipment in contacting with Covid-19 patients in China."
- patients centralized at designated hospitals in China by national policy, hospitals had designated isolation wards.
- stringent lockdowns and community measures limited community transmission.
31. Nguyen et al., “Risk of Covid-19 among front-line health-care workers and the general community: a prospective cohort study,” The Lancet Public Health, July 2020, link .
- This study examined the risk of Covid-19 among healthcare workers compared to the general public as well as the effect of personal protective equipment (PPE) on risk. Researchers used the Covid Symptom Study app which asked daily questions about symptoms, testing, PPE, and exposures.
- They found that frontline healthcare workers with inadequate PPE caring for confirmed Covid-19 patients had 5.91x higher risk of a positive test when compared to healthcare workers with adequate PPE not caring for confirmed Covid-19 patients.
32. Xue, Ming et al., “Beyond the PPE shortage: Improperly fitting personal protective equipment and Covid-19 transmission among health care professionals,” Hospital Practice, July 2020, link .
- Report on three healthcare provider infections possibly caused by ill-fitting masks in a New Jersey hospital. Three healthcare providers worked on a COVID-19 inpatient floor, all three failed fit tests that were performed after the healthcare provider was tested or became symptomatic for COVID-19. The report does not trace conduct genetic sequencing but does underline the risks when hospitals do not conduct fit testing to ensure that N95 respirators properly fit staff.
33. Marago, Italo and Minen, Isa. “Hospital-Acquired Covid-19 Infection – The Magnitude of the Problem,” The Lancet Infectious Diseases, July 2020, link .
- This study examined the prevalence of hospital-acquired Covid-19 infection or nosocomial transmission to patients in England. Patients were divided into two groups (nosocomial vs community). Cases were nosocomially acquired if a patient developed symptoms 7 or more days after hospital admission. Cases were community-acquired if a patient developed symptoms 7 days before hospital admission.
- Researchers found that 16.2% of Covid-19 patients met the criteria for nosocomial infection, the majority of which occurred in “low-risk wards” (suspected and negative Covid-19 zones).
34. Houlihan et al., “Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers,” The Lancet, July 2020, link .
- This study examined a prospective cohort study of 200 frontline healthcare workers to evaluate risks.
- Researchers found that 44% of frontline healthcare workers had evidence of SARS-CoV-2 infection either by RT-PCR or serology in London. Evidence of infection among healthcare workers was more than double that of the London population, which highlights the importance of implementing policies to better protect healthcare workers.
35. Chen et al., “To Protect Healthcare Wokers Better, To Save More Lives with Covid-19,” Anesthesia & Analgesia,” July 2020, link .
- First stage: It was unknown that a virus was the cause of the pneumonia cluster and healthcare workers were not protected. At this time, the infection rate ranged from 3.5% to 29% among healthcare workers in different hospitals in the epicenter of Wuhan.
- Second stage: Health care workers had inadequate protection due to supply shortages. During this stage, the number of confirmed cases in China was still increasing rapidly. By February 11, 2020, a total of 1,716 health care workers were confirmed with COVID-19, including five deaths.
- Third stage: disease severity acknowledged, the novel coronavirus had been identified, supply shortages were rapidly fixed thru increased manufacturing, and healthcare workers were fully protection. The highest level of precaution, so called “full precaution,” is mandatory for high-risk exposure, included a disposable surgical cap, test-fit N95 masks or respirators, gloves, goggles or face shield, gown and fluid-resistant shoe covers. “We would like to point out that the key element of full precaution is the complete coverage of the head and facial skin, which does not necessarily mean a conjoined hood or even powered air-purifying respirator system as previously reported.” No healthcare worker infections reported in this period.
36. Hou et al., “Personnel protection strategy for healthcare workers in Wuhan during the COVID-19 epidemic,” Precision Clinical Medicine, July 2020, link .
- Serological testing of healthcare workers deployed to Wuhan to assist during surge.
- Zero of the healthcare workers deployed to Wuhan tested positive by serology after the end of their deployment. Compared to 3.4% of local Wuhan healthcare workers in isolation areas and 5.4% of local Wuhan healthcare workers in non-isolation areas.
- This study reports on PPE only and the results are indicative (healthcare workers with level 2/3 PPE had much lower infection rates- this level of PPE includes N95 FFR, disposable caps, isolation gowns (fluid repellant), goggles and face shield, shoe covers). But we know that there were other controls in place that could have impacted these rates including designated “fever hospitals” or units, designated dorms for the assisting healthcare workers, shorter shifts in isolation wards in some hospitals, etc.
37. Touati et al., “Prevention of nosocomial transmission of SARS-CoV-2 using pre-operative chest CT: a monocentric study during the outbreak,” The Journal of Hospital Infection, July 2020, link .
- Reported on implementation of screening all patients before procedures using RT-PCR and chest CT at hospital in France. In addition to other preventive measures at the facility (social distancing, handwashing, PPE included gloves, goggles, face shields and masks, screening using temperature).
- Patients screened on the day before their procedure was scheduled if it could not be delayed. This led to a 7.5% change in the way that patients were handled (details not provided).
- Mean infection rate of staff decreased before vs after implementation of pre-op patient screening.
38. Chalikonda et al., “Implementation of an Elastomeric Mask Program as a Strategy to Eliminate Disposable N95 Mask Use and Resterilization: Results from a Large Academic Medical Center,” Journal of the American College of Surgeons, June 2020, link .
- This article describes the results from a Pennsylvania hospital system’s widespread implementation of elastomeric and powered air purifying respirators (PAPR) program to alleviate the issues with N95 reusage and resterilization. Researchers reported on their phased-in process, training, education, and fit testing. After conducting a conservative cost analysis, they found that implementation of the elastomeric and PAPR program was 10x cheaper per month than an N95 reuse and decontamination program.
39. Degesys et al., “Correlation Between N95 Extended Use and Reuse and Fit Failure in an Emergency Department,” JAMA, June 2020, link .
- This study examined the prevalence of N95 fit test failure while reusing 2 common types of N95 respirators at the University of California, San Francisco.
- Researchers found thatN95s worn for more hours were more likely to fail fit testing (p<0.05), N95s used for more shifts were more likely to fail fit testing (p<0.001), andN95s donned and doffed more times were more likely to fail fit testing (p<0.001).
40. Jung, Jiwon et al., “Contamination of personal protective equipment by SARS-CoV-2 during routine care of patients with mild Covid-19,” Journal of Infection, June 2020, link .
- Healthcare workers wore N95 or PAPR, face shield, double gloves, boots, coveralls for 4 hours while providing patient care to patients with mild Covid-19 (no aerosol-generating procedures performed)
- 26% top of head
- 26% top of foot
- 16% sole of foot
- “Therefore, our data support the wearing of PPE that fully cover the head and feet, as well as cautious doffing procedures."
41. Kim et al., “How South Korea Responded to the Covid-19 Outbreak in Daegu,” NEJM Catalyst, June 2020, link .
- This article described how health officials in Daegu, South Korea adopted a comprehensive package or interventions that relieved shortages, concentrated resources, isolated cases, and protected health care workers.
- Four-category risk stratification system (asymptomatic to mild, moderate, severe and critical)
- Rapid expansion of beds for isolation with use of portable negative pressure machines
- Recruitment of health care workers for both public health and medical responses. Shift lengths were closely monitored to minimize health care worker fatigue and infection risks.
- Strict screening procedures at hospital entrances were implemented. Hospital wards, emergency departments and screening clinics were organized to screen, triage and separate suspected and confirmed cases of Covid-19 in order to avoid nosocomial infections and cross-contamination.
- Universal mask policy and comprehensive use of PPE (N95 respirators, eye protection, shoe covers and coveralls). ICU hospital staff wore powered air-purifying respirators, shoe covers and coveralls.
- Screening and exposure management of hospital staff, regardless of symptoms. Health care workers were asked to self-quarantine if they had close contact with a Covid-19 patient without proper PPE, if they had traveled internationally, or if they were part of a specific religious group known to have a high incidence of infection. On the 13th day of quarantine after exposure, health care workers were tested and could return to work only on day 15 after testing negative.
42. Feldman et al., “Exposure to a Surrogate Measure of Contamination from Simulated Patients by Emergency Department Personnel Wearing Personal Protective Equipment,” JAMA, April 2020, link .
- This study assessed the protection of health care workers wearing the recommended PPE (N95 respirators, eye protection, isolation gowns, and gloves) while caring for a simulated patient with respiratory distress.
- Participating health care workers performed care tasks commonly required by patients with Covid-19 (e.g., airway management and ventilatory support) in a simulation. A nonvisible fluorescent compound as a marker of contamination was applied on predetermined surface areas (around the nose and mouth, palms, and upper chest) of the manikin and was added to the simulated secretion areas.
- Despite personal protective equipment, 7 of 8 participants had contamination (fluorescent markers) on their exposed skin. All participants had contamination in their hair; half had contamination on their shoes.
43. Wang, Xinghuan et al., “Association between 2019-nCoV transmission and N95 respirator use,” The Journal of Hospital Infection, March 2020, link .
- This study examined the infection rate in two groups of departments. Three departments were in the “mask group” because they utilized N95 respirators and also frequently performed hand hygiene (respiratory, ICU, and Infectious Disease). Three departments were in the “non-mask group” because early in the outbreak they hadn’t implemented precautions- staff did not wear masks and disinfected and cleaned hands “occasionally.”
- There were significantly more confirmed or probable Covid-19 patients cared for in the departments in the “mask group,” meaning workers in those units had significantly more exposure than the “non-mask group.” “Mask group” reported statistically significantly fewer infections than the “non-mask group.” Mask group actually reported 0 infections. They found similar results in two other hospitals- staff wearing N95s and frequently conducting hand hygiene were not infected.
Separate and dedicated Covid-19 units, including no mixed assignments, are important elements of an infection control plan.
44. Liu et al., “Protecting Healthcare Workers Amid the Covid-19 Crisis: A Safety Protocol in Wuhan,” Frontiers in Public Health, October 2020, link .
45. Glasbey et al., “Elective Cancer Surgery in COVID-19–Free Surgical Pathways During the SARS-CoV-2 Pandemic: An International, Multicenter, Comparative Cohort Study,” Journal of Clinical Oncology, October 2020, link .
46. Wang et al., “Environmental virus surveillance in the isolation ward of COVID-19,” The Journal of Hospital Infection, April 2020, link .
- This study evaluated the sites of environmental contamination on a Covid-19 isolation ward. The isolation ward was divided into three zones (clean zone, contaminated zone, and semi-contaminated zone) with two access points for patients and medical staff, respectively. Viral nucleic acid tests were performed on each ward. Health care workers took strict personal protective equipment and disinfection before entering and leaving the contaminated zone.
- Nucleic acid detection was positive in six (7.1%) locations. In the semi-contaminated zone, three sites had positive SARS-CoV-2, including the surface of the drawer in the nurse station, washstand and the drawer in the treatment room. In the contaminated zone, no sites in the patient room tested positive but one PDA was positive for the virus. In the clean zone, two sites had positive virus detection, namely the surface of the telephone receiver in the physician office and a push button of the water-free hand sanitizer.
All patients should be screened for Covid-19, considering diagnostic testing results, signs and symptoms, and recent exposure history.
47. Burns et al., “Casting the net wide: Universal testing of emergency admissions for SARS-CoV-2 to prevent onward transmission,” The Journal of Hospital Infection, November 2020, link .
- Report from 1 hospital in Ireland. ED admissions with suspected Covid are assigned to Covid pathway with infection prevention precautions. Where Covid is not suspected (no symptoms or contact), a non-covid pathway is assigned with multiple occupancy (unless other infection prevention needs exist).
- Daily onsite testing began March 16 using PCR tests, including 1 rapid test. Universal admission testing began June 1. Contacts of Covid cases identified among patients and staff were tested on day 0 and 7 and staff were advised to remain off work 14 days and patients were isolated or cohorted with other contacts for 14 days.
- 3,393 PCR tests performed June 1 o Sept 30 on non-suspected Covid patients. 9 were positive (0.26%).
- After implementation of universal admission testing, number of staff contacts was reduced, and number of patient contacts was reduced.
48. Khonyongwa et al., “Incidence and outcomes of healthcare-associated COVID-19 infections: significance of delayed diagnosis and correlation with staff absence,” The Journal of Hospital Infection, October 2020, link .
49. Piapan et al., “Covid-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy,” The Journal of Hospital Infection, August 2020, link .
50. Touati et al., “Prevention of nosocomial transmission of SARS-CoV-2 using pre-operative chest CT: a monocentric study during the outbreak,” The Journal of Hospital Infection, July 2020, link .
Contact tracing and testing are essential to effective Covid-19 infection control plans.
51. Chin et al., “Frequency of Routine Testing for Coronavirus Disease 2019 (Covid-19) in High-risk Healthcare Environments to Reduce Outbreaks,” Clinical Infectious Diseases, October 2020, link .
- Modelling study that estimates the impact of frequent surveillance testing of health care workers on health care transmission. It is a basic model that gives us an estimation of the impact.
- In an ideal case with zero delay in test results and perfect sensitivity of test, daily testing of health care workers reduced R by 98.9%.
- Longer test result delays of 3 to 5 days reduced daily testing impact from 85.3% to 56.5% and 25.9%, respectively, reduction in R.
- Optimal testing frequency was dependent on baseline R. Higher the R, the more frequent testing would need to occur to be effective.
- Assuming other measures are implemented, and R is 1.5, testing weekly would suffice to bring R below 1.
52. Rivett L, et al., “Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in Covid-19 transmission,” eLife, May 2020, link .
- “Significant differences exist in the availability of healthcare worker (HCW) SARS-CoV-2 testing between countries, and existing programmes focus on screening symptomatic rather than asymptomatic staff. Over a 3-week period (April 2020), 1,032 asymptomatic HCWs were screened for SARS-CoV-2 in a large UK teaching hospital. Symptomatic staff and symptomatic household contacts were additionally tested. Real-time RT-PCR was used to detect viral RNA from a throat + nose self-swab. 3% of HCWs in the asymptomatic screening group tested positive for SARS-CoV-2. 17/30 (57%) were truly asymptomatic/pauci-symptomatic. 12/30 (40%) had experienced symptoms compatible with coronavirus disease 2019 (COVID-19) >7 days prior to testing, most self-isolating, returning well. Clusters of HCW infection were discovered on two independent wards. Viral genome sequencing showed that the majority of HCWs had the dominant lineage B∙1. Our data demonstrates the utility of comprehensive screening of HCWs with minimal or no symptoms. This approach will be critical for protecting patients and hospital staff.”
53. Scwierzeck V, König JC, Kühn J, et al. 2020. “First Reported Nosocomial Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a Pediatric Dialysis Unit,” Clinical Infectious Diseases, April 2020, link .
- “Person-to-person transmission was at the heart of a hospital outbreak of SARS-CoV-2 between healthcare workers (HCWs) and patients in the pediatric dialysis unit at the UHM. Semi quantitative real-time RT-PCR results suggest that individuals with high viral load pose a risk to spread SARS-CoV-2 in the hospital setting. Our epidemiological observation highlights the need to develop strategies to trace and monitor SARS-CoV-2 infected HCWs in order to prevent COVID-19 outbreaks in the hospital setting.”
Surgical and cloth masks do not provide respiratory protection to the wearer. A minimum of N95 respirators is necessary to protect nurses and health care workers caring for patients with confirmed or possible Covid-19. Surgical and cloth masks may be useful for source control as they are intended to protect others from the wearer’s large respiratory droplet emissions. However, unlike N95 respirators, surgical masks are not required to be fit tested, which means that air (potentially carrying infectious particles) can leak around the edges of the mask. Surgical and cloth masks are also not made of materials that are good filters. In contrast, N95s and other respirators are subjected to rigorous filter certification by the U.S. National Institute for Occupational Safety and Health (NIOSH), which ensures they filter at least 95% of very small airborne particles or higher.
54. MacIntyre CR, et al., “A Cluster Randomised Trial of Cloth Masks Compared with Medical Masks in Healthcare Workers,” BMJ Open, March 2015, link .
- This study compared the efficacy of cloth masks to medical masks among healthcare workers in 14 hospitals in Vietnam. A total of 1,607 participants were randomized by ward into three arms: (1) medical masks at all times on their work shift; (2) cloth masks at all times on shift or (3) control arm (standard practice, which may or may not include mask use). The control arm was ‘standard practice’, which comprised mask use in a high proportion of participants.
- There were three primary end points: 1) Clinical respiratory illness; 2) influenza-like illness (ILI), defined as fever ≥38°C plus one respiratory symptom and (3) laboratory-confirmed viral respiratory infection.
- The rates of all infection outcomes were highest in the cloth mask arm, with the rate of influenza-like illness (ILI) statistically significantly higher in the cloth mask arm (relative risk (RR)=13.00, 95% CI 1.69 to 100.07) compared with the medical mask arm. Cloth masks also had significantly higher rates of ILI compared with the control arm.
- Penetration of cloth masks by particles was almost 97% and medical masks 44%. Cloth masks resulted in significantly higher rates of infection than medical masks, and also performed worse than the control arm.
55. “Covid-19 shortages of masks and the use of cloth masks as a last resort,” – a follow-up by the authors to the above article, published on March 30, 2020, link .
- “We recommend that health workers should not work during the Covid-19 pandemic without respiratory protection as a matter of work health and safety. In addition, if health workers get infected, high rates of staff absenteeism from illness may also affect health system capacity to respond...For Covid-19, wearing a mask is not enough to protect health care workers – use of gloves and goggles are also required as a minimum, as SARS-CoV-2 may infect not through the respiratory route, but also through contact with contaminated surfaces and self-contamination.”
56. Rengasamy et al., “Simple Respiratory Protection—Evaluation of the Filtration Performance of Cloth Masks and Common Fabric Materials Against 20–1000 nm Size Particles,” The Annals of Occupational Hygiene, June 2010, link .
- Household fabric materials and cloth masks were challenged with polydisperse as well as monodisperse particles in the 20–1000 nm size range, which include the size of many viruses and initial penetration levels measured and compared with those values obtained for N95 respirator filter media.
- Researchers found that cloth masks and other fabric materials tested in the study had 40–90% instantaneous penetration levels against polydisperse NaCl aerosols employed in the NIOSH particulate respirator test protocol at 5.5 cm s−1. Similarly, varying levels of penetrations (9–98%) were obtained for different size monodisperse NaCl aerosol particles in the 20–1000 nm range.
- Results obtained in the study show that common fabric materials may provide marginal protection against nanoparticles including those in the size ranges of virus-containing particles in exhaled breath.
57. Rengasamy et al., “Filtration Performance of FDA-Cleared Surgical Masks,” Journal of the International Society for Respiratory Protection, July 2009, link .
- This study investigated the filtration performance of surgical masks for a wide size range of submicron particles including the sizes of many viruses. Five models of FDA-cleared surgical masks were tested for room air particle penetrations at constant and cyclic flow conditions.
- Researchers found that many surgical masks are made of materials ineffective at filtration, with some models letting through up to 88% of particles.
58. Oberg, Tara and Lisa Brosseau, “Surgical mask filter and fit performance,” American Journal of Infection Control, May 2008, link .
- This study evaluated the filter performance and facial fit of a sample of surgical masks. Filter penetration was measured for at least 3 replicates of 9 surgical masks using monodisperse latex sphere aerosols (0.895, 2.0, and 3.1 μm) at 6 L/min and 0.075-μm sodium chloride particles at 84 L/min. Facial fit was measured on 20 subjects for the 5 masks with lowest particle penetration, using both qualitative and quantitative fit tests.
- All 20 subjects failed the unassisted qualitative fit test on the first exercise (normal breathing). Eighteen subjects failed the assisted qualitative fit tests; 60% failed on the first exercise. Quantitative fit factors ranged from 2.5 to 9.6.
- None of the surgical masks exhibited adequate filter performance and facial fit characteristics to be considered respiratory protection devices.
Children and Covid-19
Children can get infected with SARS-CoV-2 and are infected at approximately the same rate as adults. They can also transmit SARS-CoV-2 and are linked in transmission chains with household members and with other children outside their households. Many children, however, may be asymptomatic or too mildly infected to register as positive on some diagnostic tests, thus limiting case detection of children. While the clinical course for children is milder than adults, kids do transmit the virus to their teachers, parents, and grandparents with predictable consequences.
Children can also develop long-term health complications, known as long Covid or long haulers, even among those with mild or no symptoms. The implications of long Covid are still unknown. Following a Covid-19 infection, children have also developed a serious and life-threatening condition called Multisystem Inflammatory Syndrome in Children (MIS-C). It’s also worth noting that some kids have underlying conditions and are disproportionately impacted.
1. Truong et al., “Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity,” medRxiv, March 2021, link .
2. Buonsenso et al., “Preliminary Evidence on Long COVID in children,” medRxiv, January 2021, link .
- Researchers in Italy tracked 129 children diagnosed with Covid-19 between March and November 2020 (mean age of 11 ± 4.4 years, 62 (48.1%) female).
- More than half reported at least one persisting symptom even after 120 days since Covid-19, with 42.6% being impaired by these symptoms during daily activities. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems and palpitations were particularly frequent, as also described in adults.
3. Mehta et al., “SARS-CoV-2 (COVID-19): What Do We Know About Children? A Systematic Review,” Clinical Infectious Diseases, December 2020, link .
- Researchers conducted a systematic review and narrative synthesis of all literature relating to SARS-CoV-2 in pediatric populations. A total of 24 studies relating to Covid-19 were included in the review.
- They found that children are infected at approximately the same rate as adults. Though many children may be asymptomatic or too mildly infected to register as positive on some diagnostic tests, thus limiting case detection of children.
4. Laws et al., “Symptoms and Transmission of SARS-CoV-2 Among Children — Utah and Wisconsin, March–May 2020,” Pediatrics, December 2020, link .
- Researchers conducted a household transmission investigation during March to May in Milwaukee, Wisconsin, and Salt Lake City, Utah. The study enrolled individuals with Covid-19 and their household contacts, assessed daily symptoms prospectively for 14 days, and obtained specimens for SARS-CoV-2 PCR and serology testing. Among 58 households, 188 contacts were enrolled (120 adults; 68 children).
- Nineteen of the 68 pediatric contacts (28%) tested positive for SARS-CoV-2, while 36 of the 120 adult contacts (30%) tested positive, including 18 of 65 (28%) in homes with children and 18 of 55 (33%) without them.
- Covid-19 transmission from children to adults may have occurred in 2 of 10 households (20%) with susceptible household members, and children may have spread the virus to other children in 1 of 6 homes with potential child-to-child spread (17%).
5. Sharma et al., “Reversible Myocardial Injury Associated With SARS-CoV-2 in an Infant,” Journals of the American College of Cardiology, December 2020, link .
- Case report of a reversible myocardial injury and acute decompensated heart failure associated with documented SARS-CoV-2 infection in a 2-month-old infant. The patient presented with an episode of choking and cyanosis after feeding. There was no history of fever, cough, upper respiratory tract infection symptoms, diarrhea, vomiting, or decreased oral intake prior to the initial presentation.
- The 2-month-old patient initially tested negative for SARS-CoV-2, but repeat PCR was positive. He was infected by a visiting family member the week prior to presentation.
6. King et al., “Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta,” Canadian Medical Association Journal, November 2020, link .
- Of 2,463 children tested, 1,987 had a positive result and 476 had a negative result.
- Of children with a positive test result for SARS-CoV-2, 714 (35.9%) were asymptomatic. The symptoms most strongly associated with a positive test were fever (25.5%), cough (24.5%), and runny nose (19.3%).
- Cough and runny nose were also common in SARS-CoV-2–negative patients and were not predictive of a positive result; neither were sore throat, nasal congestion, or diarrhea. Loss of taste or smell, however, was highly predictive, with a likelihood of test positivity more than seven times higher than that for patients without this symptom. Other symptoms predictive of a positive result included nausea and vomiting, headache, sneezing, and fever.
- When the researchers combined loss of taste/smell, nausea/vomiting, and headache symptoms, the overall likelihood was highly predictive of a positive result, but the infrequency of loss of taste/smell in children may limit its predictive value.
7. García-Salido et al., “Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain,” Critical Care, November 2020, link .
- MIS-C seems to be the most frequent presentation among critically ill children with SARS-CoV-2 infection. MIS-C patients are older and usually healthy. They show a higher prevalence of gastrointestinal symptoms and shock and are more likely to receive vasoactive drugs and immunomodulators and less likely to need mechanical ventilation than non-MIS-C patients.
- Sixty-one percent met MIS-C definition. MIS-C patients were older than non-MIS-C patients: 9.4 years vs 3.4 years. A higher proportion of them had no previous medical history of interest (88.2% vs 51.7%). Non-MIS-C patients presented more frequently with respiratory distress (60.7% vs 13.3%). MIS-C patients showed higher prevalence of fever (95.6% vs 64.3%), diarrhea (66.7% vs 11.5%), vomits (71.1% vs 23.1%), fatigue (65.9% vs 36%), shock (84.4% vs 13.8%) and cardiac dysfunction (53.3% vs 10.3%).
8. Slaats et al., “Case report of a neonate with high viral SARSCoV-2 loads and long-term virus shedding,” Journal of Infection and Public Health, October 2020, link .
- Case report of a 7-day-old neonate who was hospitalized in the Netherlands with Covid-19. The patient had high SARS-CoV-2 viral load and prolonged shedding. Monitoring the course of this infection showed that SARS-CoV-2 RNA was detectable in the nasopharynx until day 19 and in stool until day 42 after symptom onset.
9. Ahmed et al., “Multisystem inflammatory syndrome in children: A systematic review,” EClinicalMedicine, September 2020, link .
- Of the 662 patients, almost 90% underwent an echocardiogram due to significant cardiac manifestation of the disease.
- Researchers note that some children will need lifelong monitoring and interventions due to the extent of cardiac damage.
10. Park YJ et al., “Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020,” Emerging Infectious Diseases, July 2020, link .
- Researchers identified 5,706 people who were the first to report Covid-19 symptoms in their households between January 20 and March 27, 2020, when schools were closed, and then traced the 59,073 contacts of these “index cases.” They tested all of the household contacts of each patient, regardless of symptoms, but only tested symptomatic contacts outside the household.
- Children between 10 and 19 years can transmit the virus as effectively as adults.
- Children < 9 years were about half as likely as adults to transmit. Note that fewer than 30 positive cases were included in this age group. It is possible that children in this age group may have had fewer contacts due to stay-at-home orders in the beginning of the pandemic but not because they’re less likely to transmit.
11. Heald-Sargent et al., “Age-Related Differences in Nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Levels in Patients with Mild to Moderate Coronavirus Disease 2019 (COVID-19),” JAMA Pediatrics, July 2020, link .
- Researchers examined real-time PCR cycle threshold (CT) values from nasopharyngeal swabs from 145 patients Covid-19, within 1 week of symptom onset. They compared three groups: a) young children younger than 5 years, b) older children aged 5 to 17 years, and c) adults aged 18 to 65 years.
- They found that young children had significantly lower median (interquartile range) CT values, indicating that young children have equivalent or more viral nucleic acid in their upper respiratory tract compared with older children and adults. Children < 5 years had 10 to 100 times more SARS-CoV-2 viral RNA in their upper respiratory tract than older kids and adults.
Post-Acute Sequelae of SARS-CoV-2 infection (PASC) or Long Covid is a new syndrome and is not yet fully characterized. It is not predicated on the severity of symptoms or disease. Long Covid can also occur among previously healthy individuals, across all age groups, including children. Reported symptoms range from severe fatigue, cognitive dysfunction, gastrointestinal and musculoskeletal conditions to pulmonary and cardiovascular diseases.
1. Whitaker et al., “Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people,” Imperial College of London, July 2021, link .
- Researchers from Imperial College of London estimated symptom prevalence and investigated co-occurrence of symptoms among 508,707 participants reporting symptoms lasting 12 weeks or more following suspected or confirmed Covid-19. Data from random community-based samples of the population in England were analyzed, collected between September 2020 and February 2021.
- Group 1 – respiratory symptoms such as shortness of breath, tight chest and chest pain were the most common, with more people reporting that they had severe symptoms.
- Group 2 – fatigue-related symptoms such as tiredness with muscle aches and difficulty sleeping were the most common.
- The prevalence of persistent symptoms was 1.5 times higher in women than men.
2. Vanichkachorn et al., “Post COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at the Mayo Clinic and Characteristics of the Initial Patient Cohort,” Mayo Clinic Proceedings, May 2021, link .
- This study reported on the first 100 patients who were evaluated and treated in Mayo Clinic’s Covid-19 Activity Rehabilitation program (CARP) between June 1 and December 31, 2020. Patients were evaluated a mean of 93 days after initial infection.
- Of the 100 patients in the study, 80% reported unusual fatigue, 59% had respiratory and neurologic complaints. More than one-third reported difficulties with performing basic daily living activities; only 1 in 3 had returned to unrestricted work activity. Most patients had no preexisting comorbidities prior to SARS-CoV-2 infection, and many did not experience symptoms that were severe enough to require hospitalizations.
3. Taquet et al., “6-month neurological and psychiatric outcomes in 236,379 survivors of Covid-19: a retrospective cohort study using electronic health records,” The Lancet Psychiatry, May 2021, link .
- Researchers analyzed electronic health records of 81 million U.S. patients (both insured and uninsured patients). They compared a cohort of Covid survivors to matched control cohorts (1 – patients diagnosed with influenza; 2 – patients diagnosed with respiratory tract infections). The Covid cohort was divided into subgroups of hospitalized and non-hospitalized patients.
- Among 236,379 patients who had been diagnosed with Covid-19, 1 in 3 patients experienced a psychiatric or neurological illness in 6 months following a Covid diagnosis, with 12.8% receiving their first such diagnosis. Most diagnostic categories were more common in patients who had Covid-19 than in those who had influenza and those who had other respiratory tract infections.
4. Havervall et al., “Symptoms and Functional Impairment Assessment 8 Months After Mild Covid-19 Among Health Care Workers,” JAMA, April 2021, link .
- Researchers investigated Covid related long-term symptoms in relatively young, healthy health care workers in Sweden from April 2020 to January 2021. They collected blood samples every 4 months and administered questionnaires.
- 11% of those with mild Covid-19 had at least one debilitating symptom that lasted for at least 8 months, compared to 2% of the seronegative group.
- 15% reported their long-term symptoms (lasting at least 8 months) moderately to markedly disrupted their social life, compared with 6% of the seronegative participants.
5. Al-Aly, Z., Xie, Y. & Bowe, “High-dimensional characterization of post-acute sequalae of Covid-19,” Nature, April 2021, link .
- This study analyzed over 73,000 Veterans Health Administration users who had Covid-19 and nearly 5 million VHA users who did not have Covid and were not hospitalized.
- Researchers found that Covid survivors had higher risk for death and health care utilization beyond 30 days after illness. They also found that individuals who had Covid and were not hospitalized reported excess negative health impacts over at least 6 months and that affected nearly every organ and regulatory system in the body.
- They also found increased incident use of opioids and non-opioids, antidepressants, anxiolytics, antihypertensives, and oral hypoglycemics and evidence of laboratory abnormalities in multiple organ systems.
6. Davis et al., “Characterizing Long Covid in an International Cohort: 7 Months of Symptoms and Their Impact,” medRxiv, April 2021, link .
- Researchers analyzed responses from 3,762 participants with confirmed or suspected Covid-19, from 56 countries, with illness duration of at least 28 days.
- Of the 3,762 participants, 96% reported symptoms beyond 90 days. 45% reported requiring a reduced work schedule and 22% were not working at the time of survey due to health conditions.
- A total of 205 symptoms in 10 organ systems was identified, with 66 symptoms traced over seven months. Most commonly reported symptoms were fatigue, shortness of breath, headaches, dry cough, chest tightness and muscle aches.
7. Gaber et al., “Persistent post-covid symptoms in healthcare workers,” Occupational Medicine, April 2021, link .
- Researchers investigated the long-term impact of Covid-19 in health care workers. Seropositivity for SARS-CoV-2 antibodies was evaluated for 3,759 health care workers in an English teaching hospital two months following the peak of the April 2020 wave. A questionnaire was sent electronically, which included questions about the respondents' demographics, acute symptoms and hospitalization, method of confirmation of the diagnosis, persistent symptoms, and their severity and if they sought medical help or had sick leave.
- 45% of 138 health care workers who responded to the questionnaire reported persistent symptoms, with 32% struggling to cope 3–4 months following the peak of the wave.
- Moderate-to-severe fatigue was the most disabling symptom (39%); mild-to-moderate shortness of breath, anxiety and sleep disturbance were almost universal.
- Only 16% consulted their general practitioner about their symptoms with only 2% taking sick leave after recovering from the acute illness.
8. Johansson et al., “Long-Haul Post-Covid-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience,” JACC Case Reports, March 2021, link .
- This case report presented the first case series of three Swedish patients diagnosed with postural orthostatic tachycardia syndrome (POTS) more than 3 months after initial Covid-19 infection. All three patients experienced flu-like symptoms; 2 out of the 3 patients tested negative for Covid.
- Symptoms suggestive of POTS included persistent fatigue, headache, palpitations, dizziness, brain fog, or exercise intolerance during recovery from Covid-19.
9. Huang et al., “COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler: Looking for Clarity in the Haze of the Pandemic,” medRxiv, March 2021, link .
- This study analyzed electronic medical records of 1,407 individuals in California who tested positive for Covid-19 and evaluated symptoms at presentation (days 0 – 10 following a Covid diagnosis) and at 61+ days.
- Researchers found that 27% reported persistent symptoms more than 60 days after initial infection. Five symptom clusters at day 61+ were identified: chest pain-cough, dyspnea-cough, anxiety-tachycardia, abdominal pain nausea, and low back pain-joint pain.
- Nearly one third of the patients were asymptomatic during their initial infection through the 10 days after they tested positive.
10. Nalbandian et al., “Post-acute Covid-19 syndrome,” Nature Medicine, March 2021, link .
- This study comprehensively reviewed the current literature on post-acute Covid-19, its pathophysiology, and its organ-specific sequelae. They found that symptoms range from severe fatigue, cognitive dysfunction, gastrointestinal and musculoskeletal disorders to pulmonary and cardiovascular diseases.
11. Salmon-Ceron et al., “Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: A cross-sectional study,” Journal of Infection, February 2021, link .
- This study described the clinical, biological, and imaging profile of patients with initial mild to moderate Covid-19 infections.
- Of the 70 patients included in the study, 54.3% patients had symptoms that persisted from the 1st episode, 50% that disappeared and reappeared and 75.7% presented new symptoms that were absent during the 1st episode appeared. Most commonly reported symptoms were fever, anosmia, headaches and asthenia.
12. Taquet et al., “Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62,354 Covid-19 cases in the USA,” The Lancet Psychiatry, February 2021, link .
- This study assessed whether a diagnosis of Covid-19 was associated with increased rates of subsequent psychiatric diagnoses, and whether patients with a history of psychiatric illness are at a higher risk of being diagnosed with Covid-19. Researchers looked at electronic health records in 54 health care organizations in the United States; a total of 62,354 Covid patients were included between January and August 2020.
- They found that Covid survivors have significantly higher rates of psychiatric diagnoses and psychiatric history is a potential risk factor for being diagnosed with Covid-19, independent of known physical risk factors.
13. Huang et al., “6-month consequences of Covid-19 in patients discharged from hospital: a cohort study,” The Lancet, January 2021, link .
- This study investigated the long-term health consequences and associated risk factors of previously hospitalized Covid-19 patients, discharged between January 7, 2020, and May 29, 2020. Patients in the cohort study were interviewed, underwent physical exams, received blood tests, and performed 6-minute walking tests. A total of 1,733 patients were included. A follow-up study was conducted from June 16 to September 3, 2020; the median follow-up time after symptom onset was 186 days.
- Researchers found that at 6 months following initial infection, patients most frequently experienced fatigue, sleep difficulties, and anxiety or depression. Patients who were severely ill during hospital admission had more severe impaired pulmonary diffusion capacities and abnormal chest imaging manifestations.
14. Miglis et al., “A case report of postural tachycardia syndrome after Covid-19,” Clinical Autonomic Research, September 2020, link .
- Case report of a previously healthy, 26-year-old ER nurse who developed postural orthostatic tachycardia syndrome (POTS) several months after symptom onset. The patient had no pre-existing symptoms of autonomic impairment.
SARS-CoV-2 Variants of Concern
Variants of viruses occur when there is a specific set of mutations or genetic changes. Some mutations are insignificant, impeding the virus’ ability to replicate and spread. However, some mutations confer an evolutionary advantage to the virus, which could lead to increased transmissibility, more severe disease, or other consequences. Variants are classified as “variants of concern” if there is evidence of increased transmissibility, virulence, and/or immune escape. Multiple SARS-CoV-2 variants of concern (VOC) have emerged and continue to spread in the United States and around the world. Continued widespread transmission of the virus coupled with disparate vaccination coverage globally will undoubtedly result in further evolution and spread of new variants of concern.
CDC SARS-CoV-2 Variant Classifications and Definitions https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html
Currently designated variants of concern:
- Alpha (B.1.1.7) variant, first identified in the United Kingdom in September 2020.
- Beta (B.1.351) variant, first identified in South Africa in May 2020.
- Gamma (P.1) variant, first identified in Brazil in November 2020.
- Delta (B.1.617.2) variant, first identified in India in October 2020.
- Omicron (B.1.1.529) variant, first identified in South Africa in November 2021.
As of June 2021, the Delta (B.1.617.2) variant became dominant in the United States. Several studies and pre-print papers have been published reporting on the Delta variant’s increased transmissibility, potential increased severity of disease, and ability to escape immune system recognition.
1. Bernal et al., “Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant,” New England Journal of Medicine, July 2021, link .
- This study estimated the effectiveness of the Pfizer and AstraZeneca Covid-19 vaccines against Delta (B.1.617.2) and Alpha (B.1.1.7) variants after one or two doses. Data on all symptomatic sequenced cases of Covid-19 in England from April 5 to May 16 were used to estimate the proportion of cases with either variant according to the patients’ vaccination status.
- Pfizer vaccine efficacy after one dose was 47.5% against Alpha and 35.6% against the Delta variant
- AstraZeneca vaccine efficacy after one dose was 48.7% against Alpha and 30% against the Delta variant
- Pfizer vaccine efficacy after two doses was 93.7% against Alpha and 88% against the Delta variant
- AstraZeneca vaccine efficacy after two doses was 74.5% against Alpha and 67% against the Delta variant
2. Fisman and Tuite, “Progressive Increase in Virulence of Novel SARS-CoV-2 Variants in Ontario, Canada,” medRxiv, July 2021, link .
- This study analyzed the virulence of the Delta (B.1.617.2) SARS-CoV-2 variant relative to variants with N501Y mutation. Researchers created a retrospective cohort of patients testing positive for SARS-CoV-2 in Ontario and screened for variants of concern (VOC). Case information was extracted from the Ontario provincial Case and Contact Management (CCM) database. A total of 211,197 cases was included in the study, with episode dates between February 7 and June 22, 2021.
- The adjusted elevation in risk associated with N501Y-positive variants was 59% for hospitalization, 105% for ICU admission and 61% for death
- The increase in risk associated with the Delta variant was significantly higher – 120% for hospitalization, 287% for ICU admission and 137% for death
- Researchers also found that individuals infected with VOCs were, on average, younger and less likely to have commodities than those infected with non-VOC SARS-CoV-2 strains.
3. Muecksch et al., “Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: implications for the ability of serological assays to predict immunity,” medRxiv, July 2021, link .
- This study measured antibodies in a cohort of 112 participants with a history of SARs-CoV-2 infection. Participants were surveyed to determine the date of the positive PCR test, the date of onset of symptoms, and if their symptoms required hospitalization. Serum samples were taken at a baseline visit (~3.5 to ~8.5 weeks post PCR test) and 2 weeks, 4 weeks, 8 weeks and 22 weeks later.
- Researchers found significantly reduced neutralization titers (2.5 to 5-fold reduction in sera from patients infected with Beta, Delta and Alpha variants with E484K) when compared to non-variants of concern strains.
4. Li et al., “Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant,” medRxiv, July 2021, link .
- This study investigated the first local transmission of the Delta SARS-CoV-2 variant in Guangzhou, China, from the first index case identified on May 21, 2021, to the last case reported on June 18, 2021. Researchers measured and compared the relative viral loads of 62 patients infected with the Delta variant with viral loads of 63 patients infected with earlier strains.
- Viral loads in the Delta variant infections were 1,260 times higher than those infected with previous SARS-CoV-2 strains. Researchers also found that the interval time from exposure to PCR positive was shorter for Delta infections (4 days, peak at 3.71 days) compared to previous strains (6 days, peak at 5.61 days).
- The authors noted, “This highlights more infectiousness of Delta variant during the early stage of infection is very likely, and the frequency of the population screening should be optimized for the intervention. The more infectiousness of the Delta variant infections in pre-symptomatic phase highlights the need of timely quarantine for the suspicious infection cases or closely contacts before the clinical onset or the PCR screening.”
5. Farinholt et al., “Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections,” medRxiv, July 2021, link .
- This study describes a Delta variant transmission event among family members at a wedding ceremony in Texas. All 92 wedding guests were required to be fully vaccinated and took place outdoors in a large, open-air tent.
- Researchers identified a total of 6 individuals who were fully vaccinated with Pfizer, Moderna or Covaxin Covid-19 vaccines and tested positive for SARS-CoV-2. All 6 breakthrough infections were symptomatic; one developed severe Covid-19 and one died. Two members who travelled from India to attend the wedding tested negative for SARS-CoV-2 as part of the pre-flight criteria.
6. Wall et al., “Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination,” The Lancet, June 2021, link .
- This study examined the vaccine-induced antibody neutralizing capacity against Delta (B.1.617.2), Alpha (B.1.1.7) and other variants of concern. Researchers analyzed antibodies in the blood of 250 participants who received either one or two doses of the Pfizer Covid-19 vaccine, up to three months after the first dose.
- Researchers found that fully vaccinated individuals had a 5.8-fold reduction in neutralizing antibodies against the Delta SARS-CoV-2 variant when compared to the wild-type strain, upon which current Covid-19 vaccines are based. Single-dose vaccine recipients had significantly lower neutralizing antibody levels – 79% against the wild-type strain, 50% against the Alpha variant, and 32% against the Delta variant.
7. Planas et al., “Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization,” Nature, July 2021, link .
- This study analyzed the sensitivity of the Delta (B.1.617.2), Alpha (B.1.1.7) and Beta (B.1.351) variants to monoclonal antibodies as well as the neutralization ability of sera from previously infected or vaccinated individuals.
- Some monoclonal antibodies lost binding to the spike protein and no longer neutralized the Delta variant
- Only 10 percent of the sera from single-dose (Pfizer or AstraZeneca) Covid-19 vaccine recipients neutralized the Delta variant. Administration of two vaccine doses generated a neutralizing response in 95% of individuals, with titers 3 to 5-fold lower against Delta than the Alpha variant.
- Sera from convalescent patients collected up to 12 months post onset of symptoms were 4-fold less potent against the Delta variant
8. Davis et al., “Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination,” medRxiv, June 2021, link .
- This study compared neutralizing capacity of Pfizer-BioNTech (BNT162b2) and Oxford/AstraZeneca (ChAdOx1) Covid-19 vaccines against Delta (B.1.617.2) and Beta (B.1.351) variants, and the wild-type strain. Sera was collected from 156 healthy individuals who received either one or two doses of Pfizer, or one or two doses of AstraZeneca Covid-19 vaccines. Neutralizing antibodies were measured against HIV (SARS-CoV-2) pseudotype-based system.
- Neutralizing efficacy was 4 to 6-fold lower against the Delta and Beta variants compared to the wild-type strain.
- Pfizer Covid-19 vaccine showed a 11.30-fold reduction in antibody titer against the Delta variant and a 9.56-fold reduction against the Beta variant. The mean antibody titer induced by vaccination with two doses of AstraZeneca was significantly lower than that induced by two doses of Pfizer.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 15 April 2024
COVID-19 drug discovery and treatment options
- Jasper Fuk-Woo Chan ORCID: orcid.org/0000-0001-6336-6657 1 , 2 , 3 , 4 ,
- Shuofeng Yuan ORCID: orcid.org/0000-0001-7996-1119 1 , 2 , 3 , 4 ,
- Hin Chu ORCID: orcid.org/0000-0003-2855-9837 1 , 2 , 3 , 4 ,
- Siddharth Sridhar ORCID: orcid.org/0000-0002-2022-8307 1 , 2 , 3 &
- Kwok-Yung Yuen ORCID: orcid.org/0000-0002-2083-1552 1 , 2 , 3 , 4
Nature Reviews Microbiology ( 2024 ) Cite this article
383 Accesses
26 Altmetric
Metrics details
- Antiviral agents
- Viral infection
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused substantial morbidity and mortality, and serious social and economic disruptions worldwide. Unvaccinated or incompletely vaccinated older individuals with underlying diseases are especially prone to severe disease. In patients with non-fatal disease, long COVID affecting multiple body systems may persist for months. Unlike SARS-CoV and Middle East respiratory syndrome coronavirus, which have either been mitigated or remained geographically restricted, SARS-CoV-2 has disseminated globally and is likely to continue circulating in humans with possible emergence of new variants that may render vaccines less effective. Thus, safe, effective and readily available COVID-19 therapeutics are urgently needed. In this Review, we summarize the major drug discovery approaches, preclinical antiviral evaluation models, representative virus-targeting and host-targeting therapeutic options, and key therapeutics currently in clinical use for COVID-19. Preparedness against future coronavirus pandemics relies not only on effective vaccines but also on broad-spectrum antivirals targeting conserved viral components or universal host targets, and new therapeutics that can precisely modulate the immune response during infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
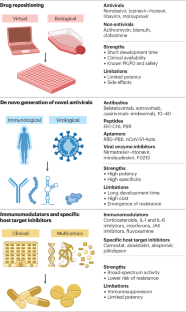
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

Age-specific nasal epithelial responses to SARS-CoV-2 infection
Maximillian N. J. Woodall, Ana-Maria Cujba, … Claire M. Smith

Mechanisms of SARS-CoV-2 entry into cells
Cody B. Jackson, Michael Farzan, … Hyeryun Choe
Zumla, A. et al. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 15 , 327–347 (2016).
Article CAS PubMed PubMed Central Google Scholar
Cheng, V. C. et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 20 , 660–694 (2007).
Chan, J. F. et al. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 28 , 465–522 (2015).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579 , 270–273 (2020).
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 , 514–523 (2020).
To, K. K. et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 10 , 507–535 (2021).
Jeon, S. et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 64 , e00819-20 (2020).
Article PubMed PubMed Central Google Scholar
Yuan, S. et al. Broad-spectrum host-based antivirals targeting the interferon and lipogenesis pathways as potential treatment options for the pandemic coronavirus disease 2019 (COVID-19). Viruses 12 , 628 (2020).
Riva, L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586 , 113–119 (2020).
Jang, W. D. et al. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl Acad. Sci. USA 118 , e2024302118 (2021).
Luttens, A. et al. Ultralarge virtual screening identifies SARS-CoV-2 main protease inhibitors with broad-spectrum activity against coronaviruses. J. Am. Chem. Soc. 144 , 2905–2920 (2022).
Gunther, S. et al. X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science 372 , 642–646 (2021).
Jin, Z. et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 582 , 289–293 (2020).
Article CAS PubMed Google Scholar
Chen, C. Z. et al. Drug repurposing screen for compounds inhibiting the cytopathic effect of SARS-CoV-2. Front. Pharmacol. 11 , 592737 (2020).
Yuan, S. et al. Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system. Pharmacol. Res. 159 , 104960 (2020).
Hung, I. F. et al. Triple combination of interferon β-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395 , 1695–1704 (2020).
Cao, B. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 382 , 1787–1799 (2020).
Article PubMed Google Scholar
Beigel, J. H. et al. Remdesivir for the treatment of COVID-19—final report. N. Engl. J. Med. 383 , 1813–1826 (2020).
Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395 , 1569–1578 (2020).
Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56 , 105949 (2020).
WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 — interim WHO Solidarity Trial results. N. Engl. J. Med. 384 , 497–511 (2021).
Article Google Scholar
Cihlar, T. & Mackman, R. L. Journey of remdesivir from the inhibition of hepatitis C virus to the treatment of COVID-19. Antivir. Ther. 27 , 13596535221082773 (2022).
Masyeni, S. et al. Molnupiravir: a lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—a narrative review. J. Med. Virol. 94 , 3006–3016 (2022).
US Food & Drug Administration. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. US Food & Drug Administration https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (2020).
Owen, D. R. et al. An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19. Science 374 , 1586–1593 (2021).
Wahl, A. et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591 , 451–457 (2021).
Valero, J. et al. A serum-stable RNA aptamer specific for SARS-CoV-2 neutralizes viral entry. Proc. Natl Acad. Sci. USA 118 , e2112942118 (2021).
de Vries, R. D. et al. Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets. Science 371 , 1379–1382 (2021).
Liu, L. et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 602 , 676–681 (2022).
Iketani, S. et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 604 , 553–556 (2022).
Liu, L. et al. An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med. 14 , eabn6859 (2022).
Tortorici, M. A. et al. Broad sarbecovirus neutralization by a human monoclonal antibody. Nature 597 , 103–108 (2021).
Xiang, Y. et al. Superimmunity by pan-sarbecovirus nanobodies. Cell Rep. 39 , 111004 (2022).
Xia, S. et al. Peptide-based pan-CoV fusion inhibitors maintain high potency against SARS-CoV-2 Omicron variant. Cell Res. 32 , 404–406 (2022).
Zhao, H. et al. Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants. Emerg. Microbes Infect. 11 , 926–937 (2022).
Yuan, S. et al. Targeting papain-like protease for broad-spectrum coronavirus inhibition. Protein Cell 13 , 940–953 (2022).
Quan, B. X. et al. An orally available M pro inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron. Nat. Microbiol. 7 , 716–725 (2022).
RECOVERY Collaborative Group et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 384 , 693–704 (2021).
Stone, J. H. et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 383 , 2333–2344 (2020).
Huet, T. et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2 , e393–e400 (2020).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370 , eabd4585 (2020).
Richardson, P. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395 , e30–e31 (2020).
Stebbing, J. et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 20 , 400–402 (2020).
Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583 , 459–468 (2020).
Bojkova, D. et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 583 , 469–472 (2020).
Yuan, S. et al. SARS-CoV-2 exploits host DGAT and ADRP for efficient replication. Cell Discov. 7 , 100 (2021).
Yuen, T. T. et al. Targeting ACLY efficiently inhibits SARS-CoV-2 replication. Int. J. Biol. Sci. 18 , 4714–4730 (2022).
Stukalov, A. et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 594 , 246–252 (2021).
Sadegh, S. et al. Exploring the SARS-CoV-2 virus–host–drug interactome for drug repurposing. Nat. Commun. 11 , 3518 (2020).
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382 , 727–733 (2020).
Chu, H. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe 1 , e14–e23 (2020).
Chu, H. et al. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat. Commun. 12 , 134 (2021).
Hoffmann, M. et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 585 , 588–590 (2020).
Wang, S. et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 31 , 126–140 (2021).
Daniloski, Z. et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell 184 , 92–105 e16 (2021).
Wang, L. et al. Susceptibility to SARS-CoV-2 of cell lines and substrates commonly used to diagnose and isolate influenza and other viruses. Emerg. Infect. Dis. 27 , 1380–1392 (2021).
Matsuyama, S. et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl Acad. Sci. USA 117 , 7001–7003 (2020).
Zhu, N. et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 11 , 3910 (2020).
Sheahan, T. P. et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12 , eabb5883 (2020).
Hou, Y. J. et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182 , 429–446.e14 (2020).
Yuan, S. et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature 593 , 418–423 (2021).
Alfi, O. et al. Human nasal and lung tissues infected ex vivo with SARS-CoV-2 provide insights into differential tissue-specific and virus-specific innate immune responses in the upper and lower respiratory tract. J. Virol. 95 , e0013021 (2021).
Chu, H. et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 71 , 1400–1409 (2020).
Chu, H. et al. SARS-CoV-2 induces a more robust innate immune response and replicates less efficiently than SARS-CoV in the human intestines: an ex vivo study with implications on pathogenesis of COVID-19. Cell Mol. Gastroenterol. Hepatol. 11 , 771–781 (2021).
Mykytyn, A. Z. et al. SARS-CoV-2 entry into human airway organoids is serine protease-mediated and facilitated by the multibasic cleavage site. eLife 10 , e64508 (2021).
Salahudeen, A. A. et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588 , 670–675 (2020).
Zhou, J. et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 26 , 1077–1083 (2020).
Kruger, J. et al. Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell-derived intestinal organoids. Cell Mol. Gastroenterol. Hepatol. 11 , 935–948 (2021).
Zhang, B. Z. et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 30 , 928–931 (2020).
Monteil, V. et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181 , 905–913.e7 (2020).
Zhao, B. et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 11 , 771–775 (2020).
Menuchin-Lasowski, Y. et al. SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids. Stem Cell Rep. 17 , 789–803 (2022).
Article CAS Google Scholar
Han, Y. et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589 , 270–275 (2021).
Chan, J. F. et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 71 , 2428–2446 (2020).
Trimpert, J. et al. The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep. 33 , 108488 (2020).
Winkler, E. S. et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 21 , 1327–1335 (2020).
Kim, Y. I. et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27 , 704–709.e2 (2020).
Munster, V. J. et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585 , 268–272 (2020).
Munoz-Fontela, C. et al. Animal models for COVID-19. Nature 586 , 509–515 (2020).
Shuai, H. et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 603 , 693–699 (2022).
Halfmann, P. J. et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603 , 687–692 (2022).
Shuai, H. et al. The viral fitness and intrinsic pathogenicity of dominant SARS-CoV-2 Omicron sublineages BA.1, BA.2, and BA.5. EBioMedicine 95 , 104753 (2023).
Hu, B. et al. Divergent trajectory of replication and intrinsic pathogenicity of SARS-CoV-2 Omicron post-BA.2/5 subvariants in the upper and lower respiratory tract. EBioMedicine 99 , 104916 (2023).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 , 271–280.e8 (2020).
Yeung, M. L. et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin–angiotensin system. Cell 184 , 2212–2228.e12 (2021).
V’Kovski, P. et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19 , 155–170 (2021).
Hassler, L. et al. A novel soluble ACE2 protein provides lung and kidney protection in mice susceptible to lethal SARS-CoV-2 infection. J. Am. Soc. Nephrol. 33 , 1293–1307 (2022).
El-Shennawy, L. et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 13 , 405 (2022).
Ikemura, N. et al. An engineered ACE2 decoy neutralizes the SARS-CoV-2 Omicron variant and confers protection against infection in vivo. Sci. Transl. Med. 14 , eabn7737 (2022).
Chen, Y. et al. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 23 , 189–199 (2022).
Takamatsu, Y. et al. Highly neutralizing COVID-19 convalescent plasmas potently block SARS-CoV-2 replication and pneumonia in Syrian hamsters. J. Virol. 96 , e0155121 (2022).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590 , 630–634 (2021).
Libster, R. et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N. Engl. J. Med. 384 , 610–618 (2021).
Sullivan, D. J. et al. Early outpatient treatment for COVID-19 with convalescent plasma. N. Engl. J. Med. 386 , 1700–1711 (2022).
Rothenberger, S. et al. The trispecific DARPin ensovibep inhibits diverse SARS-CoV-2 variants. Nat. Biotechnol. 40 , 1845–1854 (2022).
Chan, J. F. et al. A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Cell Rep. Med. 3 , 100774 (2022).
Ohashi, H. et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience 24 , 102367 (2021).
Clausen, T. M. et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183 , 1043–1057.e15 (2020).
Zhang, Q. et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 6 , 80 (2020).
Hu, Y. et al. Brilacidin, a COVID-19 drug candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E, and NL63 through targeting both the virus and the host cell. J. Med. Virol. 94 , 2188–2200 (2022).
Wang, M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30 , 269–271 (2020).
Hoffmann, M. et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 65 , 103255 (2021).
Li, K. et al. The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of COVID-19. mBio 12 , e0097021 (2021).
Takahashi, W. et al. Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation. Int. J. Infect. Dis. 102 , 529–531 (2021).
Hoffmann, M., Kleine-Weber, H. & Pohlmann, S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 78 , 779–784.e5 (2020).
Essalmani, R. et al. Distinctive roles of furin and TMPRSS2 in SARS-CoV-2 infectivity. J. Virol. 96 , e0012822 (2022).
Peacock, T. P. et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 6 , 899–909 (2021).
Chan, J. F. et al. Altered host protease determinants for SARS-CoV-2 Omicron. Sci. Adv. 9 , eadd3867 (2023).
Wang, G. et al. Dalbavancin binds ACE2 to block its interaction with SARS-CoV-2 spike protein and is effective in inhibiting SARS-CoV-2 infection in animal models. Cell Res. 31 , 17–24 (2021).
Zhao, M. M. et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal. Transduct. Target. Ther. 6 , 134 (2021).
Shang, C. et al. Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice. Virol. J. 18 , 46 (2021).
Yuan, S. et al. Viruses harness YxxO motif to interact with host AP2M1 for replication: a vulnerable broad-spectrum antiviral target. Sci. Adv. 6 , eaba7910 (2020).
Rosenke, K. et al. Hydroxychloroquine prophylaxis and treatment is ineffective in macaque and hamster SARS-CoV-2 disease models. JCI Insight 5 , e143174 (2020).
Zhao, H. et al. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 11 , 4252 (2020).
Zhao, H. et al. Cross-linking peptide and repurposed drugs inhibit both entry pathways of SARS-CoV-2. Nat. Commun. 12 , 1517 (2021).
Braga, L. et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 594 , 88–93 (2021).
Shin, D. et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 587 , 657–662 (2020).
Klemm, T. et al. Mechanism and inhibition of the papain-like protease, PL pro , of SARS-CoV-2. EMBO J. 39 , e106275 (2020).
Fu, Z. et al. The complex structure of GRL0617 and SARS-CoV-2 PL pro reveals a hot spot for antiviral drug discovery. Nat. Commun. 12 , 488 (2021).
Swaim, C. D. et al. 6-Thioguanine blocks SARS-CoV-2 replication by inhibition of PL pro . iScience 24 , 103213 (2021).
Chu, C. M. et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59 , 252–256 (2004).
Chan, J. F. et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 212 , 1904–1913 (2015).
Schoergenhofer, C. et al. Pharmacokinetics of lopinavir and ritonavir in patients hospitalized with coronavirus disease 2019 (COVID-19). Ann. Intern. Med. 173 , 670–672 (2020).
Fintelman-Rodrigues, N. et al. Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob. Agents Chemother. 64 , e00825-20 (2020).
Chaves, O. A. et al. Atazanavir is a competitive inhibitor of SARS-CoV-2 M pro , impairing variants replication in vitro and in vivo. Pharmaceuticals 15 , 21 (2021).
Ma, C. et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 30 , 678–692 (2020).
Mahmoud, A. et al. Telaprevir is a potential drug for repurposing against SARS-CoV-2: computational and in vitro studies. Heliyon 7 , e07962 (2021).
Qiao, J. et al. SARS-CoV-2 M pro inhibitors with antiviral activity in a transgenic mouse model. Science 371 , 1374–1378 (2021).
Li, Z. et al. Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs. Proc. Natl Acad. Sci. USA 117 , 27381–27387 (2020).
Ghahremanpour, M. M. et al. Identification of 14 known drugs as inhibitors of the main protease of SARS-CoV-2. ACS Med. Chem. Lett. 11 , 2526–2533 (2020).
Vuong, W. et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 11 , 4282 (2020).
Boras, B. et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID-19. Nat. Commun. 12 , 6055 (2021).
de Vries, M. et al. A comparative analysis of SARS-CoV-2 antivirals characterizes 3CL pro inhibitor PF-00835231 as a potential new treatment for COVID-19. J. Virol. 95 , e01819-20 (2021).
Wong, C. K. H. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet 400 , 1213–1222 (2022).
Wong, C. K. H. et al. Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect. Dis. 22 , 1681–1693 (2022).
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 386 , 1397–1408 (2022).
Chan, J. F. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9 , 221–236 (2020).
Sheahan, T. P. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9 , eaal3653 (2017).
de Wit, E. et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl Acad. Sci. USA 117 , 6771–6776 (2020).
Williamson, B. N. et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585 , 273–276 (2020).
White, M. A., Lin, W. & Cheng, X. Discovery of COVID-19 inhibitors targeting the SARS-CoV-2 Nsp13 helicase. J. Phys. Chem. Lett. 11 , 9144–9151 (2020).
Yuan, S. et al. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 5 , 1439–1448 (2020).
Wang, R. et al. Orally administered bismuth drug together with N -acetyl cysteine as a broad-spectrum anti-coronavirus cocktail therapy. Chem. Sci. 13 , 2238–2248 (2022).
Shu, T. et al. SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 35 , 321–329 (2020).
Zeng, J. et al. Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of nsp13 helicase. Biochem. J. 478 , 2405–2423 (2021).
Idris, A. et al. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol. Ther. 29 , 2219–2226 (2021).
Romano, M. et al. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 9 , 1267 (2020).
Hackbart, M., Deng, X. & Baker, S. C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl Acad. Sci. USA 117 , 8094–8103 (2020).
Wang, Y. et al. Coronavirus nsp10/nsp16 methyltransferase can be targeted by nsp10-derived peptide in vitro and in vivo to reduce replication and pathogenesis. J. Virol. 89 , 8416–8427 (2015).
Russ, A. et al. Nsp16 shields SARS-CoV-2 from efficient MDA5 sensing and IFIT1-mediated restriction. EMBO Rep. 23 , e55648 (2022).
Kim, Y. et al. Tipiracil binds to uridine site and inhibits Nsp15 endoribonuclease NendoU from SARS-CoV-2. Commun. Biol. 4 , 193 (2021).
Wang, X. et al. Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture. Commun. Biol. 5 , 154 (2022).
Yuan, S. et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 10 , 120 (2019).
Wong, L. R. et al. Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature 605 , 146–151 (2022).
White, K. M. et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371 , 926–931 (2021).
Mullen, P. J. et al. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat. Commun. 12 , 1876 (2021).
Acharya, A. et al. PI3K-ɑ/mTOR/BRD4 inhibitor alone or in combination with other anti-virals blocks replication of SARS-CoV-2 and its variants of concern including Delta and Omicron. Clin. Transl. Med. 12 , e806 (2022).
Rajasekharan, S. et al. Inhibitors of protein glycosylation are active against the coronavirus severe acute respiratory syndrome coronavirus SARS-CoV-2. Viruses 13 , 808 (2021).
Ambike, S. et al. Targeting genomic SARS-CoV-2 RNA with siRNAs allows efficient inhibition of viral replication and spread. Nucleic Acids Res. 50 , 333–349 (2022).
Zhao, H. et al. A trifunctional peptide broadly inhibits SARS-CoV-2 Delta and Omicron variants in hamsters. Cell Discov. 8 , 62 (2022).
Chu, H. et al. Coronaviruses exploit a host cysteine-aspartic protease for replication. Nature 609 , 785–792 (2022).
CAS PubMed Google Scholar
Chu, H. et al. Targeting highly pathogenic coronavirus-induced apoptosis reduces viral pathogenesis and disease severity. Sci. Adv. 7 , eabf8577 (2021).
Lee, S. et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 599 , 283–289 (2021).
Merad, M. et al. The immunology and immunopathology of COVID-19. Science 375 , 1122–1127 (2022).
Ye, Z. W. et al. Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection. Emerg. Microbes Infect. 10 , 291–304 (2021).
Yu, L. M. et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 398 , 843–855 (2021).
Ezer, N. et al. Inhaled and intranasal ciclesonide for the treatment of COVID-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 375 , e068060 (2021).
Bessiere, P. et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog. 17 , e1009427 (2021).
Ye, F. et al. Novaferon effectively inhibits ancestral SARS-CoV-2 and Omicron variant in vitro, 2022. China CDC Wkly. 4 , 509–512 (2022).
PubMed PubMed Central Google Scholar
Tamir, H. et al. Induction of innate immune response by TLR3 agonist protects mice against SARS-CoV-2 infection. Viruses 14 , 189 (2022).
WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 399 , 1941–1953 (2022).
Article PubMed Central Google Scholar
Tam, A. R. et al. Early treatment of high-risk hospitalized COVID-19 patients with a combination of interferon β-1b and remdesivir: a phase 2 open-label randomized controlled trial. Clin. Infect. Dis. 76 , e216–e226 (2022).
Dinnon, K. H. III et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586 , 560–566 (2020).
Chong, Z. et al. Nasally delivered interferon-λ protects mice against infection by SARS-CoV-2 variants including Omicron. Cell Rep. 39 , 110799 (2022).
Reis, G. et al. Early treatment with pegylated interferon λ for COVID-19. N. Engl. J. Med. 388 , 518–528 (2023).
Hoang, T. N. et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 184 , 460–475.e21 (2021).
Kalil, A. C. et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N. Engl. J. Med. 384 , 795–807 (2021).
Afzali, B. et al. The state of complement in COVID-19. Nat. Rev. Immunol. 22 , 77–84 (2022).
Vlaar, A. P. et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 10 , 1137–1146 (2022).
Ho, J. S. Y. et al. TOP1 inhibition therapy protects against SARS-CoV-2-induced lethal inflammation. Cell 184 , 2618–2632.e17 (2021).
Li, L. et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 324 , 460–470 (2020).
WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20 , e192–e197 (2020).
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 326 , 499–518 (2021).
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324 , 1330–1341 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04381936 (2024).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT02735707 (2024).
Sette, A. & Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184 , 861–880 (2021).
Jayk Bernal, A. et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 386 , 509–520 (2022).
Butler, C. C. et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 401 , 281–293 (2023).
Wang, L. et al. COVID-19 rebound after Paxlovid and molnupiravir during January–June 2022. Preprint at medRxiv https://doi.org/10.1101/2022.06.21.22276724 (2022).
Chen, P. et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N. Engl. J. Med. 384 , 229–237 (2021).
Arabi, Y. M. et al. Interferon β-1b and lopinavir–ritonavir for Middle East respiratory syndrome. N. Engl. J. Med. 383 , 1645–1656 (2020).
Zheng, F. et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int. J. Infect. Dis. 99 , 84–91 (2020).
Monk, P. D. et al. Safety and efficacy of inhaled nebulised interferon β-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 9 , 196–206 (2021).
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397 , 1637–1645 (2021).
Peng, J. et al. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: a systematic review and meta-analysis. Rev. Med. Virol. 32 , e2295 (2022).
Spinner, C. D. et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 324 , 1048–1057 (2020).
Skipper, C. P. et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann. Intern. Med. 173 , 623–631 (2020).
PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 397 , 1063–1074 (2021).
Butler, C. C. et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir. Med. 9 , 1010–1020 (2021).
Lopez-Medina, E. et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 325 , 1426–1435 (2021).
Weinreich, D. M. et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N. Engl. J. Med. 385 , e81 (2021).
Gottlieb, R. L. et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 325 , 632–644 (2021).
Feld, J. J. et al. Peginterferon λ for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 9 , 498–510 (2021).
Jagannathan, P. et al. Peginterferon λ-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat. Commun. 12 , 1967 (2021).
Ramakrishnan, S. et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 9 , 763–772 (2021).
Reis, G. et al. Oral fluvoxamine with inhaled budesonide for treatment of early-onset COVID-19: a randomized platform trial. Ann. Intern. Med. 176 , 667–675 (2023).
Salama, C. et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 384 , 20–30 (2021).
Salvarani, C. et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 181 , 24–31 (2021).
Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N. Engl. J. Med. 384 , 1503–1516 (2021).
Lescure, F. X. et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 9 , 522–532 (2021).
Dorward, J. et al. Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br. J. Gen. Pract. 72 , e446–e455 (2022).
Gautret, P. et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a 6-day follow up: a pilot observational study. Travel. Med. Infect. Dis. 34 , 101663 (2020).
Rosendaal, F. R. Review of: “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al. 2010”. Int. J. Antimicrob. Agents 56 , 106063 (2020).
Niburski, K. & Niburski, O. Impact of Trump’s promotion of unproven COVID-19 treatments and subsequent Internet trends: observational study. J. Med. Internet Res. 22 , e20044 (2020).
Rathi, S. et al. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect. Dis. 20 , 1118–1119 (2020).
Mehra, M. R. et al. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 395 , P1820 (2020); retraction 395 , 1820 (2020).
US Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. US Food and Drug Administration https://nycourts.gov/reporter/webdocs/Why-You-Should-Not-Use-Ivermectin-to-Treat-or-Prevent-COVID-19.pdf (2021).
Tang, C. et al. Caution against corticosteroid-based COVID-19 treatment. Lancet 395 , 1759–1760 (2020).
Download references
Acknowledgements
This study was partly supported by funding by the National Natural Science Foundation of China General Program (82272337); the Health and Medical Research Fund (20190572), Health Bureau, Government of the Hong Kong Special Administrative Region; the General Research Fund (17122322), Collaborative Research Fund (C7060-21G) and Theme-Based Research Scheme (T11-709/21-N), Research Grants Council, Government of the Hong Kong Special Administrative Region; Health@InnoHK, Innovation and Technology Commission, Government of the Hong Kong Special Administrative Region; the Partnership Programme of Enhancing Laboratory Surveillance and Investigation of Emerging Infectious Diseases and Antimicrobial Resistance for the Department of Health of the Hong Kong Special Administrative Region Government; the National Key Research and Development Program of China (projects 2021YFC0866100 and 2023YFC3041600); the Sanming Project of Medicine in Shenzhen, China (SZSM201911014); the High Level-Hospital Program, Health Commission of Guangdong Province, China; the Emergency Collaborative Project of Guangzhou Laboratory (EKPG22-01); the University of Hong Kong Outstanding Young Researcher Award; and the University of Hong Kong Research Output Prize (Li Ka Shing Faculty of Medicine); and by donations from the Shaw Foundation Hong Kong, Richard Yu and Carol Yu, Michael Seak-Kan Tong, May Tam Mak Mei Yin, Providence Foundation Limited (in memory of the late Lui Hac Minh), Lee Wan Keung Charity Foundation Limited, Hong Kong Sanatorium & Hospital, Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited, The Chen Wai Wai Vivien Foundation Limited, Chan Yin Chuen Memorial Charitable Foundation, Tse Kam Ming Laurence, Marina Man-Wai Lee, the Hong Kong Hainan Commercial Association South China Microbiology Research Fund, Pui-Sze Cheng, Perfect Shape Medical Limited, Kai Chong Tong, Tse Kam Ming Laurence, Foo Oi Foundation Limited and Betty Hing-Chu Lee, Ping Cham So, and Lo Ying Shek Chi Wai Foundation. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Author information
Authors and affiliations.
State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong, Pokfulam, Hong Kong Special Administrative Region, China
Jasper Fuk-Woo Chan, Shuofeng Yuan, Hin Chu, Siddharth Sridhar & Kwok-Yung Yuen
Carol Yu Centre for Infection, Department of Microbiology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong Special Administrative Region, China
Department of Infectious Diseases and Microbiology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, Guangdong Province, China
Centre for Virology, Vaccinology and Therapeutics, Hong Kong Science and Technology Park, Shatin, Hong Kong Special Administrative Region, China
Jasper Fuk-Woo Chan, Shuofeng Yuan, Hin Chu & Kwok-Yung Yuen
You can also search for this author in PubMed Google Scholar
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Kwok-Yung Yuen .
Ethics declarations
Competing interests.
J.F.-W.C. has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Ltd, and was an invited speaker for Gilead Sciences Hong Kong Ltd and Luminex Corporation. K.-Y.Y. is a shareholder of Hong Kong Universal Biologicals Company Ltd and Hong Kong Universal Vaccine Ltd, and collaborates with Sinovac Biotech Ltd and China National Pharmaceutical Group Co., Ltd (Sinopharm). J.F.-W.C., S.Y., H.C. and K.-Y.Y. have patent applications on a number of therapeutic candidates included in this article. S.S. declares no competing interests.
Peer review
Peer review information.
Nature Reviews Microbiology thanks Christopher Butler, Shuibing Chen, Stanley Perlman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Chan, J.FW., Yuan, S., Chu, H. et al. COVID-19 drug discovery and treatment options. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-024-01036-y
Download citation
Accepted : 28 February 2024
Published : 15 April 2024
DOI : https://doi.org/10.1038/s41579-024-01036-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Introduction
- Article Information
The date filter allows for daily selection of dates from March 1, 2020, to July 1, 2022. A slider is included under the date selection box for viewing policy progression throughout the pandemic. The health care settings filter consists of 4 checkboxes, allowing for the selection of target health care settings (general health care settings, nursing homes, home health care agencies, and both). Comprehensive definitions are found in the eTable in Supplement 1. The COVID-19 burden parameter consists of 5 checkboxes, allowing for the selection of 5 distinct categories of COVID-19 burden (cases and deaths) at the community and nursing home levels. The policy type filter allows for the selection of 5 broad policy categories. The policy subtype filter contains 38 distinct subcategories related to the broader categories. On the map, the number of policies is indicated by a color gradient, ranging from the least (light gray) to the most (dark blue). COVID-19 burden is depicted as circles of varying size, with larger diameters signifying increasing severity. Circles are red if there were deaths recorded during that period, green if there were no deaths, and gray if no data were available. The central US map can be enlarged for ease of viewing, while the 5 US territories remain fixed in size.
Maps depict May 24, 2020 (during first wave and after mandatory case and death reporting in nursing homes begins), January 12, 2021 (Alpha and Delta variants), and January 16, 2022 (Omicron variant). On the maps, the number of COVID-19 policies is indicated by a color gradient, ranging from the least (light gray color) to the most (darker blue color). All targeted health care settings (general, nursing homes, home health agencies, and both) were selected for this visual. COVID-19 burden is depicted as circles of varying size, with larger circles signifying increasing severity. The 7-day average of COVID-19 community deaths per 100 000 population was selected for this visual. Circles are red if there were deaths recorded during that period and green if there were no deaths.
eMethods. Policy Dataset and Dashboard Development
eTable. Definitions of Post–Acute Care COVID-19 Policy Categories and Subcategories
eReferences
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Stone PW , Zhao S , Chastain AM, et al. State- and Territory-Level Nursing Home and Home Health Care COVID-19 Policies and Disease Burden. JAMA Netw Open. 2024;7(4):e247683. doi:10.1001/jamanetworkopen.2024.7683
Manage citations:
© 2024
- Permissions
State- and Territory-Level Nursing Home and Home Health Care COVID-19 Policies and Disease Burden
- 1 Center for Health Policy, Columbia University School of Nursing, New York, New York
- 2 Department of Anesthesiology and Perioperative Medicine, University of Rochester School of Medicine, Rochester, New York
- 3 Department of Public Health Sciences, University of Rochester School of Medicine, Rochester, New York
- 4 RAND Health, RAND Corporation, Boston, Massachusetts
The COVID-19 pandemic disproportionately affected older persons, 1 many of whom were served by home health care agencies (HHAs) and nursing homes (NHs). The extent to which state- and territory-level COVID-19 policies reinforced or expanded federal policies is unknown. Building on the work of others, 2 we created a dataset and dashboard of state- and territory-specific NH and HHA policies linked with community and NH COVID-19 burden for researchers and public health officials to evaluate policy efficacy.
In this cross-sectional study, we used the Council of State Government’s 2020-2021 State Executive Orders website 3 and comprehensive searches of state and territory government websites to identify state- and territory-specific policies enacted from March 1, 2020, to July 1, 2022. We collected start and end dates and categorized policies as general or specific to NHs, HHAs, or both. Policies were grouped into 5 categories with 38 subcategories as (1) preventing virus transmission (n = 18), (2) expanding NH and/or HHA capacity (n = 5), (3) relaxing administrative requirements (n = 5), (4) reporting COVID-19 data (n = 3), and (5) admission and discharge policies (n = 7) (eMethods in Supplement 1 ).
We linked these policy data with community-level 4 and NH-specific COVID-19 burden (case and mortality counts) 5 and entered data into Tableau Desktop, version 2023.2. 6 We used a color gradient and circle size to visualize policy counts and COVID-19 burden, respectively. The interactive dashboard displays temporality with zoom capability of setting, policy, and COVID-19 burden.
This study was approved by the Columbia University Institutional Review Board, who waived the need for informed consent because the study was not deemed human participant research. We followed the STROBE reporting guideline.
We identified 1400 policies across 50 states and 5 territories. Most included all health care settings (n = 846), followed by NH-specific (n = 486), NH- and HHA-specific (n = 43), and HHA-specific (n = 25) policies. The most common policy category was preventing virus transmission (n = 736), followed by expanding NH and HHA capacity (n = 325), relaxing administrative requirements (n = 184), reporting COVID-19 data (n = 79), and admission and discharge (n = 54). The dashboard ( Figure 1 ) illustrates variation in the number of policies per state and severity of COVID-19 burden indicated by color gradient and circle diameter, respectively. Figure 2 highlights the dynamic change in NH and HHA policies and COVID-19 burden. For example, on May 24, 2020, Montana, Hawaii, and Alaska had no COVID-19 deaths or policies, in contrast with North Carolina’s moderate burden and several policies. By January 12, 2021, New York had a severe COVID-19 burden and the greatest number of policies, while Pennsylvania, Montana, and Florida had a similar COVID-19 burden but fewer policies.
The dataset and dashboard described in this study are potentially important tools for researchers and public health officials and could provide a template for visual platforms that may inform future efforts to manage public health crises. Variations in COVID-19 burden and state and territory policy responses displayed in the dashboard highlight the complexity of pandemic management. Exploratory analyses demonstrated that higher numbers of policies at the state and territory levels were not consistently associated with reductions in community- or NH-level COVID-19 burden, suggesting policy effectiveness may depend on implementation and compliance. We also found limited attention to HHAs compared with NHs, despite both settings serving vulnerable older populations. This suggests a gap in public health planning, raising questions about resource allocation and prioritization among health care settings during pandemics.
Study limitations include the primarily descriptive data, underlying data from various sources, and limited evaluation of efficacy of public health policies on population COVID-19 outcomes. Future public health planning and pandemic responses should include adaptive and targeted policy interventions and should consider specific needs of all health care settings. Dashboards have the potential to help formulate data-driven decision-making during public health crises.
Accepted for Publication: February 17, 2024.
Published: April 22, 2024. doi:10.1001/jamanetworkopen.2024.7683
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Stone PW et al. JAMA Network Open .
Corresponding Author: Patricia W. Stone, PhD, RN, Center for Health Policy, Columbia University School of Nursing, 560 W 168th St, New York, NY 10032 ( [email protected] ).
Author Contributions: Dr Stone had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Stone, Shang, Glance, Dick.
Acquisition, analysis, or interpretation of data: Stone, Zhao, Chastain, Perera, Shang, Dick.
Drafting of the manuscript: Stone, Zhao, Chastain, Perera, Shang, Glance.
Critical review of the manuscript for important intellectual content: Stone, Shang, Glance, Dick.
Statistical analysis: Zhao, Shang, Glance, Dick.
Obtained funding: Stone, Shang, Dick.
Administrative, technical, or material support: Stone, Chastain, Perera.
Supervision: Stone, Chastain, Perera, Shang, Dick.
Conflict of Interest Disclosures: Dr Stone reported receiving grant funding from the National Institutes of Health outside the submitted work. Ms Zhao reported participating in an internship through EmblemHealth outside the submitted work. Dr Chastain reported receiving grant funding from the National Institutes of Health outside the submitted work. Dr Perera reported receiving grant funding from the National Institutes of Health outside the submitted work. Dr Shang reported receiving grant funding from the National Institutes of Health outside the submitted work. Dr Glance reported receiving grant funding from the National Institutes of Health outside the submitted work. Dr Dick reported receiving grant funding from the National Institutes of Health outside the submitted work. No other disclosures were reported.
Funding/Support: This work was funded by grant R01NR016865 from the National Institute of Nursing Research and by grant R01AG074492 from the National Institute on Aging, the National Institute of Minority Health and Health Disparities, and the National Institute of Allergy and Infectious Diseases.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Albert Chavesta, MS, MPH, assisted in compiling and categorizing the state- and territory-level policies, as well as drafting the policy dataset development methodology. Tenzin Trinley, MPH, Jung A. Kang, MSN, RN, and Charity Ogunlusi, MD, MPH, assisted with compiling state-level policies. All those acknowledged were affiliated with the Columbia University School of Nursing during the conduct of the study and were compensated via the study funding.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Significant global variation in COVID-19 guidelines: Most countries recommend at least one treatment that doesn't work
by British Medical Journal

National clinical guidelines for the treatment of COVID-19 vary significantly around the world, with under-resourced countries the most likely to diverge from gold standard (World Health Organization; WHO) treatment recommendations, finds a comparative analysis published in the open access journal BMJ Global Health .
And nearly every national guideline recommends at least one treatment proven not to work, the analysis shows.
Significant variations in national COVID-19 treatment recommendations have been suspected since the advent of the pandemic, but these haven't been formally quantified or studied in depth, note the researchers.
And despite the fact that COVID-19 is no longer taking the toll on lives and health that it once did, the virus is still evolving and active around the globe, they emphasize. The WHO only rescinded COVID-19's status as a public health emergency in April 2023.
To assess how well national clinical practice followed the recommendations of the WHO (11th version; July 2022)—regarded as the gold standard—for the treatment of COVID-19, the researchers analyzed the content of all 194 WHO member states' most recent national guidelines at the end of 2022.
Each set of guidelines was scored according to how closely they aligned with the WHO recommendations. Extra points were awarded for those that had been updated within the preceding six months; those that made recommendations in line with the strength of evidence; and those that included assessments of the effectiveness of treatments and their side effects.
The wealth and resources of each country were then compared using per capita World Bank gross domestic product (GDP) in US dollars for 2021, the Human Development Index 2021, and the Global Health Security Index 2021.
Of the 194 countries contacted, 72 didn't respond. Of the remaining 122, nine had no formal guidelines or couldn't be accessed, and a further four didn't recommend any treatments, so these were excluded, leaving a total of 109.
The countries for which guidelines weren't obtained had, on average, smaller populations, lower GDP per head, and a lower Global Health Security Index, indicative of greater economic challenges and less ability to respond to health emergencies.
The 11th iteration of the WHO guidelines categorizes disease severity , but most of the reviewed guidelines (84%; 92) didn't define COVID-19 severity in the same way, and some didn't define severity at all (6.5%; 7). Only 10 guidelines (9%) used disease severity definitions that were comparable with those of the WHO.
Most (77%; 84) guidelines didn't include an assessment of the strength or certainty of the therapeutic recommendation. And the range of recommended drugs, irrespective of severity, varied from 1 to 22. The WHO guidelines recommend a total of 10.
In all, 105 guidelines included at least one treatment recommended by the WHO, but 4 didn't recommend any. Countries in the African region had a significantly lower proportion of therapies recommended by the WHO, compared with countries in Europe and Southeast Asia.
The most commonly recommended drugs were corticosteroids (92%;100), with 80% (88) of guidelines recommending them for the same disease severity as the WHO. But corticosteroids weren't recommended in severe disease in nearly 1 in 10 guidelines despite overwhelming evidence of their benefit.
Remdesivir was recommended for severe or critical disease in half the guidelines (51%; 72). But the WHO guidelines only indicate remdesivir conditionally for mild disease in patients at highest risk of hospital admission.
In late 2022, many guidelines continued to recommend treatments that the WHO had advised against, including chloroquine, lopinavir, ritonavir, azithromycin; vitamins and/or zinc.
One in three guidelines (36; 33%) recommended at least one neutralizing monoclonal antibody directed against SARS-CoV-2, the virus responsible for COVID-19.These guidelines were issued by wealthier countries.
But two of these monoclonal antibodies —bamlanivimab plus or minus etesivamab and regdanivimab—appeared consistently in clinical guidelines, despite not being recommended by the WHO.
Doses of the most commonly recommended drugs also varied. And many guidelines hadn't been updated for more than six months.
Guidelines from under-resourced countries diverged the most from the WHO recommendations, when stratified by annual GDP, the Human Development Index, and the Global Health Security Index.
The researchers acknowledge several limitations to their findings, including the scoring used to assess the guidelines, which hasn't been validated by other studies, and the inability to assess all national guidelines.
But they nevertheless ask, "Why do [national guidelines] differ so much in their treatment guidance for such a widespread and potentially serious infection when all have access to the same information?
"Apart from the prohibitive cost of some medications for low-resource settings we do not have a satisfactory explanation."
They offer some possible explanations, including variations in how the severity of, and therefore the most appropriate treatment for, COVID-19 is defined; the evolution of the evidence; and the research chaos and confusion of the early stages of the pandemic, leading to claims and counterclaims, compounded by intense political and media interest.
"In this 'fog of war' countries clearly felt the need to say something and do something, even if it was based on very little evidence," explain the researchers. "But why many of these unproven remedies continued to be recommended as evidence of their ineffectiveness accrued is much less clear," they add.
"There is clearly more variation in national guidelines for COVID-19 therapeutics than there should be to ensure optimum treatment," which aren't justified by significant differences between populations or geographic variation in SARS-CoV-2 antiviral susceptibility, they write.
Global health inequalities clearly have a part to play, leading to the recommendation of ineffective, unaffordable and unavailable therapies, they suggest.
"The formalization of processes in the development of [national guidelines] for COVID-19 and other infectious diseases is essential for ensuring that these guidelines are grounded in the best available evidence," they conclude.
"A systematic and structured approach would not only enhance the credibility of the guidelines but could also contribute to their effectiveness in guiding public health interventions, especially in a pandemic setting."
Explore further
Feedback to editors

Study finds suicidal behaviors increased by over 50% in Catalonia, Spain after the COVID-19 pandemic
6 minutes ago

Why do we move slower the older we get? New study delivers answers
36 minutes ago

Stress activates brain regions linked to alcohol use disorder differently for women than men, finds study
41 minutes ago

Chemical tool illuminates pathways used by dopamine, opioids and other neuronal signals

Gentle defibrillation for the heart: A milder method developed by researchers for cardiac arrhythmias

Q&A: Research shows neural connection between learning a second language and learning to code
2 hours ago

Gut microbiota acts like an auxiliary liver, study finds

Higher light levels may improve cognitive performance

Brain neurons re-entering the cell cycle age quickly and shift to senescence, particularly in neurodegenerative disease
3 hours ago

Study reveals alarming rates of pediatric injuries from mechanical bull riding
Related stories.

IDSA issues rapid guidelines for treatment of COVID-19
Apr 17, 2020

WHO recommends two new drugs to treat patients with COVID-19
Jan 13, 2022

Treatment guidelines for children hospitalized with COVID-19 were rapidly but not completely followed
Nov 16, 2022
Recommendations developed for atopic dermatitis therapies
Nov 16, 2023
WHO approves two new Covid-19 treatments
Jan 14, 2022
Expert U.S. panel develops NIH treatment guidelines for COVID-19
Apr 22, 2020
Recommended for you

Study suggests that living near green spaces reduces the risk of depression and anxiety
7 hours ago

What if flat feet were normal? Debunking a myth about injuries

Consumption of contaminated venison suspected in cases of deer hunters with prion disease
6 hours ago

Study connects enjoyment of nature to lower inflammation levels
23 hours ago

Common antibiotic may be helpful in fighting respiratory viral infections
Apr 22, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Discrimination Experiences Shape Most Asian Americans’ Lives
4. asian americans and discrimination during the covid-19 pandemic, table of contents.
- Key findings from the survey
- Most Asian Americans have been treated as foreigners in some way, no matter where they were born
- Most Asian Americans have been subjected to ‘model minority’ stereotypes, but many haven’t heard of the term
- Experiences with other daily and race-based discrimination incidents
- In their own words: Key findings from qualitative research on Asian Americans and discrimination experiences
- Discrimination in interpersonal encounters with strangers
- Racial discrimination at security checkpoints
- Encounters with police because of race or ethnicity
- Racial discrimination in the workplace
- Quality of service in restaurants and stores
- Discrimination in neighborhoods
- Experiences with name mispronunciation
- Discrimination experiences of being treated as foreigners
- In their own words: How Asian Americans would react if their friend was told to ‘go back to their home country’
- Awareness of the term ‘model minority’
- Views of the term ‘model minority’
- How knowledge of Asian American history impacts awareness and views of the ‘model minority’ label
- Most Asian Americans have experienced ‘model minority’ stereotypes
- In their own words: Asian Americans’ experiences with the ‘model minority’ stereotype
- Asian adults who personally know an Asian person who has been threatened or attacked since COVID-19
- In their own words: Asian Americans’ experiences with discrimination during the COVID-19 pandemic
- Experiences with talking about racial discrimination while growing up
- Is enough attention being paid to anti-Asian racism in the U.S.?
- Acknowledgments
- Sample design
- Data collection
- Weighting and variance estimation
- Methodology: 2021 focus groups of Asian Americans
- Appendix: Supplemental tables
Following the coronavirus outbreak, reports of discrimination and violence toward Asian Americans increased. A previous Pew Research Center survey of English-speaking Asian adults showed that as of 2021, one-third said they feared someone might threaten or physically attack them. English-speaking Asian adults in 2022 were also more likely than other racial or ethnic groups to say they had changed their daily routines due to concerns they might be threatened or attacked. 19
In this new 2022-23 survey, Asian adults were asked if they personally know another Asian person in the U.S. who had been attacked since the pandemic began.
About one-third of Asian adults (32%) say they personally know an Asian person in the U.S. who has been threatened or attacked because of their race or ethnicity since the COVID-19 pandemic began in 2020.
Whether Asian adults know someone with this experience varies across Asian ethnic origin groups:
- About four-in-ten Chinese adults (39%) say they personally know another Asian person who has been threatened or attacked since the coronavirus outbreak. Similar shares of Korean adults (35%) and those who belong to less populous Asian origin groups (39%) – those categorized as “other” in this report – say the same.
- About three-in-ten Vietnamese (31%), Japanese (28%) and Filipino (28%) Americans and about two-in-ten Indian adults (21%) say they know another Asian person in the U.S. who has been the victim of a racially motivated threat or attack.
Additionally, there are some differences by regional origin groups:
- Overall, similar shares of East and Southeast Asian adults say they know another Asian person who’s been threatened or attacked because of their race or ethnicity (36% and 33%, respectively).
- A somewhat smaller share of South Asian adults say the same (24%).
There are also differences across nativity and immigrant generations:
- U.S.-born Asian adults are more likely than Asian immigrants to say they know another Asian person who has been threatened or attacked during the COVID-19 pandemic (40% vs. 28%, respectively).
- Among immigrants, those who are 1.5 generation – those who came to the U.S. as children – are more likely than the first generation – those who immigrated as adults – to say they know someone with this experience (37% vs. 25%).
- And among U.S.-born Asian Americans, 44% of second-generation adults say this, compared with 28% of third- or higher-generation Asian adults.
Whether Asian Americans personally know another Asian person who was threatened or attacked because of their race or ethnicity since the beginning of the pandemic also varies across other demographic groups:
- Age: 44% of Asian adults under 30 years old say they know someone who has been threatened or attacked during the pandemic, compared with 18% of those 65 and older.
- Gender: Asian women are somewhat more likely than men to say they know an Asian person in the U.S. who has been threatened or attacked during the COVID-19 pandemic (35% vs. 28%, respectively).
- Party: 36% of Asian Democrats and Democratic leaners say they know another Asian person who has been threatened or attacked because of their race or ethnicity, higher than the share among Republicans and Republican leaners (25%).
Heightened anti-Asian discrimination during the COVID-19 pandemic
These survey findings follow a spike in reports of discrimination against Asian Americans during the COVID-19 pandemic. The number of federally recognized hate crime incidents of anti-Asian bias increased from 158 in 2019 to 279 in 2020 and 746 in 2021, according to hate crime statistics published by the FBI . In 2022, the number of anti-Asian hate crimes decreased for the first time since the coronavirus outbreak, to 499 incidents. Between March 2020 and May 2023, the organization Stop AAPI Hate received more than 11,000 self-reported incidents of anti-Asian bias, the vast majority of which involved harassment, bullying, shunning and other discrimination incidents.
Additionally, previous research found that calling COVID-19 the “Chinese Virus,” “Asian Virus” or other names that attach location or ethnicity to the disease was associated with anti-Asian sentiment in online discourse. Use of these phrases by politicians or other prominent public officials, such as by former President Donald Trump , coincided with greater use among the general public and more frequent instances of bias against Asian Americans.
In the 2021 Pew Research Center focus groups of Asian Americans, participants discussed their experiences of being discriminated against because of their race or ethnicity during the COVID-19 pandemic.
Participants talked about being shamed in both public and private spaces. Some Asian immigrant participants talked about being afraid to speak out because of how it might impact their immigration status:
“I was walking in [the city where I live], and a White old woman was poking me in the face saying, ‘You are disgusting,’ and she was trying to hit me. I ran away crying. … At the time, I was with my boyfriend, but he also just came to the U.S., so we ran away together thinking that if we cause trouble, we could be deported.”
–Immigrant woman of Korean origin in late 20s (translated from Korean)
“[A very close friend of mine] lived at [a] school dormitory, and when the pandemic just happened … his room was directly pasted with the adhesive tape saying things like ‘Chinese virus quarantine.’”
–Immigrant man of Chinese origin in early 30s (translated from Mandarin)
Many participants talked about being targeted because others perceive them as Chinese , regardless of their ethnicity:
“I think the crimes [that happened] against other Asian people can happen to me while going through COVID-19. When I see a White person, I don’t know if their ancestors are Scottish or German, so they will look at me and think the same. It seems that they can’t distinguish between Korean and Chinese and think that we are from Asia and the onset of COVID-19 is our fault. This is something that can happen to all of us. So I think Asian Americans should come together and let people know that we are also human and we have rights. I came to think about Asian Americans that they shouldn’t stay still even if they’re trampled on.”
–Immigrant woman of Korean origin in early 50s (translated from Korean)
“Even when I was just getting on the bus, [people acted] as if I was carrying the virus. People would not sit with me, they would sit a bit far. It was because I look Chinese.”
–Immigrant woman of Bhutanese origin in early 30s (translated from Dzongkha)
Amid these incidents, some participants talked about feeling in community and kinship with other Asian people:
“[When I hear stories about Asian people in the news,] I feel like automatically you just have a sense of connection to someone that’s Asian. … [I]t makes me and my family and everyone else that I know that is Asian super mad and upset that this is happening. [For example,] the subway attacks where there was a mother who got dragged down the stairs for absolutely no reason. It just kind of makes you scared because you are Asian, and I would tell my mom, ‘You’re not going anywhere without me.’ We got pepper spray and all of that. But there is definitely a difference because you just feel a connection with them no matter if you don’t know them.”
–U.S.-born woman of Taiwanese origin in early 20s
“[A]s a result of the pandemic, I think we saw an increase in Asian hate in the media. I think that was one time where I realized as an Asian person, I felt a lot of pain. I felt a lot of fear, I felt a lot of anger and frustration for my community. … I think it was just at that specific moment when I saw the Asian hate, Asian hate crimes, and I realized, ‘Oh, they’re targeting my people.’ I don’t know how to explain it exactly. I never really referred to myself just plainly as an Asian American, but when I saw it in that media and I saw people who looked like me or people who I related with getting hurt and mistreated, I felt anger for that community, for my community.””
–U.S.-born woman of Korean origin in late teens
Some connected discrimination during the pandemic to other times of heightened anti-Asian discrimination . For example, one woman connected anti-Asian discrimination during COVID-19 to the period after Sept. 11:
“[T]he hate crimes I’m reading about now are towards Chinese [people] because of COVID, but I remember after 9/11, that was – I remember the looks that people would give me on the subway but also reading the violent acts committed towards Indians of all types, just the confusion – I mean, I say confusion but I mean really they wanted to attack Muslims, but they didn’t care, they were just looking for a brown person to attack. So there’s always something that happens that then suddenly falls on one community or another.”
–U.S.-born man of Indian origin in late 40s
- Pew Research Center’s American Trends Panel surveys of Asian adults were conducted only in English and are representative of the English-speaking Asian adult population. In 2021, 70% of Asian adults spoke only English or said they speak English “very well,” according to a Pew Research Center analysis of the 2021 American Community Survey. By contrast, the Center’s 2022-23 survey of Asian Americans was conducted in six languages, including Chinese (Simplified and Traditional), English, Hindi, Korean, Tagalog and Vietnamese. ↩
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
- Asian Americans
- Discrimination & Prejudice
- Immigration Issues
- Race Relations
- Racial Bias & Discrimination
Key facts about Asian Americans living in poverty
Methodology: 2023 focus groups of asian americans, 1 in 10: redefining the asian american dream (short film), the hardships and dreams of asian americans living in poverty, key facts about asian american eligible voters in 2024, most popular, report materials.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
- Skip to main content
- Keyboard shortcuts for audio player
Research News
Genes play a very small role in determining left-handedness, research finds.

Ayesha Rascoe
NPR's Ayesha Rascoe speaks with Clyde Francks, a geneticist in the Netherlands, about the latest research into what makes people left or right-handed.
AYESHA RASCOE, HOST:
If you're left-handed, like me, you know you are one of a rare and special breed. Just 10% of the world's population is estimated to be left-handed, but many common conceptions about left-handedness turn out to be wrong, starting with that it's definitely hereditary.
CLYDE FRANCKS: We actually think that most of the left-handedness in the population is not caused by genetic variants.
RASCOE: Clyde Francks is a geneticist and neuroscientist at the Max Planck Institute for Psycholinguistics in the Netherlands. He led a team that just published a paper about left-handedness in the journal Nature Communications.
FRANCKS: It's probably just kind of random fluctuations of chemicals in the very early developing brain in the embryo.
RASCOE: That surprised me. My mother's right-handed, but all of her children are left-handed. We thought being left-handed must have something to do with the genes we inherited. And it's even stranger because we don't all have the same father.
FRANCKS: The heritability of left-handedness is actually quite low. So in studies of twins, it's been measured at about only 25%. So in most people who are left-handed, there will not be a simple genetic explanation just running through the generations in a clear way.
RASCOE: But in the new research, Francks and his team did discover one gene that sometimes has an effect on which hand is dominant.
FRANCKS: What we knew before this study was that there were various common variants in the genome that had very, very tiny effects on the probability of being left-handed. And so what we did in the latest study was a quite different approach. We were looking for variants in the genome that are very rare in the population and are located in the specific parts of the genome that code directly for the proteins that our bodies are made of. And those kinds of genetic variants can actually have quite large effects on human traits when they're present in a small number of people.
RASCOE: The gene they analyzed is called TUBB4B. If someone has a particular variant of this gene, Francks says that person is very likely to be left-handed. But very few people, even very few left-handed people, have this variant.
FRANCKS: They're very rare in the population, so they would only be accounting for about 1 in 1,000 left-handers at most.
RASCOE: It's still very much a mystery why the vast majority of left-handed people are that way. His idea about random fluctuations of chemicals in the embryo is also unproven. But what about this question?
I've also heard that left-handed people are more creative because the left side of the body's controlled by the right side of the brain, and that's the creative side. Is that true?
FRANCKS: Yeah. I mean, I don't think that's true. No.
RASCOE: (Laughter).
FRANCKS: I know that this is - it's a popular thing. That's too simple. The differences between the hemispheres of the brain are much more subtle and complex than that, and each side is doing important things in any particular task that you're doing.
RASCOE: OK, so - but we are very special if we're left-handed. Science has confirmed that, right?
FRANCKS: Well, 10%, you know? You decide how special that is.
RASCOE: Oh, well, thank you. I appreciate that. I'll take that. Thank you so much. Clyde Francks, a geneticist at the Max Planck Institute for Psycholinguistics in the Netherlands. Thank you so much for joining us.
FRANCKS: Thank you.
(SOUNDBITE OF LITTLE COMETS SONG, "JENNIFER")
Copyright © 2024 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.

IMAGES
VIDEO
COMMENTS
This bibliography cites and summarizes publications from American Thoracic Society journals and others centered on the year 2021 that inform our understanding of the pathophysiology, clinical manifestations, vaccines, and treatment advances in COVID-19 while considering broader effects on society, healthcare delivery, and medical education.
This bibliography accompanies the roundtable, "The Best-Laid Plans: Adapting Research to COVID-19," organized by Brooke Jespersen for the 2021 SPA meeting. During the roundtable, the contributors—Eileen Anderson-Fye, Ramsey Ismail, Brooke Jespersen, Sonya Pritzker, Julia
" Selected Bibliography of Recent Research in COVID-19." American Journal of Respiratory and Critical Care Medicine , 206(12), pp. 1548-1562 Correspondence and requests for reprints should be addressed to Benjamin D. Singer, M.D., Northwestern University Feinberg School of Medicine, 303 E. Superior Street, Simpson Querrey, 5th Floor, Chicago ...
COVID-19 Bibliography ... COVID-19: COVID-19-associated cystitis." Journal of Clinical Medicine Research 12(10): 681-682. Full Text Department of Urology Diokno AC and Devries JM (2020). "The impact of COVID-19 on urologic practice, medical education, and training." ... brains of COVID-19 patients are suggested as being a possible key to ...
Developed by McMaster University, Canada to support coordination of research activities and decision making with evidence-synthesis, technology assessment and guideline-development. University of Toronto Libraries COVID-19 Data Resources: A collection of data, map and visualization resources and tutorials related to COVID-19 compiled by the ...
The COVID-19 pandemic saw a massive mobilization of the scientific workforce. We evaluated the citation impact of COVID-19 publications relative to all scientific work published in 2020 to 2021, finding that 20% of citations received to papers published in 2020 to 2021 were to COVID-19-related papers. Across science, 98 of the 100 most-cited ...
The American Historical Association compiled a professionally vetted bibliography of historians' responses to COVID-19, published from March 2020 to March 2021, as a resource for the public, teachers, and scholars seeking historical perspectives on the crisis and its local and global impacts. The bibliography includes commentary and ...
Background The COVID-19 pandemic is the biggest worldwide health challenge in this century. Research concerning the role of children in the spread of SARS-CoV-2, and investigating the clinical effects of infection in children, has been vital. This paper describes the publication trend for pertinent scientific literature relating to COVID-19 in children during the first 6 months of the pandemic.
Abstract. The COVID-19 pandemic requires a fast response from researchers to help address biological, medical, and public health issues to minimize its impact. In this rapidly evolving context, scholars, professionals, and the public may need to identify important new studies quickly. In response, this paper assesses the coverage of scholarly databases and impact indicators during March 21 ...
Since COVID-19 literature has almost totally been written in 2020, most citations include DOIs. Additionally, many citations appear in databases during the pre-print stage, meaning they are often incomplete, so it is important to deduplicate/merge across multiple databases to create complete references. A database of this size requires a lot of ...
This site includes the Citation Quick Guide, Chicago Style Q & A, and related resources. Provides access to the online version of the 9th edition of the MLA Handbook. The site includes practice exercises, tutorials, and the MLA Guide to Undergraduate Research in Literature and MLA Guide to Digital Literacy.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download: All articles in citation management software format ... The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to ...
Explanation: The content of the Research + Activism Bibliography is kept as a group library in the Zotero bibliography manager, and then pulled into this WordPress site through the ZotPress plug-in. Showing the bibliography on our WordPress site allows us to organize and narrate tagged categories to create what amounts to a conceptual map. But search capabilities are simpler.
This is an annotated bibliography of some of the relevant scientific literature on SARS-CoV-2 and Covid-19. The nursing profession is rooted in science and the protection of nurses should also reflect both emerging scientific research and the precautionary principle. The precautionary principle asserts that we should not wait for scientific proof of harm before taking action to protect people ...
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused substantial morbidity and mortality, and serious social and ...
This cross-sectional study creates a dataset and dashboard of US state- and territory-level COVID-19 policies specific to nursing homes and home health ... Citation. Stone PW, Zhao S, ... This work was funded by grant R01NR016865 from the National Institute of Nursing Research and by grant R01AG074492 from the National Institute on Aging, ...
JongSerl Chun, Ph.D., is a Professor in the Department of Social Welfare at Ewha Womans University, where she also assumes the pivotal role of Director at the Institute for Social Work Research, located in Seoul, South Korea.With a profound commitment to advancing social welfare practices and research, Dr. Chun's scholarly pursuits encompass a wide array of critical topics, notably focusing ...
Citation 18, Citation 19 IL-8, IL-6 and IL-2R are interleukins, a kind of cytokines, were identified as significant factors in predicting COVID-19 patient outcomes, which was proved in our study. These molecules exert profound biological effects even at low concentrations and play a pivotal role in modulating immune responses and the growth of ...
A retrospective cohort study of patients hospitalized with COVID-19 found that the most common causes of death were respiratory failure and septic shock with multiorgan dysfunction syndrome because of COVID-19 or a superinfecting pathogen, underscoring the paradigm that critical COVID-19 is best understood as severe community-acquired pneumonia.
Citation: Study finds COVID-19 pandemic led to some, but not many, developmental milestone delays in infants and young children (2024, April 22) retrieved 23 April 2024 from https://medicalxpress ...
Citation: Significant global variation in COVID-19 guidelines: Most countries recommend at least one treatment that doesn't work (2024, April 22) retrieved 22 April 2024 from https://medicalxpress ...
Three years into the COVID-19 outbreak in the United States, Pew Research Center published this collection of survey findings about Americans' challenges with mental health during the pandemic.All findings are previously published. Methodological information about each survey cited here, including the sample sizes and field dates, can be found by following the links in the text.
Following the coronavirus outbreak, reports of discrimination and violence toward Asian Americans increased. A previous Pew Research Center survey of English-speaking Asian adults showed that as of 2021, one-third said they feared someone might threaten or physically attack them. English-speaking Asian adults in 2022 were also more likely than other racial or ethnic groups to say they had ...
Genes play a very small role in determining left-handedness, research finds NPR's Ayesha Rascoe speaks with Clyde Francks, a geneticist in the Netherlands, about the latest research into what ...